Abstract
Key message
Sink-specific expression of a sucrose transporter protein gene from the C4 plant maize can promote carbohydrate accumulation in target tissues and increase both fiber and seed yield of cotton.
Abstract
Sucrose is the principal form of photosynthetic products transported from source tissue to sink tissue in higher plants. Enhancing the partition of carbohydrate to the target organ is a promising way to improve crop productivity. The C4 plant Zea mays exhibits a substantially higher rate of export of photosynthates than many C3 plants, and its sucrose transporter protein ZmSut1 displays important role in sucrose allocation. To investigate how use of ZmSUT1 gene to increase the fiber and seed yield of cotton, in this study, we expressed the gene in cotton under a senescence-inducible promoter PSAG12 and a seed coat-specific promoter BAN, respectively. We show that senescence-induced expression of ZmSUT1 results in an increase of sugar accumulation in leaves. Although the leaf senescence was postponed in PSAG12::ZmSUT1 cotton, the photosynthetic rate of the leaves was decreased. In contrast, seed coat-specific expression of the gene leads to an increase of sugar accumulation in fibers and bolls, and the leaf of transgenic BAN::ZmSUT1 cotton displayed higher photosynthetic capacity than the wild type. Importantly, both fiber and seed yield of transgenic BAN::ZmSUT1 cotton are significantly enhanced. Our data indicate the potential of enhancing yield of carbohydrate crops by the regulation of sugar partitioning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sucrose is the primary product of photosynthesis and the most prevalent long-distance translocatable sugar in higher plants. It has been demonstrated that the decrease of sucrose transport from source to sink tissues can suppress photosynthesis and growth of the plant, while the increase of sink demand can enhance photosynthetic activity and crop productivity (Ainsworth and Bush 2011; Ayre 2011; Bihmidine et al. 2013; Griffiths and Paul 2017; Hall et al. 1977; Hodgkinson 1974; Kaschuk et al. 2010; McCormick et al. 2006; Paul and Foyer 2001; Paul and Pellny 2003; Sheen 1990; Stitt et al. 2010). Thus, it has been proposed that crop yields can be enhanced by promoting the photosynthate transport from the source tissue to the sink tissue (Ainsworth and Bush 2011; Ayre 2011; McCormick et al. 2006).
Plant sucrose transporter (SUT) proteins are energy-dependent sucrose/H+ symporters which mediate the transport of sucrose across plasma membranes, leading the sugar into and out of the source and sink tissues through phloem (Riesmeier et al. 1992; Sauer 2007). This property enables us to genetically regulate the distribution of photosynthesized carbon, thus enhancing the yield of the target product. For example, downregulation of StSUT4 gene in potato resulted in early flowering and increased tuber yield (Chincinska et al. 2008). The use of endosperm-specific promoter to direct the expression of HvSUT1 in barley led to an enhanced sugar-absorption capacity of the seeds and an increased content of storage protein in seeds (Weichert et al. 2010). A phloem cell-specific expression of Arabidopsis AtSUC2 to promote the sucrose load resulted in an increased yield of rice (Wang et al. 2015).
Cotton is a leading natural fiber crop and also an important oil crop in the word (Jeffrey et al. 2007). Cotton fiber is derived from single epidermal cells of seed. The fiber is composed of nearly pure cellulose, the naturally occurring polymer of glucose. During the development of cotton seeds, the phloem-unloaded sugar is transported outwards to fiber cells for cell elongation and cellulose biosynthesis while inwards to filial tissues for the development of embryo and the biosynthesis of oil and starch (Jiang et al. 2012; Ruan 2005; Xu et al. 2012). This opposite transporting direction provides a dilemma to the regulation of the phloem-unloaded assimilate partition between fibers and the seed: in the case of limited carbon supply, enhancing of the abundance of fiber usually leads to smaller seeds, while increasing of the seed size often results in a decrease of fiber productivity of the seed (Miller et al. 1958; Miller and Rawlings 1967; Zeng et al. 2007; Zhao et al. 2015). To solve this trade-off problem, the best way is providing more supply to meet the demand of both sides.
Zea mays is a C4 crop, which is featured with high water-use efficiency and high-yield potential (Usha et al. 2015). Previous studies demonstrated that C4 grasses (e.g., maize and sorghum) display a higher rate of export of photosynthates than C3 grasses (e.g., wheat and barley) (Grodzinski et al. 1998). ZmSut1, a maize sucrose/proton symporter, has an important function in sugar distribution (Aoki 1999; Baker et al. 2016). This sugar transport protein can effectively mediate sucrose uptake into the phloem in source tissue, as well as release the sugar from the phloem vessels into sink tissue (Baker et al. 2016; Carpaneto et al. 2005). Accordingly, Baker et al. (2016) suggest a potential of using SUTs from C4 plants to engineer C4 photosynthesis and carbon transport in C3 plants.
