Abstract
Key message
VyUSPA3 from the Chinese wild grape Vitis yeshanensis interacts with ERF105, PUB24 and NF-YB3, and overexpression of the VyUSPA3 gene in V. vinifera cv. 'Thompson Seedless' confers drought tolerance.
Abstract
Drought is a major abiotic stress factor that seriously affects the growth and yield of grapevine. Although many drought-related genes have been identified in Arabidopsis and other plants, the functions of only a few of their counterparts have been revealed in grape. Here, a universal stress protein (USP) A from the Chinese wild grape Vitis yeshanensis, VyUSPA3, was identified and its function was subsequently characterized by overexpressing or silencing the VyUSPA3 gene in V. vinifera cv. 'Thompson Seedless' via Agrobacterium-mediated genetic transformation. After 21 d of the drought treatment, most leaves of the untransformed (UT) 'Thompson Seedless' lines wilted, yet UT lines were less damaged compared to the RNAi-VyUSPA3 lines, nonetheless, the OE-VyUSPA3 lines were mostly unaffected. Meanwhile, OE-VyUSPA3 lines showed smaller stomatal aperture, more developed roots, higher leaf relative water content, proline content, and antioxidant enzyme activities, as well as lower malondialdehyde, H2O2 and O2•− accumulation than UT lines, but this response pattern was reversed in the RNAi-VyUSPA3 lines. Besides, the transcript levels of four drought-related genes (RD22, RD29B, DREB2A, and NCED1) in OE-VyUSPA3 lines were greater than those in the RNAi-VyUSPA3 and UT lines. In addition, a yeast two-hybrid assay and a bimolecular fluorescence complementation assay confirmed that VyUSPA3 interacted with ERF105, PUB24, and NF-YB3, respectively. This study revealed that VyUSPA3 improved drought tolerance in transgenic grapevines possibly through interaction with the hormone signaling, ubiquitination system, ethylene-responsive element binding factor and nuclear factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Grape (Vitis vinifera) is a widely cultivated fruit crop with high nutritional value and economic benefits. As one of the world’s major grape producers, China ranks first for total grape production and second for vineyard area in the world (https://www.fao.org/home/). Arid and semi-arid northwest China is one of the five main grape-growing regions of China (Cheng et al. 2022), and soil water deficit caused by sparse rain is a critical factor restricting the development of the grape industry in these regions. Drought stress affects the distribution, growth, and yield of grapevines worldwide (Fraga et al. 2016). Apart from some management practices, such as water-saving irrigation, spraying anti-transpiration agents, and covering the soil surface (with grass, straw, or a mulching film), the fundamental way to resolve this problem is to breed drought-tolerant grape varieties. Notably, wild grape germplasms are relatively tolerant to drought compared to cultivated grape varieties (Wang et al. 2004; Zhang et al. 2012). Therefore, it is important to explore key genes related to drought tolerance in wild grape resources for the molecular breeding of drought tolerance.
In the last few decades, many drought-related genes have been discovered in model plants (Chhaya et al. 2021; Yadav et al. 2021). For example, RESPONSIVE TO DEHYDRATION 22 (RD22) and RD29 are typical dehydration response genes, which are up-regulated in Arabidopsis under drought stress conditions (Yamaguchi-Shinozaki and Shinozaki 1993); DEHYDRATION-RESPONSIVE ELEMENT BINDING PROTEIN 2A (DREB2A) is strongly induced in the roots and leaves of Arabidopsis under drought and high salt stress (Sakuma et al. 2002). Moreover, the concentration of ABA was positively correlated with the expression of 9-CIS-EPOXYCAROTENOID DIOXYGENASES (NCED1) gene in grapevine, and the expression of NCED1 gene in leaves was a good marker for drought stress (Lehr et al. 2022). The discovery of drought-related genes provides valuable genetic resources for improving drought tolerance of grapevine via molecular breeding techniques.
In plants, the universal stress protein (USP) was first found in rice (Oryza sativa), and OsUSP1 functions in ethylene-mediated tolerance to waterlogging stress (Sauter et al. 2002). Some studies have confirmed that overexpression of USP genes could improve drought tolerance of transgenic plants by promoting root growth and ABA-induced stomatal closure, thereby reducing transpiration rate and leaf water loss but increasing photosynthesis, proline, total chlorophyll and soluble sugar content (Loukehaich et al. 2012; Yang et al. 2019; Hassan et al. 2021). The interaction between AnnSp2 and SpUSP suggests that the stomatal closure induced by SpUSP probably involves the Ca2+ signaling pathway since annexins have been considered targets of Ca2+ signals (Laohavisit et al. 2009; Loukehaich et al. 2012). Beside drought tolerance, USP proteins can also improve the ability of plants to resist other abiotic stresses, such as high salinity (Udawat et al. 2014; Gou et al. 2020), oxidative stress (Jung et al. 2015; Gou et al. 2020), osmotic stress (Udawat et al. 2016; Gou et al. 2020), and low temperatures (Melencion et al. 2017; Gou et al. 2020). Also, USP proteins have antifungal (Park et al. 2017) and antiparasitic (Espinola et al. 2018) functions. In addition, the USPA gene of Arabidopsis is involved in the processes of seed germination and post-germination growth (Gorshkova et al. 2018; Gorshkova and Pojidaeva 2021). Notably, the exogenous application of some hormones, such as ethylene (Sauter et al. 2002), ABA (Gorshkova et al. 2018) and gibberellin (Zahur et al. 2009) can induce the expression of USPA in plants.
USPs are small molecular weight proteins that exist in Arabidopsis in various forms, such as monomer, dimer, trimer, and oligomer, however, USPs are transformed to a high molecular weight complex once plants are subjected to an external stress factor (Jung et al. 2015). SlRd2, an ATP-binding protein containing the USP domain in tomato, forms a homodimer in plants and is phosphorylated via interaction with CIPK6, thus acting as a target of CIPK6 to regulate CIPK6-mediated reactive oxygen species (ROS) production under oxidative stress (Gutiérrez-Beltrán et al. 2017). Although the USPA genes have been studied in several plant species, to the best of our knowledge, no relevant reports yet exist for grapes.
In our previous study, the drought-responsive gene USPA was found as a differentially expressed gene of Vitis yeshanensis and V. riparia by transcriptome sequencing analysis (Cui et al. 2020). V. yeshanensis, which originated in the arid sunny slopes of Tashan Mountain, Hebei province, Northern China, is highly resistant to drought and cold (Wang et al. 2004; Zhang et al. 2012). In contrast, V. riparia is native to eastern North America, where soil moisture is higher, and thus V. riparia is more sensitive to drought (Knipfer et al. 2015). The qRT-PCR analysis showed that the transcript level of USPA was always higher in the Chinese wild V. yeshanensis accession 'Yanshan-1' than in the V. riparia acc. 'He’an (♀)' during the drought treatment (Cui et al. 2020), and it was named USPA3 after a genome-wide analysis. Heterologous expression of the USPA3 gene could increase the growth rate of E. coli under PEG, mannitol, and NaCl stress treatments (Cui et al. 2021). In this study, our aim was to overexpress and silence the VyUSPA3 gene in V. vinifera cultivar 'Thompson Seedless', which is a relatively drought-sensitive grape variety (Pouzoulet et al. 2020), to further confirm the function of VyUSPA3 in drought tolerance, and to screen and verify its interacting proteins. The findings thus provide a sound basis for understanding the regulation of VyUSPA3 in drought tolerance and thus contributing to drought tolerance molecular breeding of grape.
Materials and methods
Plant materials and treatments
Tobacco (Nicotiana benthamiana) and Arabidopsis (Arabidopsis thaliana acc. Col-0 and AtERF6 gene mutant) plants were grown in a greenhouse at the State Key Laboratory of Crop Stress Biology in Arid Areas of Northwest A&F University, Yangling, Shaanxi, China. The growth conditions inside the greenhouse were 25/23°C air temperature with a 16-h/8-h photoperiod (day/night). Potted seedlings (one seedling per pot, the diameter of the pot was 12 cm, 20 plants overall) of V. vinifera cv. 'Thompson Seedless' were maintained in the Grapevine Germplasm Resources Orchard of Northwest A and F University, Yangling, Shaanxi, China. Five-week-old seedlings of 'Thompson Seedless' grape were kept under normal conditions or treated with drought for 21 d by withholding water supply, and then the roots, stems, and leaves were collected for tissue-specific analyses. To investigate the expression pattern of USPA3, potted seedlings of 'Thompson Seedless' were treated with 4°C, 40°C, and 200 mM NaCl, and leaves of 'Thompson Seedless' were sprayed with 20 mM H2O2 or 100 μM abscisic acid (ABA), and leaf samples were collected after 0, 0.5, 1, 2, 4, 8, 12, 24, and 48 h.
