Abstract
Lichens are the dominant organisms in terrestrial Antarctic ecosystems and show a decline in species number, coverage, and growth rate from the maritime Antarctic (62°S) to the McMurdo Dry Valleys (78°S). While Livingston Island (maritime Antarctica) is a hot spot for lichen biodiversity, the McMurdo Dry Valleys (continental Antarctica) are known as one of the most extreme environments for life. Previous studies suggest the biodiversity gradient to be linked to water availability acting through length of active period, but no activity data are available for the Dry Valleys. The work presented here compares metabolic activity of lichens at Livingston Island and the Dry Valleys for 4½ months from continuous monitoring that involves concurrent measurements of chlorophyll fluorescence and microclimate. The latitudinal comparison involves two contrasting habitats for plant physiological activity and microclimate. Two species of the foliose genus Umbilicaria were monitored in both regions plus one sample of the crustose Caloplaca in the Dry Valleys. The results showed a very large difference in the duration of activity over the monitoring period, and this supports the different coverage, species abundance, and growth rates already reported for lichens between both regions. Despite this large difference in activity, and in habitat conditions, analysis of the activity behaviour of the two Umbilicaria species shows interesting common features, while the crustose Caloplaca had additional strategies to improve hydration. This offers one explanation for the abundance of crustose lichens inside the Valleys, indicating better adaptation strategies to a polar desert.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although Antarctica is the coldest, driest and windiest continent on Earth, its vast territory encompasses a wide range of climatic and geographical conditions, milder and wetter in the North, coldest, driest, highest and windiest towards South. The McMurdo Dry Valleys (78°S, Victoria Land, Ross Sea region) are considered as one of the most extreme environments for life on Earth. The mean annual temperature is −20 °C, the annual precipitation between 50 and 100 mm (Fritsen et al. 2000), and the relative humidity is extremely low, which means that the region is a Polar Desert (Ross Desert). In contrast, the mean annual temperature for the South Shetland Islands is between −2 and −3 °C, with an annual precipitation of at least 400–500 mm (Øvstedal and Smith 2001) and high mean relative humidity values (Longton 1988). Extrapolating this climatic contrast to biodiversity reveals that Livingston Island has 187 lichen species in 3 km2 of ice free land, around one half of all the lichen species in the Antarctic region (Søchting et al. 2004), while a recent work lists 27 lichen species for the Dry Valleys (Pérez-Ortega et al. 2012). The ecosystems in the Dry Valleys are simple and are normally restricted to microniches where the climatic conditions are milder such as the slopes of the surrounding mountains, where clouds and snow falls are more frequent. The floor of the Dry Valleys represents a region where life approaches to its environmental limit (Doran et al. 2010).
Green et al. (2011a) proposed two zones to understand Antarctic terrestrial biodiversity, a macroenvironmental zone north of about 72°S where biodiversity is coupled with mean annual temperature and a microenvironmental zone south of about 72°S where species richness is determined by light, water availability and temperature occurring at different sheltered microsites. Reasonably high biodiversity of lichens can occur such as in the proximity of the Beardmore Glacier, Transantarctic Mountains, 84°S, where the total of close to 40 species recorded (Green et al. 2011b) is a higher species abundance than in most of other northern locations inside the Ross Sea region.
The latitudinal cline not only affects species number but is also linked with a clear decrease in vegetation cover, from almost complete cover in the north of maritime Antarctica to isolated patches within the continent (Green et al. 2011a). There is also a marked decrease in growth rates and Sancho et al. (2007) showed differences in growth rates of two orders of magnitude between crustose lichens in the South Shetland Islands and in the McMurdo Dry Valleys. Very low growth rates have been reported also for mosses in the continent (Selkirk and Skotnicki 2007). This wide range of biological responses along the latitudinal gradients opens interesting opportunities for research in the area. The Intergovernmental Panel for Climate Change (www.ipcc.ch) considers the polar and alpine regions to be especially vulnerable to environmental changes. Maritime Antarctica is one of the world’s most threatened area by these changes, and has already experienced temperature rises and severe ice melting (Convey 2006). The fact that terrestrial life is strongly coupled to macroenvironmental conditions in a part of Antarctica positions cryptogamic vegetation as bioindicators in a pristine and fragile environment where other drivers for change are absent.