To see whether and how the ectopic expression of a C4 SUT in cotton is able to increase the efficacy of sucrose transport, thus enhancing the fiber and seed yield, in this study, we constructed transgenic cotton in which the ZmSUT1 gene is under control of a senescence-inducible and seed coat-specific promoter, respectively. Our data demonstrated that seed coat-specific expression of ZmSUT1 led to an increase of sugar accumulation in fibers and bolls, and elevated both fiber and seed yield of cotton; while senescence-induced expression of the gene resulted in an increase of sugar accumulation in leaves and a delay of leaf senescence, but decreased the yield.
Materials and methods
Plasmid construction and plant transformation
An 1812 bp cDNA including 1638 bp open-reading frame (ORF) of ZmSUT1 gene was cloned from Zea mays and the cDNA clone was inserted into pUCm-T vector (Promega, USA). For PSAG12::ZmSUT1 construct, the leaf senescence-specific promoter PSAG12 (2180 bp) was cloned from Arabidopsis and inserted into pMD19-T vector (Takara, Japan). A modified pCambia2300 vector pLGN which contains NPTII::GUS fusion gene driven by CaMV35S promoter was used to construct plant expression vectors. PSAG12 promoter was inserted in pLGN vector using BamHI and SalI restriction sites and the cDNA of ZmSUT1 gene was inserted the downstream of PSAG12 promoter. For BAN::ZmSUT1 construct, the promoter of BAN gene that is specifically expressed in seed coat and early fibers (Zhang et al. 2011) was amplified from Arabidopsis. BAN promoter was inserted in pLGN vector using HindIII and EcoRI restriction sites and the ZmSUT1 cDNA was inserted the downstream of BAN promoter. All primers used for PCR were listed in Table S1. These two constructs were transformed into an upland cotton (Gossypium hirsutum L., cultivar ‘Jimian 14′) by Agrobacterium tumefaciens strain LBA4404 (Luo et al. 2010). Kanamycin-resistant and GUS-positive plants were screened out and grown in glasshouse at Southwest University, Chongqing, China.
Isolation of total RNA and qRT-PCR analysis
Total RNA was extracted according to the manual of EASY Spin Plant RNA Kit (Aidlab, China), and cDNA was synthesized using a PrimeScript™ RT reagent kit with gDNA eraser (TaKaRa, China). qRT-PCR assay was performed on the CFX96 real-time PCR system (Bio-Rad, USA) with 1 × iQ™ SYBR Green Supermix (Bio-Rad, USA) according to the manual, and data were analyzed by the native software Bio-Rad CFX Manager 2.0 (Bio-Rad, USA). GhHIS3 and GhUBQ14 were used as the reference genes. Primers used for qRT-PCR are listed in Table S1.
Sugar determination
Tissues or organs were collected at indicated time for sugar content measurement. In brief, the cotton leaves or bolls (100 mg, dry weight) were ground on a MM301 ball grinder (Retsch, Germany), and extracted with 5 mL of 80% ethanol. The extraction was then heated for 10 min in 80 °C water. After cooling, samples were centrifuged at 12,000g for 10 min the supernatant was collected into a new tube. The extraction step was repeated three times. Then, the supernatant was collected to determine the amount of total soluble sugars by the anthrone-sulfuric acid method. Sucrose amount determination is followed by the method described previously (Zhao et al. 2010). For the sugar determination in seed coat and fibers, seed coat and fibers of 10-DPA (day after anthesis) ovules were separated with tweezers, then frozen in liquid nitrogen. Samples (100 mg, fresh weight) were homogenized. The methods for soluble sugar extraction and sucrose content determination were as the same as that in leaves.
In situ hybridization
In situ hybridization of ZmSUT1 mRNA was followed the method described previously (Zhang et al. 2017). In brief, gene-specific probe was prepared according to DIG Northern Starter Kit manual (Roche, USA). For ZmSUT1, DNA template of the probe was amplified from the cDNA of ZmSUT1 with sequence-specific primers (Table S1). Paraffin sections of leaves were deparaffinized and incubated with Dig-labeled RNA probe. Then, the section was incubated with anti-Dig-AP conjugate (Roche, USA) and the signal was detected by NBT/BCIP solution (Roche, USA). Sections incubated with the sense RNA probe were used as the negative control. Images were captured using a CKX41 microscope (Olympus, Japan).
Dark-induced senescence
For dark-induced senescence treatment, plants at 65 days after sow (DAS) grown in natural conditions were divided randomly into two groups and transferred them into growth chambers: one group was under normal condition (16 h light/8 h dark) and other group was under dark condition. 4 days after treatment, mature leaves in the same position of the plant were used for the determination of ZmSUT1 transcription.
Aging-related physiological change assay
The chlorophyll content determination was followed by the method described previously (Chory et al. 1994; Zhao et al. 2015). The malondialdehyde (MDA) content kit (Comin Biotechnology Co., Ltd. Suzhou, China) was used to determine MDA content. The superoxide dismutase (SOD) activity kit and the peroxidase (POD) activity kit (Comin Biotechnology Co., Ltd. Suzhou, China) were used to determine SOD and the POD activities.
Dry matter measurement
Five plants (125 DAS) were harvested from each transgenic cotton line in the growth chamber. Leaves, stems and fruits were dried in a forced draft oven at 70 °C and then weighted.