To confirm the biological function of VyUSPA3, the untransformed (UT), overexpressed (OE-VyUSPA3), and silenced (RNAi-VyUSPA3) lines of 'Thompson Seedless' grape seedlings were subjected to drought treatment. For this, previously well-watered plants were initially watered to runoff (i.e., the relative water content of the soil was about 60%, Fig. 4b), and then water supply was withheld for 21 d (i.e., the relative water content of the soil was maintained below 5%, Fig. 4b). Phenotypes of the experimental plants were observed and photographed just before the withholding of water and again 21 d later. Similarly, drought treatment was applied to wild-type (WT) and the erf6 mutants of Arabidopsis for 14 d.
Isolation and sequence analysis of the VyUSPA3 gene
Based on the sequence of VvUSPA of V. vinifera cv. 'Pinot Noir' (accession number XM_002283354), the full-length CDS of VyUSPA3 was isolated from the cDNA of the leaves of 'Yanshan-1' grape by homologous cloning. The Grape Genome Browser (https://www.genoscope.cns.fr/blat-server/cgi-bin/vitis/webBlat) was used to predict the chromosome location of VyUSPA3. A conserved domain analysis of the VyUSPA3 protein was conducted by utilizing SMART (http://smart.embl-heidelberg.de), and its secondary structure was analyzed with SOPMA (https://npsa-prabi.ibcp.fr/cgi-bin/secpred_sopma.pl). The software tool DNAMAN (Lynnon Biosoft, San Ramon, CA, USA) was used to align the amino acid sequences of VyUSPA3 to several homologous ones from V. vinifera 'Pinot noir' and 'Thompson Seedless', V. riparia, model plant A. thaliana, other economical crops like Hordeum vulgare, Castanea mollissima, and Juglans regia. The primers used are shown in Supplementary Table S1.
Plasmid construction
The coding sequence (CDS) without the stop codon of VyUSPA3 was inserted into the Kpn I and BamH I sites of the pCAMBIA2300-GFP vector as the overexpression vector (Fig. 2a). For the RNAi vector, the specific fragments of VyUSPA3 were cloned from the CDS of VyUSPA3 with Xba I/BamH I and Kpn I/EcoR I, respectively, and inserted into the vector pKANNIBAL (Wesley et al. 2001) to generate the plasmid pKANNIBAL-VyUSPA3. Then, the interfering cassette was removed with Not I from the vector pKANNIBAL-VyUSPA3 and cloned into the site for Not I in the vector pART27 (Gleave 1992) to generate the vector pART27-VyUSPA3.
Subcellular localization of VyUSPA3
The pCAMBIA2300-USPA3-GFP and non-modified control pCAMBIA2300-GFP were introduced into Agrobacterium tumefaciens GV3101 and the transformed bacteria were then separately injected into N. benthamiana leaves (Wang and Wang 2019). The two plasmids (pCAMBIA2300-USPA3-GFP and pCAMBIA2300-GFP) were transformed into Arabidopsis protoplasts by implementing the polyethylene glycol-mediation method (Zhao et al. 2016); these transformed protoplasts were cultured at room temperature for 20 h before the observation. The distribution of GFP was visualized under a laser confocal microscope (TCS SP8SR, Leica, Germany). The primers used are shown in Supplementary Table S1.
Agrobacterium-mediated genetic transformation of the grape callus and identification of transgenic plants
Agrobacterium-mediated genetic transformation (Shu et al. 2021) was carried out using the callus from stem segments of 'Thompson Seedless' grape, which was preserved in our laboratory. GV3101, carrying the plasmid pCAMBIA2300-USPA3-GFP or RNAi-USPA3, was activated and adjusted to OD600 = 0.8. The callus was then cut into 0.5 mm3 pieces before they were infected in the bacterial suspension for 10 min. Then, each infected and sterilized callus was cultivated on the Murashige & Skoog Basel Medium (MS) with 30 g/L sucrose, 4 mg/L benzylaminopurine (6-BA), 0.02 mg/L indole-3-butyric acid (IBA), 7 g/L agar, 300 mg/L ceftiofur, 200 mg/L carbenicillin, and 75 mg/L kanamycin for 2 months and thereafter sub-cultured monthly. The resistant callus was then induced into seedlings on the Lloyd & McCown wood plant basal medium (WPM) (2.41 g WPM + 30 g/L sucrose + 0.2 mg/L 6-BA + 0.2 mg/L IBA + 7 g/L agar + 1.5 g/L activated carbon).
Real-time fluorescence quantitative PCR (qRT-PCR) and western blot analyses were used to identify the positive seedlings of transgenic plants. We used the Plant RNA Kit (Omega) for the total RNA extraction from samples of each grape plant. Next, 1 μg RNA was used to synthesize the first strand of cDNA with the FastKing RT Kit (Tiangen, Beijing, China). Oligonucleotide primers (Table S1) for qRT-PCR were designed online at the NCBI website (https://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_ LOC = BlastHome) and synthesized by the Shaanxi Zhongke Yutong Biotechnology Co., Ltd (Xi’an, China). The qRT-PCR reaction volume was 20 μL, consisting of 1 μL cDNA template, 0.8 μL of each forward and reverse primer, 10 μL of SYBR Green fluorescent dye, and 7.4 μL of ddH2O. The reaction profile was as follows: 95°C for 1 min, followed by 40 cycles of 95°C for 10 s, 58°C for 20 s, and 72°C for 20 s. Three biological replicates were used for each treatment and their relative gene expression levels were determined by the 2−ΔΔCT method. Grape Actin7 gene (accession no. XM_002282480) served as an internal reference. The positive plants identified by qRT-PCR were confirmed using western blot. From each sample, its total protein was extracted following Méchin (2006), resolved by a 10% SDS-PAGE gel, then transferred to a PVDF membrane, and detected with the anti-GFP antibody (ABclonal). The primers used are shown in Supplementary Table S1.
Measurement of stomatal aperture
After 21 d of drought treatment, the detached leaves of drought-treated grapevines were incubated in a buffer solution (10 mM MES-KOH, pH 6.15; 10 mM KCl; 50 μM CaCl2) for 2 h under light conditions. Then its Ca2+ concentration was increased to 2 mM and the incubation period was extended for another 2 h. To observe the stomatal aperture, the transparent sticky-tape method (Wang et al. 2020) was applied under a microscope (ECLIPSE 50i, Nikon, Japan). The Nano Measurer software tool was used to measure stomatal pore diameter. The average of nine pore diameters from three individual plants was recorded, i.e. three stomata per leaf and one leaf per plant.
Observation of roots
After 21 d of imposed drought stress, the potted seedlings of the UT, OE-VyUSPA3, and RNAi-VyUSPA3 lines were carefully pulled out from their pots together with the substrate, and the substrate was removed. The roots were carefully cleaned under running water to ensure their integrity, then scanned by a root scanner (Epson Perfection V700 Photo, Epson, Japan) and analyzed by WinRHIZO (Regent, Canada) software program.
Quantification of physiological and biochemical indicators
The leaf relative water content was measured by the method of Zhang et al. (2022), and the content of proline was determined by the acidic-ninhydrin method (Ábrahám et al. 2010), while malondialdehyde (MDA) content was determined by the thiobarbituric acid method (Zhang et al. 2020). Electrolyte leakage was measured using a previously described method (Campos et al. 2003). POD activity was assayed by the method of Fang and Kao (2000), SOD activity was determined by measuring the reduction of nitro blue tetrazolium at 560 nm, and CAT activity was analyzed by measuring the H2O2 consumption at 240 nm (Mellacheruvu et al. 2016). The H2O2 content was quantified by the titanium sulfate method (Wang et al. 2009), and the O2•− content was measured by applying the method of Elstner and Heupel (1976).