If lichens and mosses are to be used to follow the impact of possible climatic changes in Antarctica then one method of detecting these changes is by obtaining large datasets linking microclimate and physiological performance in the field. Being poikilohydric, cryptogams are only active when wet, and this means that they are typically metabolically inactive under the harshest climatic conditions and active when the environment allows physiological activation. Microclimatic conditions become relevant because vegetation in many parts of Antarctica is active under more moderate conditions than those suggested by mesoclimate (Green et al. 2007). The development of robust equipment at the beginning of the 1990s with the capability of measuring chlorophyll a fluorescence in the field allowed monitoring of plant activity in Antarctica (see Schroeter et al. 1991). Since then, several studies has been developed as technology has improved (Schroeter et al. 1995, 1997; Pannewitz et al. 2003, 2006), adding new information about lichens’ ecophysiological performance in the field in Antarctica. Schroeter et al. (2010, 2011) have reported the longest period of lichen monitoring in Antarctica and also along the largest climatic differences, measuring microclimate at samples level and metabolic activity through an on/off fluorescence signal due to battery limitations related with the long monitoring period.
In this research, we continue the approach of long-term plant monitoring in Antarctica using the last technological improvements, in particular the MoniDA, a device able to measure concurrently the metabolic activity and the microclimate of the sample and to report the data immediately to a web page through satellite connection. In addition to new equipment, we include new species, new growth form and a more extreme site that in previous works. We chose Livingston Island and the Dry Valleys as our monitoring sites because they are good examples of Antarctic extremes for life; one appears to be near optimal for cryptogams development, while the other represents the edge for terrestrial life on Earth. The comparison proposed tries to solve the existing gaps in the understanding of vegetation patterns between both regions.
Materials and methods
Localities and species chosen
Livingston Island is the second largest of the South Shetland Islands, in the northern part of maritime Antarctica. Since 1988 the Island holds the Spanish Antarctic Base Juan Carlos I. The vegetation, climate, geology and geomorphology of the Island have been already well described (López-Martínez et al. 1991; Sancho et al. 1999; Søchting et al. 2004). The monitoring device was installed between the base and the sea, on a rock with coordinates 62°39′46′′S 60°23′20′′W. The species monitored was Umbilicaria antarctica Frey and I.M. Lamb, a foliose lichen which is an Antarctic endemic and is widely distributed through maritime Antarctica. A description of the microhabitat chosen for the study can be found in Schroeter et al. (2010).
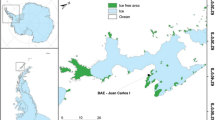
The McMurdo Dry Valleys are in continental Antarctica, southern Victoria Land. They contain many valleys of different size that together form the largest ice free area in Antarctica (Pointing et al. 2009) and are protected as an Antarctic Special Managed Area (ASMA) by the Scientific Committee for Antarctic Research (SCAR, see www.scar.com). The research effort developed by the New Zealand Latitudinal Gradient Project (www.lgp.aq) is providing considerable amounts of information concerning the characteristics of these special ecosystems (see Howard-Williams et al. 2006, 2010), and the region is becoming well studied and documented (Hogg et al. 2006; Cary et al 2010; Doran et al. 2010; Cowan et al. 2011). The monitoring station was installed in Garwood Valley, 78°01′36.4′′S 163°53′51′′, close to a frozen lake. The samples chosen were three thalli of the well-known continental Antarctic lichen Umbilicara aprina (Nyl.) situated in a big stone with some level of inclination and one sample of the orange and widespread crustose genus Caloplaca sp. present in a flat and smaller stone nearby the Umbilicaria population. Both locations have been depicted in a map for geographical comparisons (Fig. 1, ArcGIS 10.1 program).