Photosynthesis measurement
Photosynthetic rate was measured by the Li-6400 portable photosynthesis system (LI-COR, Lincoln, NE, USA) set at saturating light of 1200 µmol/m2 s and a CO2 concentration of 400 ppm. The fourth main-stem leaf from the apex (functional leaf) of four plants at 125 DAS grown in greenhouse was used for the photosynthetic rate determination.
Field experiment
The T3 generation lines of transgenic plant and the wild type were planted in the green house. The boll number was counted at 135 DAS. After harvest, fibers and seeds were weighed separately, and the number of seeds per boll, and seed cotton weight (seed plus lint weight) per boll were determined. The seed cotton yield per plant (g) = boll number per plant × seed cotton weight per boll; the fiber yield per plant (g) = boll number per plant × lint fiber weight per boll. In 2018 field trial, line BU-21 (T4) that showed best performance in the green house experiment and wild-type control were grown in the experimental farm of Southwest University (Chongqing, China) using randomized block design with three replications. Each block was 18 m2 and contained 60 plants in 4 rows with 1.0 m apart. The space between two neighboring plants in a row was 0.30 m. For eliminating the edge effect of the plot, open bolls were harvested from the plants growing in the central two rows. The cotton harvest began on 28th July and ended on 30th August.
Results
Senescence-induced expression of ZmSUT1 in cotton promotes sugar accumulation in leaves and delays leaf senescence
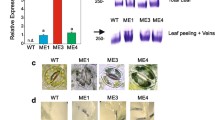
To see the alteration of sugar allocation by senescence-inducible expression of ZmSUT1 in the aged leaf, we constructed transgenic cotton in which ZmSUT1 gene is under the control of a senescence-specific promoter PSAG12 (Aoki 1999; Baker et al. 2016; Carpaneto et al. 2005; Slewinski et al. 2010). Based on the transcription level, two lines, i.e., SU-8 and SU-14 which showed the highest expression of ZmSUT1 in aged leaves among nine PSAG12::ZmSUT1 transformants (Fig. 1a) were selected for further investigation. A high transcript level of ZmSUT1 was detected in aged leaves. However, the level was nearly undetectable in mature leaves, ovules and fibers (Fig. 1b). The etiolation treatment could significantly increase the expression of the gene in mature leaf (Fig. 1c), indicating a senescence-inducible expression pattern of PSAG12::ZmSUT1 in the transgenic cotton. According to the previous study (Baker et al. 2016), ZmSut1 located mainly in the phloem, xylem and vascular bundle sheath cells, but not in mesophyll cells of the maize leaf. However, our mRNA in situ hybridization showed that ZmSUT1 transcription signal appeared in all type cells, including mesophyll cells, of the aged leaves of PSAG12::ZmSUT1 cotton (Fig. 1d).
Senescence-induced expression of ZmSUT1 in cotton promotes sugar accumulation in aged leaves. aZmSUT1 transcription level in PSAG12::ZmSUT1 transgenic lines. RNAs of the 8th leaf from apex of the plants (90 DAS) were isolated and analyzed by qRT-PCR. GhHIS3 was used as the reference gene. Error bars indicate standard deviation (SD) of three replicates. WT, wild type. b Expression of ZmSUT1 in various tissues of transgenic cotton. GhUBQ14 was used as the reference gene. Mature leaf, the 4th leaf from the apex; aged leaf, the 16th leaf from the apex; DPA, day after anthesis. c The transcription level of ZmSUT1 in the mature leaves after 4-day dark treatment. GhUBQ14 was used as the reference gene. dZmSUT1 mRNA in situ hybridization of ZmSUT1 in the aged leaf of PSAG12::ZmSUT1 transgenic cotton and wild-type control. Sense probe was used as the negative control. Bar 100 μm. Aged leaf, the 16th leaf from the apex. Black arrows point to the hybridization signal. e, f Sucrose content and total soluble sugar content in aged leaves of SU-8, SU-14 and wild type at 125 DAS. g Sucrose content of mature leaves of 4-day dark-treated plants. SU-8 and SU-14 represent PSAG12::ZmSUT1 transgenic lines #8 and #14. Results are presented as mean ± SD (n = 3). Asterisks indicate significant differences (Student’s t test, *p ≤ 0.05, **p ≤ 0.01)
Sugar determination showed that sucrose content in transgenic SU-8 and SU-14 lines was increased by 21.6% and 34.4%, significantly higher than that of the wild-type control (Fig. 1e), and the total soluble sugar increased by 5.8% and 10.2%, respectively (Fig. 1f). To confirm that the senescence-induced expression of ZmSUT1 could promote sucrose accumulation in leaves, we compared the sugar content of leaves of darkness-treated (etiolated) plants with that of the control. The sucrose content in the etiolated leaves of transgenic cotton was 6.5 ± 0.2 mg/g, showing a 41.3% increase than that of the wild-type control (4.6 ± 0.4 mg/g) (Fig. 1g). These results indicated that the ectopic expression of ZmSUT1 in cotton promotes the sucrose accumulation rather than the efflux of sucrose in transgenic leaves.