DAB and NBT staining
Grape leaves were immersed in diaminobenzidine (DAB) (1 mg/mL, pH 3.8) and nitro blue tetrazolium (NBT) (5 mg/mL) dye under dark conditions for 8 h, and then transferred to 95% ethanol for de-staining and subsequent imaging.
Yeast two-hybrid assay
Yeast two-hybrid assay was performed according to the method of Chen et al. (2021) as follows. The CDS of VyUSPA3 was transformed into the vector pGBKT7 to form a bait vector, which was co-transformed into Y2HGold strain with yeast cDNA library, and obtained three stress-related interacting proteins: ERF105 (accession no. XM_002281876), PUB24 (accession no. XM_002265021), and NF-YB3 (accession no. XM_003635491). The corresponding CDS of ERF105, PUB24, and NF-YB3 was separately inserted into the vector pGADT7, and then pGBKT7-VyUSPA3 was co-transformed with pGADT7-X (the X denoting genes encoding the three interacting proteins). In parallel, the co-transformation of pGADT7-T with pGBKT7-VyUSPA3 was used as a negative control. The transformed Y2HGold strain was spread onto SD/-Trp/-Leu/-Ade/-His/AbA/X-α-Gal medium containing 200 ng/mL AbA and cultured at 28°C for 3 d. The primers used are shown in Supplementary Table S1.
Bimolecular fluorescence complementation assay to verify protein interactions
Bimolecular fluorescence complementation (BiFC) assay was carried out according to the method of Chen et al. (2021) as follows. The CDS of VyUSPA3 was inserted separately into the vectors pBI221NE, pSPYNE, and pSPYCE. The corresponding CDS of candidate genes ERF105, PUB24, and NF-YB3 was inserted into the pBI221CE vector, respectively. Next, pBI221NE-VyUSPA3 was co-transformed with pBI221CE-X into Arabidopsis protoplasts, while co-transformation of pBI221NE-VyUSPA3 with pBI221CE was carried out as a control. The transformed protoplasts were cultured and wrapped in tinfoil at 22°C in an incubator for 20 h, and their yellow fluorescent protein (YFP) fluorescence was observed under a laser confocal microscope (TCS SP8 SR, Leica, Germany). Both pSPYNE-VyUSPA3 and pSPYCE-VyUSPA3 were each transformed into GV3101, separately, and the recombinant bacteria were then injected into N. benthamiana leaves. YFP was observed under a laser confocal microscope (TCS SP8 SR, Leica, Germany) after 48 h. The primers used are shown in Supplementary Table S1.
Identification and functional analysis of the ERF6 Arabidopsis mutant
To reveal the biological function of VvERF105, we procured the Arabidopsis AtERF6/103 mutant AT4G17490 (donor stock no. SALK_087356.10.b) from AraShare (https://www.arashare.cn/index/Product/index.html) because AtERF6/103 is the homologous gene of VvERF105 and they have a 39.06% amino acid identity. Genomic DNA was extracted from leaves of WT and the mutant Arabidopsis by the CTAB method (Murray and Thompson 1980), and PCR-amplified using the primers LBb1.3, LP, and RP. Then, qRT-PCR was performed to determine the expression of AtERF6 in the two types of lines using primers qRT-AtERF6-F, and qRT-AtERF6-R, for which Arabidopsis Actin2 gene (accession no. NM_001338359) served as an internal reference. The primers used are shown in Supplementary Table S1. After 14 d of drought treatment, phenotypic and physiological and biochemical changes including proline content, MDA content, electrolyte leakage and antioxidant enzyme activity (POD, SOD, and CAT) of WT and ERF6 mutant were assessed.
Statistical analysis
Three biological replicates were performed for each set of data, and the results were expressed as mean ± standard error (SE). The SPSS 23.0 software (IBM, New York, USA) was used for the statistical analysis of data. Significant differences among means were determined by one-way ANOVA followed by Tukey’s-b (K) test (P < 0.05).
Results
Cloning and sequence analysis of VyUSPA3
VyUSPA3 is located on chromosome 1 (Fig. 1a) and has a length of 1931 bp. The CDS of VyUSPA3 is 495 bp in length and encodes 164 amino acids (aa); the carboxyl terminal (residues 13–161 aa) of VyUSPA3 contains a USP-like domain. VyUSPA3 has an amino acid sequence identical to its homologue in V. vinifera cv. 'Pinot Noir', yet one amino acid difference from that of V. riparia acc. 'He’an (♀)', and two of V. vinifera cv. 'Thompson Seedless', which is highly similar to that for USPA of C. mollissima, J. regia, H. vulgare, and A. thaliana (Fig. 1b). Secondary structure analysis revealed that VyUSPA3 has five β-folds separated by four α-helices as well as an ATP-binding site sequence G-(2X)-G-(9X)-G(S/T), indicating that VyUSPA3 is a type of ATP-binding protein (Fig. 1b).
Sequence analysis of VyUSPA3. a Chromosome location analysis of VyUSPA3; its location was predicted at 3,338,954 to 3,340,884 on chromosome 1. The USP-like domain is situated from 13 to 161 aa. b Multiple sequence alignments between VyUSPA3 and its homologous proteins from other plant species. Protein information for the sequence alignments: V. vinifera cv. 'Pinot Noir' (PN) VvUSPA (XM_002283354), V. riparia VrUSPA (XM_034828214), V. vinifera cv. 'Thompson Seedless' (TS) VvUSPA (SRP026420), C. mollissima CmUSPA (KAF3949527), J. regia JrUSPA (XP_018806489), H. vulgare HvUSPA (ADB54810), and A. thaliana AtUSPA (NP_564927). Differential amino acids between different grapes are represented by red ovals. The α-helix and β-fold components are indicated above the sequence as blue and orange lines, respectively, with ATP-binding sites in red boxes
VyUSPA3 is localized to the nucleus and cytoplasm, and may exist in plants as a homodimer
Green fluorescent protein (GFP) appeared throughout the cells of tobacco leaves and protoplasts of Arabidopsis transformed with the empty vector pCAMBIA2300, but appeared only in the nucleus and cytoplasm when transformed by the recombinant plasmid VyUSPA3-pCAMBIA2300 (Fig. 2). This confirms that VyUSPA3 is localized to the nucleus and cytoplasm. Yeast two-hybrid (Fig. S1a) and BiFC assays (Fig. S1b, c) showed that VyUSPA3 interacts with itself and may form a homodimer in plants.
Expression pattern of USPA3
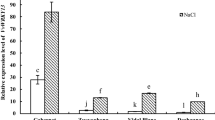
As shown in Fig. 3, USPA3 was expressed in the roots, stems, and leaves of 'Thompson Seedless', but its transcript level in leaves was significantly higher than that in roots or stems under normal and drought conditions. The transcript level of USPA3 peaked after 2 h of the low temperature (4°C) treatment, suggesting that USPA3 is potentially an early response gene to low temperature, while its expression remained higher at all sampling times under high temperature (40°C) than that of control (25°C). The transcript level of USPA3 increased with the prolongation of salt stress, peaking at 12 h. Both oxidative stress and the plant hormone abscisic acid (ABA) were also able to induce the expression of USPA3, although the expression level was almost unchanged in the early stage of the treatments. In a previous study, we analyzed the promoter of the USPA3 gene and found the presence of an ABA response element (ABRE) (Cui et al. 2021). Based on these results, it can be concluded that USPA3 responds to temperature, salt, oxidative stress and ABA treatment, and thus it appears to be a general stress-response gene, and may be related to the ABA signaling pathway.