MoniDA, description of the monitoring station
The device is small, portable and powered by an internal battery boosted by an external solar panel (Fig. 2). It sends the data recorded daily through satellite connection to a web page, which is an improvement compared with previous works in Antarctica regarding plant physiological performance. Each of the four probes available monitors one sample and is equipped with a light and temperature sensor to measure microclimate and one fibre optic to monitor the physiological performance. The methodology used to track the metabolic activity through chlorophyll a fluorescence is the widespread saturation pulse method (see Schreiber et al. 1994; Maxwell and Johnson 2000). At a selected time interval of 60 min, the sample is illuminated with a low-intensity modulated measuring light and the resulting chlorophyll a fluorescence is recorded (F t). A high-intensity, actinic flash of light (duration about 1 s, 4000 μmol m−2 s−1) is then applied, and the resulting maximal fluorescence is recorded (F′m). The effective quantum use efficiency of PSII (Φ PSII, Yield) can then be calculated as Φ PSII or Yield = (F′m−F t)/F′m. When this ratio is calculated after a period of darkness, in the field situation this means at night or over winter, it represents the maximum quantum use efficiency. In addition to F t, F′m and yield, the relative electron transport rate of the PSII (ETR = Yield × Light × 0.5 × 0.84) is also calculated, and this can provide qualitative information about photosynthetic CO2 fixation.
The MoniDA has an internal data filter to avoid false activity values based on F t (F 0 in the dark) and F′m (F m under dark) lower limits. If F t/F 0 is below 10 measuring units and if/or F′m/F m is below 50 U, the device does not report any yield values. There are cases with no reported metabolic activity (which means yield = 0) when the F t/F 0 and F′m/F m values were consistently different. We have, therefore, applied a second filter based in the differences between F′m/F m and F t/F 0 during inactive periods. To overcome the inherent variability at low signal levels, we have calculated the means for all these measuring points. By these means, we have avoided possible false activity values due to the low F t/F 0 and F′m/F m signal obtained in the Dry Valleys.
In addition to the MoniDA, we have used data from two other devices at both monitoring areas. First, in Livingston Island, we have used a relative humidity sensor placed between lacinia of a thalii of Usnea aurantiaco-atra (Jacq.) Bory close to where the U. antarctica was monitored. In the Dry Valleys, we used a photographic camera (placed in the nearby Miers Valley) to take pictures at 30 min intervals of the area. This helped us to identify snow events that are likely to be common between neighbouring Valleys.
Results
The F′m (F m in the dark), the Yield, the temperature (T) and the photosynthetic photon flux density (PPFD) for U. antarctica in Livingston Island over the whole measuring period are plotted in Fig. 3. Metabolic activity occurred over the whole measuring period (Fig. 3a, d). Yield values were slightly lower during January and most of February, reaching maximal values at the end of the later month and remaining high afterwards. The microclimate also showed clear trends, with the highest T being at the same time as high PPFD values. Linkages between microclimate and metabolism can also be seen with periods of lowest activity occurring when the T and the PPFD were highest, a result of the drying of the lichen monitored due to those environmental conditions. The lower temperature limit for activity was at about −4 °C (Fig. 4a, b).
Diel patterns of thallus temperature (T, black line) and metabolic activity (Yield, dotted grey line) from April 24 to May 7 for Umbilicaria antarctica in Livingston Island, maritime Antarctica (a) and Yield and temperature for the same thallus over the complete measuring period (January 20–June 2, b)
Metabolic activity and microclimate are shown in Fig. 5 for one sample of the foliose U. aprina and for the crustose Caloplaca sp. in the Dry Valleys. The measurement period is the same as at Livingston Island. The two other samples of U. aprina monitored in the Dry Valleys did not show metabolic activity at all during the whole measuring period (see electronic supplementary material, ESM). Metabolic activity as indicated by elevated F′m and Yield (Fig. 5a, d) was sporadic and rare for both species but especially for U. aprina. The latter species was only active at two isolated times at the end of January with two F′m peaks linked to Yield values. After these events, the F′m baseline returned to low and steady values indicating that the activity period of the U. aprina was over, probably until the next summer when ice melting could initiate new activity. The situation for the crustose Caloplaca sp. was different. Although the metabolic activity was also concentrated in isolated events (groups of activity periods), the frequency of metabolic activation was higher and continued until the late autumn (Fig. 5d).