The decrease of chlorophyll content is a sign of leaf senescence (Kusaba et al. 2007). Under darkness, the top leaves of wild-type cotton became yellow and the chlorophyll content in the leaves was dramatically decreased (Fig. 2a). However, the leaf etiolation was much less apparent in the transgenic lines and the chlorophyll content in the transgenic cotton leaves was significantly higher than that of the wild type (Fig. 2b). The increase of malonaldehyde (MDA) content, and the decrease of superoxide dismutase (SOD) and peroxidase (POD) activities are usually recognized as indicators of plant senescence (Dhindsa et al. 1981). In the lower leaves of transgenic cotton lines, the chlorophyll content, and SOD and POD activities were significantly higher, while the content of MDA was significantly lower than that of the wild-type cotton leaves (Fig. 2c–f), confirming the delay of senescence in PSAG12::ZmSUT1 cotton lines. Interestingly, photosynthetic rate of SU-8 and SU-14 leaves was noticeably decreased (Fig. 2g). These data suggested that the increased accumulation of sugar in PSAG12::ZmSUT1 transgenic leaves could compensate the loss of carbohydrates in the aging leaves in some degree, thus postponing the leaf senescence. However, the increased sugar accumulation might in turn account for the decreased photosynthetic capacity of the leaves.
Senescence-induced expression of ZmSUT1 delays leaf senescence while decreases photosynthetic efficiency. a The phenotype of SU-8 and wild type in leaves after 4-day dark treatment of whole plant (65-DAS plants). Bar 15 cm. b Chlorophyll content of young leaves (65-DAS plants) after 4-day dark treatment. c Chlorophyll content of aged leaves (125-DAS plants) in PSAG12::ZmSUT1 transgenic lines and wild type in the field conditions. d MDA content of aged leaves (125-DAS plants) in PSAG12::ZmSUT1 transgenic lines and wild type. e, f Activities of SOD and POD in aged leaves (125-DAS plants) of PSAG12::ZmSUT1 transgenic lines and wild type. g Photosynthetic rate of the 4th leaf from the apex at 125 DAS. Error bars indicate SD of four randomly selected plants of each line. Young leaves, 1st and 2nd leaves from the apex; aged leaves, 16th leaf from the apex. Results are presented as mean ± SD (n = 3). Asterisks indicate significant differences (Student’s t test, *p ≤ 0.05, **p ≤ 0.01)
Seed coat-specific expression of ZmSUT1 promotes sucrose accumulation in cotton fibers and increases seed and lint yield
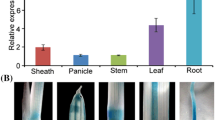
Our data had demonstrated that the expression of ZmSUT1 promotes the sucrose accumulation rather than the efflux of the sugar in PSAG12::ZmSUT1 transgenic leaves. Cotton fiber is derived from a single epidermal cell of the seed coat. To investigate the possibility of increasing fiber yield by enhancing carbon sink strength of fibers, we used a seed coat-specific promoter BAN (Debeaujon et al. 2003; Zhang et al. 2011) to direct the expression of ZmSUT1 in the epidermis of ovule or in the fibers. From the resulting nine BAN::ZmSUT1 transformants, we selected two lines, i.e., BU-21 and BU-59 which showed relatively high expression level of the gene in the 0-DPA ovules (Fig. 3a) as representative lines to investigate the sugar allocation in the target tissue. Transcript assay showed that ZmSUT1 was preferentially expressed in the rapid elongating fibers (10-DPA, Fig. 3b). As expected, sucrose content was significantly increased in the ovule epidermis and fibers (10-DPA, Fig. 3c). In addition, photosynthetic rate of BU-21 and BU-59 leaves was enhanced, showing a 5.5% and 5.1% increase relative to that of the wild type (Fig. 3d).
Seed coat-specific expression of ZmSUT1 enhances sucrose accumulation in the seed coat and fibers, and improves photosynthetic efficiency of leaves. aZmSUT1 transcription level in the BAN::ZmSUT1 transgenic lines. RNAs were isolated from 0-DPA ovules of transgenic cotton plants and ZmSUT1 transcript was analyzed by qRT-PCR. GhHIS3 was used as the reference gene. Error bars indicate SD of three replicates; WT, wild type. b Seed coat-specific expression of ZmSUT1 in various tissues of transgenic cotton. GhUBQ14 was used as the reference gene. c Sucrose content in the seed coat (without fiber) and fiber of BU-21, BU-59 and wild type at 10 DPA. Error bars indicate SD of three replicates. d Photosynthetic rate of the 4th leaf from the apex at 125-DAS cotton plants. Error bars indicate SD of four randomly selected plants of each line. e The total soluble sugar content of different tissues between wild-type cotton and BAN::ZmSUT1 transgenic lines. Error bars indicate SD of three replicates. BU-21 and BU-59 represent BAN::ZmSUT1 transgenic lines #21 and #59. Asterisks indicate significant differences (Student’s t test, *p ≤ 0.05, **p ≤ 0.01)
In growth chamber condition, BAN::ZmSUT1 transgenic cotton had more soluble sugar in bolls (Fig. 3e), and more dry matters of the bolls (Table 1). The content of total soluble sugars in bolls of BU-21 and BU-59 lines was increased by 4.2% (p = 0.062459402) and 20.0% (p = 0.000196135), respectively. Meanwhile the content in leaves was significantly decreased compared with the control (Fig. 3e). The dry weight of boll per plant of line BU-21 and BU-59 was enhanced by 21.6% and 15.0%, respectively (Table 1). The total dry biomass per plant of the two lines was increased by 18.8% and 17.1%, compared with the wild-type control. However, no obvious change in the dry weight of leaves between transgenic BAN::ZmSUT1 and wild type was observed (Table 1). In contrast to BAN::ZmSUT1 lines, the dry weight of bolls per plant of PSAG12::ZmSUT11 lines was significantly decreased (Table 1).