Transcript quantification of USPA3 in different plant tissues and treatments. The USPA3 gene expression was analyzed in roots, stems, and leaves of 'Thompson Seedless' grape under normal conditions and after 21 d of drought treatment by qRT-PCR. Potted seedlings of 'Thompson Seedless' were treated with 4℃, 40℃, or 200 mM NaCl, and leaves were sprayed with 20 mM H2O2 and 100 μM ABA and collected at 0, 0.5, 1, 2, 4, 8, 12, 24, and 48 h post-treatment. The analysis employed qRT-PCR with the grapevine Actin7 gene (accession no. XM_002282480) as an internal control. Relative expression levels were calculated using the 2−ΔΔCT method. Data are the mean (± SE) of three biological replicates. Significant differences among means were determined by one-way ANOVA followed by Tukey’s-b (K) test (P < 0.05)
VyUSPA3 improves drought tolerance of transgenic ‘Thompson Seedless’ grape
Agrobacterium-mediated genetic transformations were carried out using the meristematic callus of the 'Thompson Seedless' as the recipient material (Fig. S2a). Three overexpression lines OE-60, OE-66, and OE-68, and two RNAi-silencing lines RNAi-7 and RNAi-15 were identified by qRT-PCR and the western blot (Fig. S2b, c). To explore the relationship between VyUSPA3 and drought tolerance, the UT, OE-VyUSPA3, and RNAi-VyUSPA3 'Thompson Seedless' lines with comparable and robust growth were selected and subjected to 21 d of a drought treatment (Fig. 4a). There was no significant difference in soil relative water content among the grape genotypes before and after the drought treatment (Fig. 4b). After drought stress, nearly all the leaves of UT lines appeared withered, while the overexpressed lines displayed negligible or no damage, in stark contrast to the severe leaf wilting and curling of RNAi-VyUSPA3 lines (Fig. 4a). Also, the leaf relative water content was similar among all genotypes before the drought treatment, but after 21 d of the drought treatment, the leaf relative water content of OE-VyUSPA3 lines was significantly higher than that of RNAi-VyUSPA3 lines and the UT lines (Fig. 4c). Stomatal observations after 21 d of drought stress revealed a smaller stomatal aperture in the OE-VyUSPA3 lines than in the UT lines and a bigger stomatal aperture in the RNAi-VyUSPA3 lines than in the UT lines (Fig. 4d, e), indicating that the VyUSPA3 gene can induce a decrease in stomatal aperture under drought stress conditions. We also found that the OE-VyUSPA3 lines had more developed roots than the RNAi-VyUSPA3 lines and UT lines (Fig. 4f–i), indicating that the VyUSPA3 gene activity and protein product are potentially related to root growth under drought stress. Overall, these results suggested that VyUSPA3 improves the drought tolerance of grape plants, possibly by reducing the stomatal aperture and promoting root growth.
Plant phenotypes, soil relative water content, leaf relative water content, stomatal aperture, and root morphology of the untransformed (UT), overexpression (OE-VyUSPA3) and silenced (RNAi-VyUSPA3) grape lines before (0 d) and after (21 d) the drought treatment. a Plant phenotypes, b soil relative water content, and c leaf relative water content in UT lines, OE-VyUSPA3 and RNAi-VyUSPA3 grapes at 0 and 21 d under drought treatment. d, e Stomata, f root tips, g roots, h root forks, and i root crossings of UT, OE-VyUSPA3 and RNAi-VyUSPA3 grapes after 21 d of drought treatment. Data are the mean (± SE) of three biological replicates. Significant differences among means were determined by one-way ANOVA followed by Tukey’s-b (K) test (P < 0.05)
VyUSPA3 alleviates oxidative damage under drought stress
There was no significant difference in the proline content among the grape genotypes before the drought stress treatment. After 21 d of treatment, the proline content increased, but it was significantly higher in the OE-VyUSPA3 lines than in RNAi-VyUSPA3 lines and UT lines (Fig. 5a). Before the drought treatment, except for the OE-VyUSPA3 line OE-66, which had a lower MDA content, there were no significant differences among the other lines. But after the drought treatment, the MDA content of RNAi-VyUSPA3 line RNAi-15 was significantly higher than that of other genotypes, while OE-VyUSPA3 lines had the lowest MDA content (Fig. 5b). The POD, SOD, and CAT activities of all plants had no or little difference just prior (0 d) to the drought treatment. By contrast, after 21 d of drought treatment, POD and SOD activities of the OE-VyUSPA3 lines significantly surpassed those of the UT lines, with the significantly lower in the RNAi-VyUSPA3 lines (Fig. 5c, d). There was lower CAT activity in the RNAi-VyUSPA3 lines than either in the UT lines or OE-VyUSPA3 lines at 21 d of drought treatment (Fig. 5e). Before incurring drought stress, the contents of H2O2 and O2•− were similar among all genotypes, however, after drought stress, ROS content was significantly lower in the OE-VyUSPA3 lines than in the RNAi-VyUSPA3 lines and the UT lines, except for the content of O2•− in OE-66 (Fig. 5f, g). The results of DAB and NBT staining were consistent with the H2O2 and O2•− contents. Leaf ROS accumulation as observed in the DAB and NBT staining was the lowest in the OE-VyUSPA3 lines and highest in the RNAi-VyUSPA3 lines (Fig. 5h, i). The above results indicated that the VyUSPA3 gene is responsible for promoting the accumulation of proline and the activity of antioxidant enzymes while reducing the accumulation of ROS and MDA under drought stress.
Changes in osmoregulatory substances, lipid peroxidation, antioxidant enzyme activity and ROS accumulation in the UT, OE-VyUSPA3 and RNAi-VyUSPA3 grape lines before (0 d) and after (21 d) the drought treatment. a Proline content. b MDA content. c POD activity. d SOD activity. e CAT activity. f H2O2 content. g O2•− content. h DAB staining and i NBT staining of UT, OE-VyUSPA3 and RNAi-VyUSPA3 grape leaves at 0 and 21 d under drought stress. Data are the mean (± SE) of three biological replicates. Significant differences among means were determined by one-way ANOVA followed by Tukey’s-b (K) test (P < 0.05)
Overexpression of VyUSPA3 up-regulates the transcript levels of some drought-related genes
In this experiment, the transcript levels of four drought-related genes (RD22, RD29B, DREB2A, and NCED1) in the UT, OE-VyUSPA3 and RNAi-VyUSPA3 lines before and after drought treatment were quantified. The results showed that the four genes were all up-regulated after the drought treatment in the UT and OE-VyUSPA3 lines, with their transcript levels significantly higher in OE-VyUSPA3 plants than in the UT and RNAi-VyUSPA3 lines, especially RD29B (Fig. 6), further indicating a role for VyUSPA3 in drought stress response.
Expression of four stress-responsive genes (RD22, RD29B, DREB2A, and NCED1) in the UT, OE-VyUSPA3 and RNAi-VyUSPA3 lines, before (0 d) and after (21 d) the drought treatment. Relative expression levels were calculated using the 2−ΔΔCT method. Quantitative data are the mean (± SE) of three biological replicates. Significant differences among means were determined by one-way ANOVA followed by Tukey’s-b (K) test (P < 0.05)
VyUSPA3 interacts with ERF105, PUB24, and NF-YB3 in yeast and Arabidopsis protoplasts
The pGADT7-ERF105, pGADT7-PUB24, and pGADT7-NF-YB3 were each co-transformed with pGBKT7-VyUSPA3 into the Y2HGold strain, with co-transformed pGBKT7-VyUSPA3 and pGADT7 as the negative control. Except for the negative control, other co-transformed strains were able to grow on the SD/-Trp/-Leu/-Ade/-His/AbA/X-α-Gal medium containing 200 ng/mL AbA; that is, VyUSPA3 was found to interact with ERF105, PUB24, and NF-YB3 in yeast (Fig. 7a). Next, pBI221NE-VyUSPA3 and pBI221CE-X (Fig. 7b) were each co-transformed into Arabidopsis protoplasts. We found that YFP (Fig. 7c) appeared in all protoplasts co-transformed with recombinant plasmids except for the negative control after 20 h of culture, confirming that VyUSPA3 interacted with each of the above three proteins in Arabidopsis protoplasts. Moreover, the interaction between VyUSPA3 and ERF105 or PUB24 primarily occurred in the nucleus, while interaction with NF-YB3 occurred mainly in the cytoplasm (Fig. 7c).
The interactions of VyUSPA3 with ERF105, PUB24, and NF-YB3 were verified by yeast two-hybrid and bimolecular fluorescence complementation (BiFC) assays. a Yeast two-hybrid. The pGBKT7-VyUSPA3 and pGADT7 vectors were co-transformed as the controls. b Depicted genetic construction used for BiFC. c BiFC assay. Scale bars = 50 μm
To further study the interactions between the above-mentioned interacting proteins and VyUSPA3, the expression patterns of ERF105, PUB24, and NF-YB3 under drought stress were analyzed based on the results of previous transcriptome data (Cui et al. 2020). From the results shown in Fig. S3, we can see that NF-YB3 and USPA3 showed the same expression pattern under drought stress, and the expression levels were up-regulated after drought treatment and reached a peak at 16 d. In contrast, ERF105 and PUB24 showed downregulation in response to drought stress.