Maximum fluorescence (F′m, F m during dark, a), thallus temperature (T, b), photosynthetic photon flux density (PPFD, c) and metabolic activity (Yield, d) for Umbilicaria aprina (black line) and Caloplaca sp. (dotted grey line) in the Dry Valleys, continental Antarctica, over the whole monitoring period (January 20–June 2). The different activity events detected are indicated with numbers
In order to understand the overall physiological behaviour of the samples, the recorded activities have been grouped into four events. Event 1 occurred only in Caloplaca sp. between January 21st and 25, with clear F′m and Yield peaks on those 2 days. The common features of the activity pattern were initiation of activity during night with low Yield, T and PPFD values followed by a peak of Yield in the early morning coinciding with increases in T and PPFD. Event 2 had similar activity features to event 1: they both occurred in January (mid-Antarctic summer), and they both showed very clear increases in F′m. Event 2 had the highest Yield values of the total measuring period, and most importantly, it was the only period when the two different species activated their metabolism concurrently. In order to understand this event better it is amplified and plotted in Fig. 6. It ran from January 28 until January 31, with both species active on the first and the last day of the event. The first day, the 28, had microclimatic features common to both samples which were depressed and stable T and PPFD over the whole day (Fig. 6b, c), very different from the normal daily microclimatic pattern of midday peaks in temperature and PPFD. The individual activity patterns for U. aprina and Caloplaca sp. during event 2 were different. While U. aprina only had isolated Yield values during the day, Caloplaca sp. shows a regular and daily activity pattern of the same form seen in event 1 with activity starting during night at low T and PPFD and then activity ending as PPFD and T increased in the early morning (Fig. 6a, d).
Maximum fluorescence (F′m, F m during dark, a), thallus temperature (T, b), photosynthetic photon flux density (PPFD, c) and metabolic activity (Yield, d) for Umbilicaria aprina (normal line) and Caloplaca sp. (dotted line) in the Dry Valleys, continental Antarctica, during event 2 (28–31 January 2010)
Events 3 and 4 also shared common characteristics: they were both late in the summer season, they showed an unusual microclimate, and the activity pattern was not clear because although F′m was generally elevated there was a lack of obvious peaks. Temperature and PPFD were both stable during these events (Fig. 5b–d). The PPFD values during event 3 were low but recovered to previous levels at the end of the event. Event 4 occurred at the end of March and beginning of April, which is early winter. It had very low T (around −20 °C) and also very low PPFD that do not recover after the event indicating the beginning of the Antarctic night, which started just a few weeks later. (Note that for each of the different figures plotted, the scaling of the axis is adjusted to the maximum values obtained for each time period depicted.)
In Table 1, it can be seen how, while the mean T during activity is higher than during the complete measuring period in U. antarctica from Livingston and in U. aprina from the Dry Valleys, they are equal in the case of Caloplaca sp. from the Dry Valleys. The PPFD is lower during activity than during the complete measuring period in Livingston, but the opposite in the two lichens from the Dry Valleys, especially in U. aprina. Activity in Livingston occurred for half of the measured time period, with a half of that activity occurring during the night. The activity periods in the Dry Valleys are very much shorter, being two orders of magnitude lower in U. aprina compared with U. antarctica in Livingston. Activity without light is also lower in the Dry Valleys, with U. aprina showing no activity at all during night. Finally, the sum of ETR, a qualitative indicator of photosynthetic performance, is higher in Livingston compared with the Dry Valleys, result of the very much longer activity period for the former site.
Discussion
The results from over 4 months of monitoring of lichen activity at Livingston Island (62°S Antarctic Peninsula) and Garwood Valley (78°S McMurdo Dry Valleys) show around two orders of magnitude difference in length of activity period. The actual differences between U. antarctica in the Peninsula with activity of 45.6 % of total time were 250 times for U. aprina (0.2 % active) and 25 times for Caloplaca sp. (1.7 % active) in the Dry Valleys. It is a weakness of the comparison reported here that different lichen species had to be used at the two sites. However, Schlensog et al. (2013) compared several different species at one site (Leonie Island, 65°S) and found only small differences in active time for similar species; three Umbilicaria species, for example, had activities that fell within a range of 22.97–25.03 %. The substantial differences found here are real and reflect differences in environmental drivers between the sites. Sancho et al. (2007) found two orders of magnitude difference in growth rate for crustose lichens across Antarctica and the similar magnitude of difference in the results presented here for folious lichens provide strong support that length of active time is an important driver of differences in productivity and consequential growth. They also support the proposals of Green et al. (2011a) that activity period related to degree days (an indicator of liquid water availability) is more important than differences in actual photosynthetic parameters of the lichens. A very interesting aspect is the finding that despite the Dry Valleys being one of the more extreme desert environments in the world the two lichens studied appear to show different strategies in their water relations.