To investigate the agronomic performance of PSAG12::ZmSUT1 and BAN::ZmSUT1 transgenic cotton lines, we grew them in green house. The seed cotton weight per boll of transgenic BU-21 and BU-59 plants was increased by 26.7% and 23.3%, significantly higher than that of the wild type (Table 2). Consequently, seed cotton yield per plant and lint yield per plant of BU-21 and BU-59 lines were increased by 31.2% (p = 0.000836) and 29.6% (p = 0.001207), and 13.2% (p = 0.049015) and 11.8% (p = 0.072847), respectively, relative to the wild-type control. Conversely, the senescence-induced expression of ZmSUT1 gene led a decrease in seed cotton weight per boll. The lint yield per plant of PSAG12::ZmSUT1 lines SU-8 and SU-14 was decreased by 26.2% and 25.6%, respectively, compared with that of wild-type control. To further assess the value of BAN::ZmSUT1 transgenes in yield improvement, we selected BU-21 (T4) as the best line for field trial in 2018. As in displayed T3 generation, the transgenic line showed higher productivity in seed yield and lint fiber yield, increased by 13.8% and 8.4%, respectively, as compared to the wild type (Table 3).
Discussion
In maize leaves, ZmSUT1 is expressed in phloem companion cells, and xylem and vascular bundle sheath cells, but not in mesophyll cells (Baker et al. 2016). The cellular location pattern of ZmSUT1 indicates its biological function: loading sucrose in phloem companion cells, while retrieving the sugar in other cell types from the apoplasm (Baker et al. 2016). It was reported that loss of ZmSut1 in maize resulted in an increased sugar accumulation in the leaves, indicating the defect of loading functions of the protein in the mutant. In this study, the senescence-induced ectopic expression of ZmSUT1 in cotton significantly increased the sugar accumulation in leaves (Fig. 1). SAG12, encoding a cysteine protease, shows an expression pattern in senescent tissues including mesophyll cytoplasm of aged leaves (Noh and Amasino 1999; Otegui et al. 2005). Our in situ mRNA hybridization indicated that under the control of a senescence-activated promoter PSAG12, the transcript signal of ZmSUT1 appeared not only in the phloem, xylem and vascular bundle sheath cells, but also in mesophyll cells (Fig. 1d). We also found a decreased expression of endogenous SUT and SWEET in the aged leaves of transgenic PSAG12::ZmSUT1 cotton (Supplementary Fig. 1). This indicates that the ectopic expression of ZmSUT1 may interrupt endogenous sugar transport system including the native phloem upload from apoplast (mediated by SUTs) and the sucrose outflow from parenchyma cells (mediated by SWEETs). We suggest that the promoted loading of sucrose into phloem resulted from the ectopic expression of ZmSUT1 may be compromised by the decreased expression of native SUT in phloem companion cells and the decreased of native SWEET in parenchyma cells. In the meantime, the mis-expression of ZmSUT1 gene in mesophyll cells can facilitate the retrieving of sucrose from the apoplasm into the cells, which may account for the increased sugar content in the leaves.
During leaf senescence process, nutrients are remobilized from senescing leaves to other parts of the plant (Ori et al. 1999). It has been shown that low sugar level can increase ethylene production or ethylene sensitivity, thus accelerating leaf senescence (Grbić and Bleecker 1995; Thimann et al. 1977); whereas, the increase of sugar supply can inhibit leaf senescence. Interestingly, the expression of SAG12 can be induced by sugar starvation or suppressed by sugar supply (Noh and Amasino 1999). Accordingly, we suggest that the increased sugar accumulation in transgenic PSAG12::ZmSUT1 cotton can suppress the expression of senescence-related genes (Kong et al. 2013), including endogenous SAG12 of cotton and thus postpone the leaf senescence (Supplementary Fig. 2). However, high level of sugars in leaves would repress the expression of photosynthetic genes and thereby inhibit net photosynthesis (Marschall et al. 1998; Rook and Bevan 2003). Therefore, retaining more sugar in the leaf would produce two negative consequences for the plant: decreasing the photosynthetic capacity of leaves, and reducing the nutrient remobilization from senescing leaves to the sink organs. As the result, the yield of transgenic cotton would be decreased (Table 2).