ERF6 negatively regulates drought tolerance in Arabidopsis
Since AtERF6/103 is a homologous gene of VvERF105, we revealed the biological function of VvERF105 by detecting the changes in phenotype and some physiological and biochemical indexes of Arabidopsis erf6 mutant under drought stress. Genomic DNA and qRT-PCR assays confirmed the veracity of the erf6 mutant and that it is a homozygous mutant (Fig. S4a-c). After incurring the drought stress treatment, almost all leaves of the WT plants wilted at 14 d, whereas the erf6 mutant grew vigorously (Fig. S4d, e). In addition, compared with WT, the erf6 mutant had a higher proline content (Fig. S4f), lower MDA content (Fig. S4g) and electrolyte leakage (Fig. S4h), and higher antioxidant enzyme activity (Fig. S4j-k), implying that ERF6 aggravates drought-induced oxidative stress in Arabidopsis. Taken together, these results suggest that the ERF6 gene is a negative regulator of drought tolerance in Arabidopsis.
Discussion
In this study, a differentially expressed gene, USPA, was cloned from the extremely drought-resistant Chinese wild grape V. yeshanensis acc. 'Yanshan-1' based on our previous transcriptome data (Cui et al. 2020). According to the distribution of that gene on the chromosome, it was named VyUSPA3 (Cui et al. 2021). Heterologous expression of the VyUSPA3 gene in E. coli can improve the tolerance of the strain TOP10 to PEG, mannitol, and NaCl (Cui et al. 2021). These results coupled with previous research results (Yang et al. 2019; Gou et al. 2020; Hassan et al. 2021) motivated us to infer that VyUSPA3 is a drought-related gene. To verify the function of the VyUSPA3 gene in drought tolerance, VyUSPA3 overexpression and RNAi-silencing 'Thompson Seedless' grape lines were executed in the present study. After a 21-d drought treatment, we found that the OE-VyUSPA3 lines feature the best growth status, while both the RNAi-VyUSPA3 lines and the UT lines presented wilting phenotype, and the wilting degree of the former was slightly higher than that of the latter. The non-significant difference between the RNAi-VyUSPA3 lines and the UT lines may be due to the existence of multiple genes similar in structure and function to VyUSPA3 in the grape genome, which might compensate VyUSPA3 to maintain drought tolerance in grapes when the expression of VyUSPA3 is suppressed. However, overexpression of VyUSPA3 increased drought tolerance in the 'Thompson Seedless' grape cultivar.
Roots and leaves are essential organs for maintaining water balance and many of their traits are closely related to plant growth and stress resistance. Plants with longer and denser root systems are more likely to survive in the face of drought stress (He et al. 2021). Leaf water loss is mainly determined by transpiration, and stomatal conductance is closely related to plants’ transpiration rate and water use efficiency (Egea et al. 2011). Overexpressing the Malus sieversii MsUSPA gene in Arabidopsis induces longer roots, and a more compact leaf cell structure than UT lines under extreme drought conditions (Yang et al. 2019). Similarly, overexpression of Gossypium arboreum GUSP1 gene in G. hirsutum can increase the leaf water content, chlorophyll content, stomatal conductance, and root length of transgenic cotton plants under drought stress (Hassan et al. 2021). Our study also yielded similar results. Compared with the UT lines or RNAi-VyUSPA3 lines, the OE-VyUSPA3 lines had smaller stomatal aperture, more developed roots and higher leaf relative water content. This indicates that the VyUSPA3 gene could improve drought tolerance of grapes by promoting root growth and reducing stomatal openings and delaying leaf water loss.
Under drought stress, the changes in some important physiological and biochemical indexes, such as proline content, MDA accumulation and antioxidant enzyme activity in plants can reflect the drought tolerance ability of plants (Kar and Mishra 1976; Davey et al. 2005; Shi et al. 2018). Our results showed that the proline content in the OE-VyUSPA3 lines was much higher than either UT lines or RNAi-VyUSPA3 lines after the drought treatment, and the MDA content was lower in the OE-VyUSPA3 lines. Proline, a crucial osmotic regulator, accumulates quickly in plants in response to drought stress (Shi et al. 2018). Under drought stress, a higher accumulation of proline can lower cell osmotic potential and decrease leaf water loss, thus enhancing the drought tolerance of the plant. MDA accumulation reflects the level of lipid peroxidation of the plant cell membrane, and thus a lower MDA content is a signature of the stability of the cell membrane (Sudhakar et al. 2001). Moreover, excessive ROS accumulation can be toxic to plant cells, nonetheless, a higher antioxidant enzyme activity can ensure a stronger ROS scavenging capacity (Kapoor et al. 2019). Compared with the UT or RNAi-VyUSPA3 lines, the contents of H2O2 and O2•− in the OE-VyUSPA3 lines were lower and the activities of POD, SOD, and CAT in the OE-VyUSPA3 lines were higher under drought treatment. The insignificant difference in CAT activity between the two OE-VyUSPA3 lines and the UT lines may be attributed to the overlapping functions of CAT and POD in certain contexts. The main role of SOD is to dismutate O2•− into H2O2 (Kapoor et al. 2019), while the main function of POD and CAT is to remove H2O2, and ascorbate peroxidase also catalyzes the production of H2O from H2O2 (Nakano and Asada 1981; Kapoor et al. 2019). These results show that the VyUSPA3 gene functions in removing ROS by increasing the activity of antioxidant enzymes. Apart from physiological and biochemical processes, plants can also increase their resistance to stress by regulating the expression of certain stress-related genes. The transcript levels of RD22, RD29B, DREB2A, and NCED1, especially RD29B were always greater in OE-VyUSPA3 plants than in the UT and RNAi-VyUSPA3 lines. Previous studies have shown that these genes are strongly associated with ABA to varying degrees, for example, RD22 and RD29B are ABA-dependent (Virlouvet et al. 2014), NCED1 is involved in ABA synthesis (Liu et al. 2016), and DREB2A is induced by ABA (Kim et al. 2011). Our results suggest that VyUSPA3 improved the drought tolerance of transgenic grapes probably by regulating ABA signaling pathway.
To further clarify the drought tolerance mechanism of VyUSPA3, we carried out yeast two-hybrid and BiFC experiments, and uncovered three proteins such as ERF105, PUB24, and NF-YB3 that interacted with VyUSPA3. ERF105 is a member of the AP2/ERF family, and ERF transcription factors are known to be involved in the regulation of various developmental processes and biotic and abiotic stresses (Joo et al. 2013; Bolt et al. 2017; Xie et al. 2019). Arabidopsis ERF6/ERF103 is the ortholog of VvERF105, and responds to oxidative stress (Vermeirssen et al. 2014) and cold (Xin et al. 2007), but its involvement in drought resistance has not been reported. We found that erf6 mutants of Arabidopsis are more tolerant to drought stress than the UT lines, indicating that AtERF6 negatively regulates drought tolerance in Arabidopsis. This study suggests that VvERF105 may negatively regulate the drought tolerance of grapes as well. It is highly likely that some regulatory relationships between USPA3 and ERF105 may exist except for physical interaction. It is also possible that the presence of other interacting proteins influences their binding. PUB24 is a U-box type E3 ubiquitin ligase. The ubiquitination system plays a prominent role in plant growth and development, immune regulation, and resistance to abiotic stresses (Gong et al. 2020). So far, PUB genes have been reported to positively regulate the resistance of plants to cold and bacterial pathogen Xanthomonas euvesicatoria (Yao et al. 2017; Liu et al. 2021), while certain PUB genes act as negative regulators of drought stress tolerance (Cho et al. 2008; Seo et al. 2021). The Nuclear Factor (NF) belongs to the CCAAT-box binding factor (CBF) and consists of three subunits, namely NF-YA, NF-YB, and NF-YC (Kim et al. 1996). Overexpression of the Picea wilsonii NF-YB3 gene enhances the tolerance of Arabidopsis to salt and drought stress by regulating the expression of genes that depend on the CBF pathway (Zhang et al. 2015). Overexpressing the wheat NF-YB3:l gene in tobacco improves the tolerance of transgenic tobacco to drought stress by regulating the ABA signaling pathway (Yang et al. 2017). Based on the expression pattern of the three genes under drought stress and the relationship between the three interacting proteins and plant drought resistance, we speculate that VyUSPA3 cooperates with NF-YB3 and antagonizes ERF105 and PUB24 to regulate the drought tolerance of grape plants.