The latitudinal range studied here spans 16° and the northern site, Livingston Island, lies in the maritime Antarctic, an area much warmer and wetter than continental Antarctica. The high activity period for U. antarctica, of 45.6 % is, therefore, not surprising as similar magnitude values have already been reported (Schroeter et al. 2010). High and almost continuous values for yield suggests that hydration of U. antarctica was by rain or melting snow for long periods in March, April and May (Fig. 3). However, yields in late January and February were lower and more sporadic indicating sub-optimal hydration possibly through high humidity or sporadic but not heavy rain events. Mean temperature when active was 1.1 °C, slightly warmer than the overall mean of 0.5 °C and the minimum was higher and the maximum lower showing a clear tendency to more moderate conditions. This is similar to the results from Schlensog et al. (2013) and Schroeter et al. (2010) and conforms to their suggestions that lichen temperatures when active are very similar across Antarctica. Mean PPFD when active was lower than totals, which is a reflection of the very cloudy climate of the northern Antarctic Peninsula and adjacent islands.
The activity periods were considerably shorter in the Dry Valleys. Of the three thalli of U. aprina chosen for the study, only one showed any metabolic activity and then only 0.2 % of the total measurement period. The low rates are not unexpected as the valleys are extremely dry with an erratic precipitation of around 50–100 mm rain. The activity of U. aprina was confined to one event on 28 January and two on 31 January. The marked depression in PPFD and stability in temperature on 28 and 29 January, and also the high PPFD associated with the events (1214 µmol m−2 s−1) all suggest that the activation was by a snow fall/melt (Fig. 6). It is known that hydrated U. aprina thalli are very tolerant of high PPFD at levels approaching full sunlight in Granite Harbour (Kappen et al. 1998a). Hydration predominantly by snow melt is reported for the crustose lichen Buellia frigida and the U. aprina at Granite Harbour (Kappen et al. 1998b; Schroeter et al. 2010). The percentage of activity for U. aprina in the Dry Valleys is lower than the 7 % reported by Schroeter et al. (2011) for the same species in Botany Bay (Continental Antarctica), but it cannot be forgotten that the periods reported in both researches are different. Another important question is until which extent the activity reported in the Dry Valleys could be related with real carbon fixation (i.e: when activity moments with PPFD coincides with light higher than the compensation points, LCP, of each species). Although we do not have the LCP points of our species and these are also highly T dependent and species and habitat specific, LCP from 25 to 100 µmol m−2 s−1 at a T range of −5 to 15 °C have been reported for lichens from Continental Antarctica. (Kappen 1989; Kappen et al. 1998a). This information, together with the proven high photo-tolerance (Kappen et al. 1998a), point strongly to real carbon fixation in events 1 and 2, with some possibilities also during Event 3.
The other species monitored in the Dry Valleys, Caloplaca sp., had a longer active time of 1.7 % which occurred in four events (events 1–4, Fig. 5). The second of these events, Event 2, coincided with the activity of U. aprina and close examination shows that Caloplaca was exploiting an additional hydration source apparently unavailable to U. aprina (Fig. 5). In Event 2, U. aprina had three yield peaks and about four of the yield peaks of Caloplaca sp. coincided with these indicating snow melt hydration. However, many of the remaining yield peaks of Caloplaca sp. occurred in the early morning with activity being initiated during the night (night means low PPFD but not darkness). The activity coincided with the lowest temperatures and PPFD on 29 and 30 January. The most probable explanation of these yield peaks was that they represented frost deposition which then became available to Caloplaca sp. either by water vapour transfer or by melt as PPFD increased. Event 1, which occurred a few days earlier than event 2 (Fig. 5), only had activity by Caloplaca sp. indicating that it was also probably frost initiated. The very similar PPFD when active and inactive (216 and 129 µmol m−2 s−1, respectively) suggests that different hydration strategies are being used compared to U. aprina (Table 1).