Contrast to PSAG12 promoter, seed coat-specific promoter controlling expression of ZmSUT1 enables us to promote more sugar into developing fiber and ovule. As desired, sugar content in seed coat and fiber was significantly increased (Fig. 3). It has been known that the promotion of carbon partition from vegetative to reproductive structures can result in an increase of the yield of cotton (Meredith and Wells 1989). In our experiment, the increased sugar supply to fibers and ovules consequently increased the yield of seed cotton and lint fiber (Tables 2, 3). It is worth mentioning that the enhancement of sink strength can promote the transport of photosynthetic assimilates from source leaves to the sink tissues. The increased photosynthetic rate does not result in an increase of sugar content in transgenic leaves of BAN::ZmSUT1 cotton (Fig. 3e), indicating the promoted sugar efflux from the leaf. This will in turn benefit the source capacity by relieving the feedback-inhibition of sugar accumulation imposed on photosynthesis (Iglesias et al. 2002), and, therefore, increasing photosynthetic rate of the transgenic cotton leaves (Fig. 3d). As the result of the enhanced sink strength accompanied with the increased photosynthesis, the yield of transgenic BAN::ZmSUT1 cotton is elevated.
Taken together, our data indicated that seed coat-specific expression of ZmSUT1 could promote the accumulation of sugar in bolls and fibers thus increasing the yield of lint fibers and seeds; whereas, senescence-induced expression of this gene led to a decreased sugar content in bolls and a decreased lint and seed yield, although the leaf senescence was delayed. We show that sink-specific expression of ZmSUT1 is capable of increasing sugar content in the target organ. This strategy is particularly feasible for the yield enhancement of carbohydrate crops (e.g., tomato, rice, potato, wheat, oilseed rape, cotton, etc.), the target products of which are sugar, starch, oil, or cellulose. It is worthwhile to note that, however, sugars serve not only as an energy source but also as signal materials (Hc and Van den Ende 2018; Li and Sheen 2016; Smeekens 2000), and high concentration of sugar will result in osmotic stress. To avoid the possible negative impact from the excess sugars on plant growth, an acute spatiotemporal manipulation of sugar transporters or the promotion of sugar utilization in target tissues should be taken into consideration.
Author contribution statement
YP conceived and planned the study and wrote the manuscript. XD, JZ, LH, XL and SQ performed the experiments. All the authors read and approved the final manuscript.
References
Ainsworth EA, Bush DR (2011) Carbohydrate export from the leaf: a highly regulated process and target to enhance photosynthesis and productivity. Plant Physiol 155:64–69. https://doi.org/10.1104/pp.110.167684
Aoki N (1999) Molecular cloning and expression analysis of a gene for a sucrose transporter in maize (Zea mays L.). Plant Cell Physiol 40:1072–1078
Ayre BG (2011) Membrane-transport systems for sucrose in relation to whole-plant carbon partitioning. Mol Plant 4:377–394. https://doi.org/10.1093/mp/ssr014
Baker RF, Leach KA, Boyer NR, Swyers MJ, Benitez-Alfonso Y, Skopelitis T, Luo A, Sylvester A, Jackson D, Braun DM (2016) Sucrose transporter ZmSut1 expression and localization uncover new insights into sucrose phloem loading. Plant Physiol 172:1876–1898. https://doi.org/10.1104/pp.16.00884
Bihmidine S, Hunter CT 3rd, Johns CE, Koch KE, Braun DM (2013) Regulation of assimilate import into sink organs: update on molecular drivers of sink strength. Front Plant Sci 4:177. https://doi.org/10.3389/fpls.2013.00177
Carpaneto A, Geiger D, Bamberg E, Sauer N, Fromm J, Hedrich R (2005) Phloem-localized, proton-coupled sucrose carrier ZmSUT1 mediates sucrose efflux under the control of the sucrose gradient and the proton motive force. J Biol Chem 280:21437. https://doi.org/10.1074/jbc.M501785200
Chincinska IA, Liesche J, Krügel U, Michalska J, Geigenberger P, Grimm B, Kühn C (2008) Sucrose transporter StSUT4 from potato affects flowering, tuberization, and shade avoidance response. Plant Physiol 146:515–528. https://doi.org/10.1104/pp.107.112334
Chory J, Reinecke D, Sim S, Washburn T, Brenner M (1994) A role for cytokinins in de-etiolation in Arabidopsis (det mutants have an altered response to cytokinins). Plant Physiol 104:339–347
Debeaujon I, Nesi N, Perez P, Devic M, Grandjean O, Caboche M, Lepiniec L (2003) Proanthocyanidin-accumulating cells in Arabidopsis testa: regulation of differentiation and role in seed development. Plant Cell 15:2514–2531. https://doi.org/10.1105/tpc.014043