In summary, the function of the VyUSPA3 gene from V. yeshanensis was confirmed by analyzing the OE-VyUSPA3 and RNAi-VyUSPA3 grape lines in the current study. The results indicate that the VyUSPA3 gene from Chinese wild grapes can positively regulate drought tolerance in cultivated grapes. Furthermore, VyUSPA3 interacted physically with ERF105, PUB24, and NF-YB3, thus regulating the drought tolerance of the grapevine (Fig. 8). These findings advance our current understanding of the regulation network of drought tolerance and provide new insights into promising genetic resources for grapevine breeding with drought tolerance.
A hypothetical working model of VyUSPA3 regulating and enhancing drought tolerance. On the one hand, VyUSPA3 can accelerate root growth and the accumulation of osmoregulatory substance proline, reduce harmful malondialdehyde accumulation, and increase the activity of antioxidant enzymes (POD, SOD and CAT) to remove ROS. On the other hand, VyUSPA3 can up-regulate the transcription of genes involved in ABA synthesis or signaling pathways and decrease the stomatal aperture. In addition, VyUSPA3 can physically interact with ERF105, PUB24, NF-YB3 and itself to regulate the drought tolerance in grape
Author contributions statement
ZJX designed the experiments and revised the manuscript. CXY carried out the experiments and drafted the manuscript. ZPY and CCC performed the experiments. All authors read and approved the manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ábrahám E, Hourton-Cabassa C, Erdei L, Szabados L (2010) Methods for determination of proline in plants. In: Sunkar R (ed) Plant Stress Tolerance. Methods in Molecular Biology. Humana Press, Totowa, pp 317–331
Bolt S, Zuther E, Zintl S, Hincha DK, Schmülling T (2017) ERF105 is a transcription factor gene of Arabidopsis thaliana required for freezing tolerance and cold acclimation. Plant Cell Environ 40:108–120. https://doi.org/10.1111/pce.12838
Campos PS, Quartin VN, Ramalho JC, Nunes MA (2003) Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. plants. J Plant Physiol 160:283–292. https://doi.org/10.1078/0176-1617-00833
Chen CC, Cui XY, Zhang PY, Wang Z, Zhang JX (2021) Expression of the pyrroline-5-carboxylate reductase (P5CR) gene from the wild grapevine Vitis yeshanensis promotes drought resistance in transgenic Arabidopsis. Plant Physiol Bioch 168:188–201. https://doi.org/10.1016/j.plaphy.2021.10.004
Cheng X, Sun S, Liu ZJ, Yang XG (2022) Drought analysis during the growth stages of grape in the main grape-growing regions in China. Theor Appl Climatol 149:1497–1502
Chhaya YB, Jogawat A, Gnanasekaran P, Kumari P, Lakra N, Lal SK, Pawar J, Narayan OP (2021) An overview of recent advancement in phytohormones-mediated stress management and drought tolerance in crop plants. Plant Gene 25:100264. https://doi.org/10.1016/j.plgene.2020.100264
Cho SK, Ryu MY, Song C, Kwak JM, Kim WT (2008) Arabidopsis PUB22 and PUB23 are homologous U-Box E3 ubiquitin ligases that play combinatory roles in response to drought stress. Plant Cell 20:1899–1914. https://doi.org/10.1105/tpc.108.060699
Cui XY, Xue JN, Zhang B, Chen CC, Tang YY, Zhang PY, Zhang JX (2020) Physiological change and screening of differentially expressed genes of wild Chinese Vitis yeshanensis and American Vitis riparia in response to drought stress. Sci Hortic Amst 266:109140. https://doi.org/10.1016/j.scienta.2019.109140
Cui XY, Zhang PY, Hu YF, Chen CC, Liu QY, Guan PY, Zhang JX (2021) Genome-wide analysis of the Universal stress protein A gene family in Vitis and expression in response to abiotic stress. Plant Physiol Bioch 165:57–70. https://doi.org/10.1016/j.plaphy.2021.04.033
Davey MW, Stals E, Panis B, Keulemans J, Swennen RL (2005) High-throughput determination of malondialdehyde in plant tissues. Anal Biochem 347:201–207. https://doi.org/10.1016/j.ab.2005.09.041
Egea G, Verhoef A, Vidale PL (2011) Towards an improved and more flexible representation of water stress in coupled photosynthesis-stomatal conductance models. Agr Forest Meteorol 151:1370–1384. https://doi.org/10.1016/j.agrformet.2011.05.019
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620. https://doi.org/10.1016/0003-2697(76)90488-7
Espinola SM, Cancela MP, Corrêa LB, Zaha A (2018) Evolutionary fates of universal stress protein paralogs in Platyhelminthes. BMC Evol Biol 18(10):10. https://doi.org/10.1186/s12862-018-1129-x
Fang WC, Kao CH (2000) Enhanced peroxidase activity in rice leaves in response to excess iron, copper and zinc. Plant Sci 158:71–76. https://doi.org/10.1016/S0168-9452(00)00307-1
Fraga H, de Cortázar Atauri IG, Malheiro AC, Santos JA (2016) Modelling climate change impacts on viticultural yield, phenology and stress conditions in Europe. Glob Change Biol 22:3774–3788. https://doi.org/10.1111/gcb.13382
Gleave AP (1992) A versatile binary vector system with a T-DNA organisational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol Biol 20:1203–1207. https://doi.org/10.1007/BF00028910
Gong M, Li ZP, Wan JN, Chen MJ, Wang H, Shang JJ, Zhou SC, Tan Q, Wang Y, Bao DP (2020) Chilling stress reduced protein translation by the ubiquitination of ribosomal proteins in Volvariella volvacea. J Proteomics 215:103668. https://doi.org/10.1016/j.jprot.2020.103668
Gorshkova DS, Getman IA, Voronkov AS, Chizhova SI, Kuznetsov VIV, Pojidaeva ES (2018) The gene encoding the universal stress protein AtUSP is regulated by phytohormones and involved in seed germination of Arabidopsis thaliana. Dokl Biochem Biophys 479:105–107. https://doi.org/10.1134/S1607672918020151
Gorshkova DS, Pojidaeva ES (2021) Members of the universal stress protein family are indirectly involved in gibberellin-dependent regulation of germination and post-germination growth. Russ J Plant Physiol 68:451–462. https://doi.org/10.1134/S1021443721030055
Gou LM, Zhuo CL, Lu SY, Guo ZF (2020) A universal stress protein from Medicago falcata (MfUSP1) confers multiple stress tolerance by regulating antioxidant defense and proline accumulation. Environ Exp Bot 178:104168. https://doi.org/10.1016/j.envexpbot.2020.104168
Gutiérrez-Beltrán E, Personat JM, de la Torre F, del Pozo O (2017) A universal stress protein involved in oxidative stress is a phosphorylation target for protein kinase CIPK6. Plant Physiol 173:836–852. https://doi.org/10.1104/pp.16.00949
Hassan S, Ahmad A, Batool F, Rashid B, Husnain T (2021) Genetic modification of Gossypium arboreum universal stress protein (GUSP1) improves drought tolerance in transgenic cotton (Gossypium hirsutum). Physiol Mol Biol Pla 27:1779–1794. https://doi.org/10.1007/s12298-021-01048-5
He J, Jin Y, Siddique KHM, Li FM (2021) Trade-off between root efficiency and root size is associated with yield performance of soybean under different water and phosphorus levels. Agriculture 11:1–11. https://doi.org/10.3390/agriculture11060481
Joo J, Choi HJ, Lee YH, Kim YK, Song SI (2013) A transcriptional repressor of the ERF family confers drought tolerance to rice and regulates genes preferentially located on chromosome 11. Planta 238:155–170. https://doi.org/10.1007/s00425-013-1880-6