Event 3 occurred in late February and event 4 around the beginning of April (Fig. 5). For both events, the steady temperature with no diurnal warming, and the low PPFD, especially clear for event 3, suggest snow falls and subsequent disappearance of the snow. An automatic photographic camera placed in the neighbouring Miers Valley reported big snow events on these days with subsequent disappearance on these days. Caloplaca sp., and less so U. aprina, showed elevated F′m at these times, but positive yields were seen only for Caloplaca sp. There was no possibility of snow melt in event 3 (around −10 °C) or event 4 (around −30 °C) so hydration was clearly by water vapour transfer from the snow, a phenomenon demonstrated for green algal lichens by Schroeter et al. (1994) and Scheidegger et al. (1995).
Lichens in the Dry Valleys seem to exploit three sources for hydration, snow melt, water vapour transfer from snow and dew/frost events. The latter is utilised only by Caloplaca sp. possibly because of its more horizontal position compared with the more vertical U. aprina. Such a horizontal situation would give greater exposure to the cold sky overnight and lead to lower surface temperatures and finally frost formation. Our interpretation of these results is that thin ice sheets form over the horizontal surface of the sample during the night followed by the melting of the ice during sunrise, providing the liquid water necessary for the peaks in the yield. Büdel et al. (2008) showed how endolithic cyanobacterial communities became active through dew and frost in the Dry Valleys. Kieft (1988) proposed that ice nucleation agents on the lichen thalli could enhance the deposition of thin ice layers over their surface in cold environments in a form of water capture. The combination of activity at very low water potentials and temperatures, surface area reduced to a minimum (Kappen 1988), aridity tolerance and the ability of enhance ice formation and taking profit from it could explain why crustose lichens tend to dominate the landscape in a polar desert such as the McMurdo Dry Valleys.
The minimum temperature recorded for activity in Caloplaca sp. was −29.4 °C (event 4), but the low F t and F m signals linked to that activity must make us cautious considering this data. In order to put the −29.4 °C in context in relation with the literature, Schroeter et al. (1994) reported positive net photosynthesis in the field in Continental Antarctica at −17 °C with the value being set by instrumental limitations. In the laboratory, Lange (1965) measured positive net photosynthesis at −24 °C. This ability by Caloplaca sp. would be an extraordinary adaptation phenomenon to dryness and freezing and would possibly allow some metabolic activity such as protection of important structures to occur.
The differences in growth rate of two orders of magnitude proposed by Sancho et al. (2007) have been shown to be correlated with differences of two orders of magnitude in metabolic activity between both regions, even taking into account the highest activity during dark in maritime Antarctica. Our results provide a physiological basis to understand the differences in growth rates, biomass and coverage reported between both extremes in the continent and suggest that change in productivity is a major contributor to the biodiversity gradients. The large difference in growth rates and percentage activity confirm that lichens are excellent organisms to monitor and predict possible consequences of environmental changes in Antarctica. Lichens are well known to be classified as stress organisms on the C-S-R strategy template of Grime (Grime and Pierce 2012). However, it is perhaps surprising that even in the extreme habitat of the Antarctic Dry Valleys at least three strategies were found to enhance hydration and that Caloplaca sp. managed almost an order of magnitude greater activity by utilising frost and water vapour transfer as water sources in addition to snow melt.