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Grbić V, Bleecker AB (1995) Ethylene regulates the timing of leaf senescence in Arabidopsis. Plant J 8:595–602
Griffiths CA, Paul MJ (2017) Targeting carbon for crop yield and drought resilience. J Sci Food Agr. https://doi.org/10.1002/jsfa.8501
Grodzinski B, Jiao J, Leonardos ED (1998) Estimating photosynthesis and concurrent export rates in C3 and C4 species at ambient and elevated CO2. Plant Physiol 117:207–215. https://doi.org/10.1104/pp.117.1.207
Hall AJ, Brady CJ, Hall AJ, Brady CJ (1977) Assimilate source-sink relationships in Capsicum annuum L. II. Effects of fruiting and defloration on the photosynthetic capacity and senescence of the leaves. Funct Plant Biol 4:771–783
Hc JVR, Van den Ende W (2018) UDP-glucose: a potential signaling molecule in plants? Front Plant Sci 8:2230. https://doi.org/10.3389/fpls.2017.02230
Hodgkinson K (1974) Influence of partial defoliation on photosynthesis, photorespiration and transpiration by lucerne leaves of different ages. Funct Plant Biol 1:561–578. https://doi.org/10.1071/PP9740561
Iglesias DJ, Lliso I, Tadeo FR, Talon M (2002) Regulation of photosynthesis through source: sink imbalance in citrus is mediated by carbohydrate content in leaves. Physiol Plantarum 116:563–572. https://doi.org/10.1034/j.1399-3054.2002.1160416.x
Jeffrey ZC, Scheffler BE, Elizabeth D, Triplett BA, Tianzhen Z, Wangzhen G, Xiaoya C, Stelly DM, Rabinowicz PD, Town CD (2007) Toward sequencing cotton (Gossypium) genomes. Plant Physiol 145:1303–1310. https://doi.org/10.1104/pp.107.107672
Jiang Y, Guo W, Zhu H, Ruan YL, Zhang T (2012) Overexpression of GhSusA1 increases plant biomass and improves cotton fiber yield and quality. Plant Biotechnol J 10:301–312. https://doi.org/10.1111/j.1467-7652.2011.00662.x
Kaschuk G, Hungria M, Leffelaar PA, Giller KE, Kuyper TW (2010) Differences in photosynthetic behaviour and leaf senescence of soybean (Glycine max [L.] Merrill) dependent on N2 fixation or nitrate supply. Plant Biol 12:60–69
Kong X, Luo Z, Dong H, Eneji AE, Li W, Lu H (2013) Gene expression profiles deciphering leaf senescence variation between early- and late-senescence cotton lines. PLoS One 8:e69847. https://doi.org/10.1371/journal.pone.0069847
Kusaba M, Ito H, Morita R, Iida S, Sato Y, Fujimoto M, Kawasaki S, Tanaka R, Hirochika H, Nishimura M (2007) Rice NON-YELLOW COLORING1 is involved in light-harvesting complex II and grana degradation during leaf senescence. Plant Cell 19:1362–1375. https://doi.org/10.1105/tpc.106.042911
Li L, Sheen J (2016) Dynamic and diverse sugar signaling. Curr Opin Plant Biol 33:116–125. https://doi.org/10.1016/j.pbi.2016.06.018
Luo M, Xiao Y, Li X, Lu X, Deng W, Li D, Hou L, Hu M, Li Y, Pei Y (2010) GhDET2, a steroid 5α-reductase, plays an important role in cotton fiber cell initiation and elongation. Plant J 51:419–430. https://doi.org/10.1111/j.1365-313X.2007.03144.x
Marschall M, Proctor MCF, Smirnoff N (1998) Carbohydrate composition and invertase activity of the leafy liverwort Porella platyphylla. New Phytol 138:343–353. https://doi.org/10.1046/j.1469-8137.1998.00102.x
McCormick AJ, Cramer MD, Watt DA (2006) Sink strength regulates photosynthesis in sugarcane. New Phytol 171:759–770. https://doi.org/10.1111/j.1469-8137.2006.01785.x
Meredith WR, Wells R (1989) Potential for increasing cotton yields through enhanced partitioning to reproductive structures. Crop Sci 29:636–639. https://doi.org/10.2135/cropsci1989.0011183X002900030017x
Miller PA, Rawlings JO (1967) Selection for increased lint yield and correlated responses in upland cotton, Gossypium hirsutum L. Crop Sci 7:637–640. https://doi.org/10.2135/cropsci1967.0011183X000700060024x
Miller DA, Williams JC, Robinson HF, Comstock JB (1958) Estimates of genotypic and environmental variances and co variances in upland cotton and their implication in selection. Agron J 50:126–131. https://doi.org/10.2134/agronj1958.00021962005000030004x
Noh YS, Amasino RM (1999) Identification of a promoter region responsible for the senescence-specific expression of SAG12. Plant Mol Biol 41:181–194
Ori N, Juarez MT, Jackson D, Yamaguchi J, Banowetz GM, Hake S (1999) Leaf senescence is delayed in tobacco plants expressing the maize homeobox gene knotted1 under the control of a senescence-activated promoter. Plant Cell 11:1073–1080. https://doi.org/10.1105/tpc.11.6.1073