Jung YJ, Melencion SMB, Lee ES, Park JH, Alinapon CV, Oh HT, Yun DJ, Chi YH, Lee SY (2015) Universal stress protein exhibits a redox-dependent chaperone function in Arabidopsis and enhances plant tolerance to heat shock and oxidative stress. Front Plant Sci 6:1141. https://doi.org/10.3389/fpls.2015.01141
Kapoor D, Singh S, Kumar V, Romero R, Prasad R, Singh J (2019) Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 19:100182. https://doi.org/10.1016/j.plgene.2019.100182
Kar M, Mishra D (1976) Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol 57:315–319. https://doi.org/10.1104/pp.57.2.315
Kim IS, Sinha S, de Crombrugghe B, Maity SN (1996) Determination of functional domains in the C subunit of the CCAAT-binding factor (CBF) necessary for formation of a CBF-DNA complex: CBF-B interacts simultaneously with both the CBF-A and CBF-C subunits to form a heterotrimeric CBF molecule. Mol Cell Biol 16:4003–4013. https://doi.org/10.1128/MCB.16.8.4003
Kim JS, Mizoi J, Yoshida T, Fujita Y, Nakajima J, Ohori T, Todaka D, Nakashima K, Hirayama T, Shinozaki K, Yamaguchi-Shinozaki K (2011) An ABRE promoter sequence is involved in osmotic stress-responsive expression of the DREB2A gene, which encodes a transcription factor regulating drought-inducible genes in Arabidopsis. Plant Cell Physiol 52:2136–2146. https://doi.org/10.1093/pcp/pcr143
Knipfer T, Eustis A, Brodersen C, Walker AM, McElrone AJ (2015) Grapevine species from varied native habitats exhibit differences in embolism formation/repair associated with leaf gas exchange and root pressure. Plant Cell Environ 38:1503–1513. https://doi.org/10.1111/pce.12497
Laohavisit A, Mortimer JC, Demidchik V, Coxon KM, Stancombe MA, Macpherson N, Brownlee C, Hofmann A, Webb AAR, Miedema H, Battey NH, Davies JM (2009) Zea mays annexins modulate cytosolic free Ca2+ and generate a Ca2+-permeable conductance. Plant Cell 21:479–493. https://doi.org/10.4161/psb.4.5.8297
Lehr PP, Hernández-Montes E, Ludwig-Müller J, Keller M, Zörb C (2022) Abscisic acid and proline are not equivalent markers for heat, drought and combined stress in grapevines. Aust J Grape and Wine Res 28:119–130. https://doi.org/10.1111/ajgw.12523
Liu SA, Li MJ, Su LC, Ge K, Li LM, Li XY, Liu X, Li L (2016) Negative feedback regulation of ABA biosynthesis in peanut (Arachis hypogaea): a transcription factor complex inhibits AhNCED1 expression during water stress. Sci Rep - UK 6:37943. https://doi.org/10.1038/srep37943
Liu X, Meng G, Wang MR, Qian ZL, Zhang YX, Yang WC (2021) Tomato SlPUB24 enhances resistance to Xanthomonas euvesicatoria pv. perforans race T3. Hortic Res Engl 8:30. https://doi.org/10.1038/s41438-021-00468-4
Loukehaich R, Wang TT, Ouyang B, Ziaf K, Li HX, Zhang JH, Lu YE, Ye ZB (2012) SpUSP, an annexin-interacting universal stress protein, enhances drought tolerance in tomato. J Exp Bot 63:5593–5606. https://doi.org/10.1093/jxb/ers220
Méchin V, Damerval C, Zivy M (2006) Total protein extraction with TCA-acetone. Plant Proteomics 355:1–8. https://doi.org/10.1385/1-59745-227-0:1
Melencion SMB, Chi YH, Pham TT, Paeng SK, Wi SD, Lee C, Ryu SW, Koo SS, Lee SY (2017) RNA chaperone function of a universal stress protein in Arabidopsis confers enhanced cold stress tolerance in plants. Int J Mol Sci 18:2546. https://doi.org/10.3390/ijms18122546
Mellacheruvu S, Tamirisa S, Vudem DR, Khareedu VR (2016) Pigeonpea hybrid-proline-rich protein (CcHyPRP) confers biotic and abiotic stress tolerance in transgenic rice. Front Plant Sci 6:1167. https://doi.org/10.3389/fpls.2015.01167
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4326. https://doi.org/10.1093/nar/8.19.4321
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Park SC, Jung YJ, Lee Y, Kim IR, Seol MA, Kim EJ, Jang MK, Lee JR (2017) Functional characterization of the Arabidopsis universal stress protein AtUSP with an antifungal activity. Biochem Bioph Res Co 486:923–929. https://doi.org/10.1016/j.bbrc.2017.03.126
Pouzoulet J, Pivovaroff AL, de Guzman ME, Rolshausen PE, Santiago LS (2020) Contrasting adaptation of xylem to dehydration in two Vitis vinifera L. sub-species. Vitis 59:53–61. https://doi.org/10.5073/vitis.2020.59.53-61
Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, Yamaguchi-Shinozaki K (2002) DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem Biophy Res Co 290:998–1009. https://doi.org/10.1006/bbrc.2001.6299
Sauter M, Rzewuski G, Marwedel T, Lorbiecke R (2002) The novel ethylene-regulated gene OsUsp1 from rice encodes a member of a plant protein family related to prokaryotic universal stress proteins. J Exp Bot 53:2325–2331. https://doi.org/10.1093/jxb/erf096
Seo DH, Lee A, Yu SG, Cui LH, Min HJ, Lee SE, Cho NH, Kim S, Bae H, Kim WT (2021) OsPUB41, a U-box E3 ubiquitin ligase, acts as a negative regulator of drought stress response in rice (Oryza Sativa L.). Plant Mol Biol 106:463–477. https://doi.org/10.1007/s11103-021-01158-4
Shi WY, Du YT, Ma J, Min DH, Jin LG, Chen J, Chen M, Zhou YB, Ma YZ, Xu ZS, Zhang XH (2018) The WRKY transcription factor GmWRKY12 confers drought and salt tolerance in soybean. Int J Mol Sci 19:4087
Shu X, Ding L, Gu B, Zhang HJ, Guan PY, Zhang JX (2021) A stress associated protein from Chinese wild Vitis amurensis, VaSAP15, enhances the cold tolerance of transgenic grapes. Sci Hortic Amst 285:110147. https://doi.org/10.1016/j.scienta.2021.110147
Sudhakar C, Lakshmi A, Giridarakumar S (2001) Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci 161:613–619. https://doi.org/10.1016/S0168-9452(01)00450-2
Udawat P, Jha RK, Sinha D, Mishra A, Jha B (2016) Overexpression of a cytosolic abiotic stress responsive universal stress protein (SbUSP) mitigates salt and osmotic stress in transgenic tobacco plants. Front Plant Sci 7:518. https://doi.org/10.3389/fpls.2016.00518
Udawat P, Mishra A, Jha B (2014) Heterologous expression of an uncharacterized universal stress protein gene (SbUSP) from the extreme halophyte, Salicornia brachiata, which confers salt and osmotic tolerance to E. coli. Gene 536:163–170. https://doi.org/10.1016/j.gene.2013.11.020
Vermeirssen V, De Clercq I, Van Parys T, Van Breusegem F, Van de Peer Y (2014) Arabidopsis ensemble reverse-engineered gene regulatory network discloses interconnected transcription factors in oxidative stress. Plant Cell 26:4656–4679. https://doi.org/10.1105/tpc.114.131417
Virlouvet L, Ding Y, Fujii H, Avramova Z, Fromm M (2014) ABA signaling is necessary but not sufficient for RD29B transcriptional memory during successive dehydration stresses in Arabidopsis thaliana. Plant J 79:150–161. https://doi.org/10.1111/tpj.12548
Wang L, Wang YJ (2019) Transcription factor VqERF114 regulates stilbene synthesis in Chinese wild Vitis quinquangularis by interacting with VqMYB35. Plant Cell Rep 38:1347–1360. https://doi.org/10.1007/s00299-019-02456-4
Wang PF, Mu XP, Gao YG, Zhang JC, Du JJ (2020) Successful induction and the systematic characterization of tetraploids in cerasus humilis for subsequent breeding. Sci Hortic Amst 265:109216. https://doi.org/10.1016/j.scienta.2020.109216