References
Büdel B, Bendix J, Bicker FR, Green TGA (2008) Dewfall as a water source frequently activates the endolithic cyanobacterial communities in the Granites of Taylor Valley, Antarctica. J Phycol 44:1415–1424
Cary SC, McDonald IR, Barret JE, Cowan DA (2010) On the rocks: the microbiology of Antarctic Dry Valleys soils. Nature Rev Microbiol 8:129–138
Convey P (2006) Antarctic climate change and its influences on terrestrial ecosystems. In: Bergstrom DM, Convey P, Huiskes HL (eds) Trends in Antarctic terrestrial and limnetic ecosystems. Springer, Dordrecht, pp 253–272
Cowan DA, Pointing SB, Stevens MI, Cary SC, Stomeo F, Tuffin IM (2011) Distribution and abiotic influences on hypolithic microbial communities in an Antarctic Dry Valley. Polar Biol 34:307–311
Doran PT, Lyons WB, McKnight DM (2010) Life in Antarctic deserts and other cold dry environments: astrobiological analogs. Cambridge University Press, Cambridge
Fritsen CH, Grue AM, Priscu JC (2000) Distribution of organic carbon and nitrogen in surface soils in the McMurdo Dry Valleys, Antarctica. Polar Biol 23:121–128
Green TGA, Schroeter B, Sancho LG (2007) Plant life in Antarctica. In: Pugnaire FI, Valladares F (eds) Functional plant ecology, 2nd edn. CRC Press, Boca Ratón, pp 389–434
Green TGA, Sancho LG, Pintado A, Schroeter B (2011a) Functional and spatial pressures on terrestrial vegetation in Antarctica forced by global warming. Polar Biol 34:1643–1656
Green TGA, Sancho LG, Türk R, Seppelt RD, Hogg ID (2011b) High diversity of lichens at 84°S, Queen Maud Mountains, suggest preglacial survival of species in the Ross Sea region, Antarctica. Polar Biol 34:1211–1220
Grime JP, Pierce S (2012) The evolutionary strategies that shape ecosystems. Wiley-Blackwell, Oxford
Hogg I et al (2006) Biotic interaction in terrestrial Antarctic ecosystems. Are they a factor? Soil Biol Biochem 38:3035–3040
Howard- Williams C, Peterson D, Lyons WD, Cattaneo-Vietti R, Gordon S (2006) Measuring ecosystem response in a rapidly changing environment: the latitudinal gradient project. Antarct Sci 18:465–471
Howard- Williams C, Hawes I, Gordon S (2010) The environmental basis of ecosystem variability in Antarctica: research in the latitudinal gradient project. Antarct Sci 22:591–602
Kappen L (1988) Ecophysiological relationships in different climatic regions. In: Galun M (ed) Handbook of lichenology, vol II. CRC Press, Boca Ratón, pp 37–100
Kappen L (1989) Field measurements of carbon dioxide exchange of the Antarctic lichen Usnea sphacelata in the frozen state. Antarct Sci 1:31–34
Kappen L, Schroeter B, Green TGA, Seppelt RD (1998a) Chlorophyll a fluorescence and CO2 exchange of Umbilicaria aprina under extreme light stressing the cold. Oecologia 113:325–331
Kappen L, Schroeter B, Green TGA, Seppelt RD (1998b) Microclimatic conditions, meltwater moistening, and the distributional pattern of Buellia frigida on rock in a southern continental Antarctic habitat. Polar Biol 19:101–106
Kieft TL (1988) Ice nucleation activity in lichens. App Environ Microbiol 54:1678–1681
Lange OL (1965) Der CO2 Gaswechsel Von Flechten Bei Tiefen Temperaturen. Planta 64:1–19
Longton RE (1988) Biology of polar bryophites and lichens. Cambridge University Press, Cambridge
López-Martínez J, Martínez de Pisón E, Arche A (1991) Pulsaciones glaciares y terrazas marítimas escalonadas en los alrededores de la Base Antártica Española, Isla Livingston, Shetland del Sur. Geogaceta 9:44–47
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence-a practical guide. J Exp Bot 51:659–668
Øvstedal DO, Lewis Smith RI (2001) Lichens of Antarctica and South Georgia. A guide to their identification and ecology. Cambridge University Press, Cambridge
Pannewitz S, Schlensog M, Green TGA, Sancho LG, Schroeter B (2003) Are lichens active under snow in continental Antarctica? Oecologia 135:30–38
Pannewitz S, Green TGA, Schlensog M, Seppelt R, Sancho LG, Schroeter B (2006) Photosynthetic performance of Xanthoria mawsonii CW Dodge in coastal habitats, Ross Sea region, continental Antarctica. Lichenol 38:67–81
Pérez-Ortega S, Ortiz-Álvarez R, Green TGA, De Los Ríos A (2012) Lichen myco- and photobiont diversity and their relationships at the edge of life (McMurdo Dry Valleys, Antarctica). FEMS Microbiol Ecol 82:429–448
Pointing SB, Chan Y, Lacap DC, Lau MCY, Jurgens JA, Farrell RL (2009) Highly specialized microbial diversity in hyper-arid polar desert. Proc Natl Acad Sci USA 106:19964–19969