Otegui MS, Noh YS, Martinez DE, Vila Petroff MG, Staehelin LA, Amasino RM, Guiamet JJ (2005) Senescence-associated vacuoles with intense proteolytic activity develop in leaves of Arabidopsis and soybean. Plant J 41:831–844. https://doi.org/10.1111/j.1365-313X.2005.02346.x
Paul MJ, Foyer CH (2001) Sink regulation of photosynthesis. J Exp Bot 52:1383–1400. https://doi.org/10.1093/jexbot/52.360.1383
Paul MJ, Pellny TK (2003) Carbon metabolite feedback regulation of leaf photosynthesis and development. J Exp Bot 54:539–547. https://doi.org/10.1093/jxb/erg052
Riesmeier JW, Willmitzer L, Frommer WB (1992) Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J 11:4705. https://doi.org/10.1002/j.1460-2075.1992.tb05575.x
Rook F, Bevan MW (2003) Genetic approaches to understanding sugar-response pathways. J Exp Bot. https://doi.org/10.1093/jxb/erg054
Ruan YL (2005) Recent advances in understanding cotton fibre and seed development Seed. Sci Res 15:269–280. https://doi.org/10.1079/ssr2005217
Sauer N (2007) Molecular physiology of higher plant sucrose transporters. FEBS Lett 581:2309–2317. https://doi.org/10.1016/j.febslet.2007.03.048
Sheen J (1990) Metabolic repression of transcription in higher plants. Plant Cell 2:1027. https://doi.org/10.1105/tpc.2.10.1027
Slewinski TL, Garg A, Johal GS, Braun DM (2010) Maize SUT1 functions in phloem loading. Plant Signal Behavior 5:687–690. https://doi.org/10.4161/psb.5.6.11575
Smeekens S (2000) Sugar-induced signal transduction in plants. Annu Rev Plant Mol Bio 51:49–81. https://doi.org/10.1146/annurev.arplant.51.1.49
Stitt M, Lunn J, Usadel B (2010) Arabidopsis and primary photosynthetic metabolism—more than the icing on the cake. Plant J 61:1067–1091. https://doi.org/10.1111/j.1365-313X.2010.04142.x
Thimann KV, Tetley RM, Krivak BM (1977) Metabolism of oat leaves during senescence: V. senescence in light. Plant Physiol 59:448–454. https://doi.org/10.1104/pp.65.5.855
Usha B, Bordoloi D, Parida A (2015) Diverse expression of sucrose transporter gene family in Zea mays. J Genet 94:151–154. https://doi.org/10.1007/s12041-015-0491-3
Wang L, Lu Q, Wen X, Lu C (2015) Enhanced sucrose loading improves rice yield by increasing grain size. Plant Physiol 169:2848–2862. https://doi.org/10.1104/pp.15.01170
Weichert N, Saalbach I, Weichert H, Kohl S, Erban A, Kopka J, Hause B, Varshney A, Sreenivasulu N, Strickert M, Kumlehn J, Weschke W, Weber H (2010) Increasing sucrose uptake capacity of wheat grains stimulates storage protein synthesis. Plant Physiol 152:698–710. https://doi.org/10.1104/pp.109.150854
Xu SM, Brill E, Llewellyn DJ, Furbank RT, Ruan YL (2012) Overexpression of a potato sucrose synthase gene in cotton accelerates leaf expansion, reduces seed abortion, and enhances fiber production. Mol Plant 5:430–441. https://doi.org/10.1093/mp/ssr090
Zeng LH, Meredith WR, Boykin DL, Taliercio E (2007) Evaluation of an exotic germplasm population derived from multiple crosses among Gossypium tetraploid species. J Cotton Sci 11:118–127
Zhang M, Zheng X, Song S, Zeng Q, Hou L, Li D, Zhao J, Wei Y, Li X, Luo M (2011) Spatiotemporal manipulation of auxin biosynthesis in cotton ovule epidermal cells enhances fiber yield and quality. Nat Biotechnol 29:453–458. https://doi.org/10.1038/nbt.1843
Zhang M, Zeng J, Long H, Xiao Y, Yan X, Pei Y (2017) Auxin regulates cotton fiber initiation via GhPIN-mediated auxin transport. Plant Cell Physiol 58:385–397. https://doi.org/10.1093/pcp/pcw203
Zhao DL, Mackown CT, Starks PJ, Kindiger BK (2010) Rapid analysis of nonstructural carbohydrate components in grass forage using microplate enzymatic assays. Crop Sci 50:1537–1545. https://doi.org/10.2135/cropsci2009.09.0521
Zhao J, Bai W, Zeng Q, Song S, Mi Z, Li X, Lei H, Xiao Y, Ming L, Li D (2015) Moderately enhancing cytokinin level by down-regulation of GhCKX expression in cotton concurrently increases fiber and seed yield. Mol Breed 35:60. https://doi.org/10.1007/s11032-015-0232-6
Acknowledgements
This study was funded by National Major Project of Breeding of China (2016YFD0100505 to YP and 2018YFD0100403 to XL), National Transgenic New Species Breeding Major Project of China (2016ZX08005-003-004 to YP) and Natural Science Foundation of China (31130039 to YP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Tarek Hewezi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ding, X., Zeng, J., Huang, L. et al. Senescence-induced expression of ZmSUT1 in cotton delays leaf senescence while the seed coat-specific expression increases yield. Plant Cell Rep 38, 991–1000 (2019). https://doi.org/10.1007/s00299-019-02421-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-019-02421-1