Wang YJ, Yang YZ, Zhang JX, Pan XJ, Wan YZ (2004) Preliminary identification of drought resistance of Chinese wild Vitis species and its interspecific hybrids. (in Chin) Acta Hortic Sin 6:711–714
Wang Y, Yang ZM, Zhang QF, Li JL (2009) Enhanced chilling tolerance in Zoysia matrella by pre-treatment with salicylic acid, calcium chloride, hydrogen peroxide or 6-benzylaminopurine. Biol Plantarum 53:179–182. https://doi.org/10.1007/s10535-009-0030-2
Wesley SV, Helliwell CA, Smith NA, Wang MB, Rouse DT, Liu Q, Gooding PS, Singh SP, Abbott D, Stoutjesdijk PA, Robinson SP, Gleave AP, Green AG, Waterhouse PM (2001) Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J 27:581–590. https://doi.org/10.1046/j.1365-313X.2001.01105.x
Xie ZL, Nolan T, Jiang H, Tang BY, Zhang MC, Li ZH, Yin YH (2019) The AP2/ERF transcription factor TINY modulates brassinosteroid-regulated plant growth and drought responses in Arabidopsis. Plant Cell 31:1788–1860
Xin ZG, Mandaokar A, Chen JP, Last RL, Browse J (2007) Arabidopsis ESK1 encodes a novel regulator of freezing tolerance. Plant J 49:786–799. https://doi.org/10.1111/j.1365-313X.2006.02994.x
Yadav B, Jogawat A, Rahman MS, Narayan OP (2021) Secondary metabolites in the drought stress tolerance of crop plants: a review. Gene Rep 23:101040. https://doi.org/10.1016/j.genrep.2021.101040
Yamaguchi-Shinozaki K, Shinozaki K (1993) The plant hormone abscisic acid mediates the drought-induced expression but not the seed-specific expression of rd22, a gene responsive to dehydration stress in Arabidopsis thaliana. Molec Gen Genet 238:17–25
Yang ML, Che SY, Zhang YX, Wang HB, Wei T, Yan GR, Song WQ, Yu WW (2019) Universal stress protein in Malus sieversii confers enhanced drought tolerance. J Plant Res 132:825–837. https://doi.org/10.1007/s10265-019-01133-7
Yang MY, Zhao YJ, Shi SY, Du XM, Gu JT, Xiao K (2017) Wheat nuclear factor Y (NF-Y) B subfamily gene TaNF-YB3:l confers critical drought tolerance through modulation of the ABA-associated signaling pathway. Plant Cell Tiss Org 128:97–111. https://doi.org/10.1007/s11240-016-1088-0
Yao WK, Wang L, Wang J, Ma FL, Yang YZ, Wang C, Tong WH, Zhang JX, Xu Y, Wang XP, Zhang CH, Wang YJ (2017) VpPUB24, a novel gene from Chinese grapevine, Vitis pseudoreticulata, targets VpICE1 to enhance cold tolerance. J Exp Bot 68:2933–2949. https://doi.org/10.1093/jxb/erx136
Zahur M, Maqbool A, Irfan M, Barozai MYK, Rashid B, Riazuddin S, Husnain T (2009) Isolation and functional analysis of cotton universal stress protein promoter in response to phytohormones and abiotic stresses. Mol Biol 43:578–585. https://doi.org/10.1134/S0026893309040086
Zhang JX, Wu XC, Niu RX, Liu Y, Liu N, Xu WR, Wang YJ (2012) Cold-resistance enaluation in 25 wild grape species. Vitis 51:153–160
Zhang SJ, Li YL, Song GQ, Gao J, Zhang RZ, Li W, Chen ML, Li GY (2020) Heterologous expression of the ThIPK2 gene enhances drought resistance of common wheat. J Integr Agr 19:941–952. https://doi.org/10.1016/S2095-3119(19)62714-0
Zhang T, Zhang D, Liu YJ, Luo CB, Zhou YN, Zhang LY (2015) Overexpression of a NF-YB3 transcription factor from Picea wilsonii confers tolerance to salinity and drought stress in transformed Arabidopsis thaliana. Plant Physiol Bioch 94:153–164. https://doi.org/10.1016/j.plaphy.2015.05.001
Zhang Y, Zhang HZ, Fu JY, Du YY, Qu J, Song Y, Wang PW (2022) The GmXTH1 gene improves drought stress resistance of soybean seedlings. Mol Breeding 42:3. https://doi.org/10.1007/s11032-021-01258-5
Zhao FL, Li YJ, Hu Y, Gao YR, Zang XW, Ding Q, Wang YJ, Wen YQ (2016) A highly efficient grapevine mesophyll protoplast system for transient gene expression and the study of disease resistance proteins. Plant Cell Tiss Org 125:43–57. https://doi.org/10.1007/s11240-015-0928-7
Funding
This study was supported by the National Science-Technology Support Plan Projects of the Ministry of Science and Technology of the People’s Republic of China [grant number 2013BAD02B04-06], and the Shaanxi Province Key Project-Agriculture of the People’s Republic of China [grant number 2017ZDXM-NY-026].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by Leandro Peña.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
299_2022_2943_MOESM2_ESM.tif
Supplementary file2 VyUSPA3 may exist as a homodimer. a Yeast two-hybrid. Co-transformation of pGADT7-T with pGBKT7-p53 was used as a positive control, and pGADT7-T with pGBKT7-lam was used as a negative control, meanwhile, pGADT7-T and pGBKT7-VyUSPA3 were transformed to eliminate the possibility of interaction between pGADT7 vector and pGBKT7-VyUSPA3. b Depicted genetic construction for the BiFC. C BiFC assay. Scale bars = 40 μm (TIF 25250 KB)
299_2022_2943_MOESM3_ESM.tif
Supplementary file3 Transformation and identification of transgenic VyUSPA3 'Thompson Seedless' grape plants. a Genetic transformation process of 'Thompson Seedless' grape. b The qRT-PCR detection of the OE-VyUSPA3 and RNAi-VyUSPA3 lines. Relative expression levels were calculated using the 2-ΔΔCT method. Data are the mean (±SE) of three biological replicates. Significant differences among means were determined by one-way ANOVA followed by Tukey’s-b (K) test (P < 0.05). c Western blot detection of the OE-VyUSPA3 lines using antibodies specific for GFP (TIF 1373 KB)
299_2022_2943_MOESM4_ESM.tif
Supplementary file4 Expression levels of USPA3, ERF105, PUB24 and NF-YB3 under normal conditions and drought stress in 'Yanshan-1'. The bar on the right represents relative expression values, and the different expression levels are represented using FPKM (fragments per kilobase of transcript sequence per million base pairs sequenced) and different colors (TIF 41980 KB)
299_2022_2943_MOESM5_ESM.doc
Supplementary file5 Identification, phenotype, and physiological and biochemical indicators of the WT lines and erf6 (ERF6 Arabidopsis mutant). a Protocol for SALK T-DNA primer design. WT represents wild-type, HZ represents heterozygous mutants, and HM represents homozygous mutants. b Detection of genomic DNA. Lane 1 is the DL5000 marker, lane 2 is the WT lines, lanes 3–12 is the ERF6 Arabidopsis mutant. c Analysis of three selected mutants by qRT-PCR. Phenotypes of the WT lines and mutant d prior to and e after the 14-day drought treatment. The quantified level of f proline content, g MDA content, h electrolyte leakage, i POD activity, j SOD activity, and k CAT activity. Relative expression levels were calculated using the 2-ΔΔCT method. Data are the mean (±SE) of three biological replicates. Significant differences among means were determined by one-way ANOVA followed by Tukey’s-b (K) test (P < 0.05) (DOC 60 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cui, X., Zhang, P., Chen, C. et al. VyUSPA3, a universal stress protein from the Chinese wild grape Vitis yeshanensis, confers drought tolerance to transgenic V. vinifera. Plant Cell Rep 42, 181–196 (2023). https://doi.org/10.1007/s00299-022-02943-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-022-02943-1