Sancho LG, Schulz F, Schroeter B, Kappen L (1999) Bryophite and lichen flora of South Bay (Livingston Island, South Shetland Islands, Antarctica). Nova Hedwigia 68:301–337
Sancho LG, Green TGA, Pintado A (2007) Slowest to fastest: extreme range in lichen growth rate supports their use as an indicator of global change in Antarctica. Flora 202:667–673
Scheidegger C, Schoreter B, Frey B (1995) Structural and functional processes during water vapour uptake and desiccation in selected lichens with green alga photobionts. Planta 197:399–409
Schlensog M, Green TGA, Schroeter B (2013) Life form and water source interact to determine active time and environment in cryptogams: an example from the maritime Antarctic. Oecologia 173:59–72
Schreiber U, Bilger W, Neubauer C (1994) Chlorophyll fluorescence as a non intrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze ED, Cadwell MM (eds) Ecophysiology of Photosynthesis. Springer, Berlin, pp 49–70
Schroeter B, Kappen L, Moldaenke C (1991) Continuous in situ recording of the photosynthetic activity of Antarctic lichens. Established methods and a new approach. Lichenologist 23:253–265
Schroeter B, Green TGA, Kappen L, Seppelt RD (1994) Carbon dioxide exchange at subzero temperatures. Field measurements on Umbilicaria aprina in Antarctica. Cryptogam Bot 4:233–241
Schroeter B, Olech M, Kappen L, Heitland W (1995) Ecophysiological investigations of Usnea antarctica in maritime Antarctic. I Annual microclimatic conditions and potential primary production. Antarc Sci 7:251–260
Schroeter B, Kappen L, Schulz F (1997) Long-term measurements of microclimatic conditions in the fruticose lichen Usnea aurantiaco-atra in the maritime Antarctic. Actas del V Simposio de Estudios Antárticos, Barcelona, pp 63–69
Schroeter B, Green TGA, Pannewitz S, Schlensog M, Sancho LG (2010) Fourteen degrees of latitude and a continent apart: comparison of lichen activity over two years at continental and maritime Antarctic sites. Antarc Sci 22:681–690
Schroeter B, Green TGA, Pannewitz S, Schlensog M, Sancho LG (2011) Summer variability, winter dormancy: lichen activity over 3 years at Botany Bay 77°S latitude, continental Antarctica. Polar Biol 34:13–22
Selkirk PM, Skotnicki ML (2007) Measurement of moss growth in continental Antarctica. Polar Biol 30:407–413
Søchting U, Øvstedal DO, Sancho LG (2004) The lichens of Hurd Peninsula, Livingston Island, South Shetlands, Antarctica. Bibl Lichenol 88:607–658
Acknowledgments
This research was funded by a grant of the Spanish Ministerio de Ciencia (2007–2011) CGL2006-12179-CO2-01. We are indebted to the Spanish Antarctic program, all the staff from Unidad de Tecnología Marina (UTM) and Juan Carlos I Spanish Antarctic Base, and also to the New Zealand Antarctic program, Antarctica New Zealand and Scott Base staff. Part of the research is in the frame of the New Zealand Latitudinal Gradient Project (LGP, www.lgp.aq) and we have to thank to the New Zealand Government the financial support and the logistics for the expedition to the Dry Valleys. The New Zealand Foundation for Research, Science and Technology (FRST) and the Department of Biological Sciences, University of Waikato provided financial support. The research was supported by the FRST IPY grant, Understanding, valuing and protecting Antarctica’s unique terrestrial ecosystems: predicting biocomplexity in Dry Valley ecosystems. We also thank to Dr. Roman Türk for all his help in the field and for finding the population of Umbilicaria aprina inside Garwood Valley and to Alberto Benavent for his help with Fig. 1.
Conflict of interest
The authors declare that they have not conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is an invited contribution on Life in Antarctica: Boundaries and Gradients in a Changing Environment as the main theme of the XIth SCAR Biology Symposium. J.M. Gili and R. Zapata Guardiola (Guest Editors).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Raggio, J., Green, T.G.A. & Sancho, L.G. In situ monitoring of microclimate and metabolic activity in lichens from Antarctic extremes: a comparison between South Shetland Islands and the McMurdo Dry Valleys. Polar Biol 39, 113–122 (2016). https://doi.org/10.1007/s00300-015-1676-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-015-1676-1