Abstract
Key message
Strigolactones inhibit bud growth by negatively regulating the auxin transport without changing the auxin biosynthesis and suppressing the expression of A-ARR in buds.
Abstract
Strigolactones (SLs) are important phytohormones associated with regulation of shoot branching in rice. Rice shoot branching is persuasively mediated by plant hormones like auxin, cytokinins (CKs) and SLs. The interactions among these hormones were diversely investigated by many researchers but remained a subject of debate. In the present study, the removal of panicle and application of subsequent synthetic SLs were used to regulate rice bud growth on node 2 (the second node from panicle) at full heading stage. The bud growth was significantly induced after panicle removal but GR24 (synthetic SLs) application inhibited it, along with variations in endogenous hormone contents in bud. RNA samples from buds were subjected to RNA sequencing through Illumina HiSeq 2000 (RNA-seq). Comparison of transcript expression levels among three treatments, viz. (1) intact (Co), (2) removed panicle (RP) and (3) RP combined with synthetic SL GR24 (GR) revealed the involvement of numerous genes associated with hormone signal transduction. GR24 supply minimized the RP-induced enhancement of auxin early response genes, independent of ARF. CK signal transduction was also induced by RP, but type-A ARR were the only genes responding to GR without any other CK signal associated genes. Additionally, RP and GR can also modulate auxin transport and CK degradation by regulating the genes’ expression involved in the biosynthesis of flavonoid, phenylpropanoid and benzoxazinoid. Contemplating the results obtained so far, it is possible to open new vistas of research to reveal the interactions among SLs, auxin and CK in controlling the shoot branching of rice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa L.) is among the key food crops and used as staple food in many areas of the world. Tillering of rice plant is among the crucial agronomic traits contributing to rice yield. It is also a model system to investigate molecular mechanism of branching in monocotyledonous plants. Rice tillers develop from the tiller bud, located at the leaf axil, which is dynamic and adjustable under the impact of various environmental factors. Bud outgrowth is a two-stage process including bud initiation and its subsequent outgrowth (Oikawa and Kyozuka 2009). The primary shoot or inflorescence is considered to be the major factor of inhibiting axillary growth. Normally, rice tiller buds emerge on the elongated upper internodes and become dormant during stem differentiation into its panicles (Hanada 1993). In a previous study, removal of panicle was found to be associated with growth stimulation of dormant buds located at the second node from panicle (Arite et al. 2007; Liu et al. 2011). Exogenous cytokinin (CK) supply was reported to be releasing the axillary bud from dormancy (Xu et al. 2014).
Plant hormones play remarkable roles during plant development including lateral bud outgrowth. The interaction between auxin, CK and strigolactones (SLs) on bud growth regulation have been discussed for years (Beveridge and Kyozuka 2010; Domagalska and Leyser 2011; Dun et al. 2012; Muller and Leyser 2011; Wang et al. 2018; Xu et al. 2015). Axillary meristem activity is regulated by a network of systemic signals, where auxin plays a pivotal role. Two hypotheses, the second messenger model and the auxin transport canalization-based model, regarding an indirect action of auxin in regulating shoot branching have been proposed (Domagalska and Leyser 2011). In intact plants, auxin synthesized in the shoot apex moves basipetally and inhibits outgrowth of axillary buds indirectly (Booker 2003; Petrasek and Friml 2009). Removal of apical meristem, for inhibition of polar auxin transport stream, leads to the axillary bud outgrowth, while, application of auxin to the stump prevents it (Thimann and Skoog 1933). The auxin signals are relayed by several downstream messengers like CK and SLs, which regulate outgrowth upon entering the axillary buds (Brewer et al. 2015; Domagalska and Leyser 2011; Muller and Leyser 2011; Tanaka et al. 2006). Concurrently, auxin transport out of the bud may be allowed due to decapitation-induced low auxin levels in the main stem, by enhancing stem sink strength for auxin, and thereby allowing the bud outgrowth (Balla et al. 2011; Domagalska and Leyser 2011).
On the contrary, CK biosynthesis is down-regulated by auxin in the main stem, and the direct application of CK to buds induced outgrowth even in an intact plant (Nordstrom et al. 2004; Tanaka et al. 2006). In the apical dominance context, two genes encoding the ISOPENTENYL TRANSFERASE (IPT) enzyme, PsIPT1 and PsIPT2, were rapidly up-regulated in the nodal stem after decapitation (Tanaka et al. 2006). It suggests that auxin inhibits bud outgrowth partly by modulating local CK levels. Function of CK in direct bud promotion can be correlated with the genes of cell-cycle regulation (Dewitte et al. 2007; Dun et al. 2012; Riou-Khamlichi et al. 1999). However, a study on arr and ipt multiple mutants suggested that impaired CK response did not affect auxin-mediated inhibition, and elevated IPT expression was not needed for bud release, following decapitation (Muller et al. 2015).
SLs, as phytohormones, play both systemic and local role in directly suppressing axillary bud outgrowth (Gomez-Roldan et al. 2008). Both, the SLs biosynthesis mutant d10 and the SL-signaling mutant d3, have shown an increase in rice tillers count (Ishikawa et al. 2005). SLs were also demonstrated to be involved in phosphorus and nitrogen deficiency-induced tiller inhibition in rice (Luo et al. 2018; Sun et al. 2014; Umehara et al. 2010). Direct application of exogenous SLs to bud caused bud growth arrest in wild-type plants and SL-deficiency mutants (Brewer et al. 2009, 2015; Dun et al. 2012, 2013). SLs signaling is mediated by the proteasome system-induced D53 protein degradation, which in turn releases the repression of IPA1-regulated gene expression (Jiang et al. 2013; Zhou et al. 2013; Song et al. 2017). SLs biosynthesis is mediated by auxin, which is evident from the positive regulation of MAX3/D17 and MAX4/D10 (SLs biosynthesis genes) by auxin, in both rice and pea (Hayward et al. 2009; Johnson et al. 2006; Waters et al. 2012). It indicates that the SLs act as a second message signal for downstream of auxin to inhibit bud outgrowth (Domagalska and Leyser 2011; Muller and Leyser 2011). SLs can rapidly deplete the auxin transporter PIN1 on the plasma membrane, contributing to the canalization of auxin transport from bud into the main stem (Bennett et al. 2006; Crawford et al. 2010; Shinohara et al. 2013). In addition, the bud activity is also considered to be determined by the ratio of CK and SLs in buds (Braun et al. 2012; Dun et al. 2012).
Besides, various reports have identified some other signals, functioning in the regulation of bud growth. The bud inhibition function of ABA had been evidenced in early works (Mader et al. 2003; Reddy et al. 2013; Yao and Finlayson 2015). Most recently study has shown that ABA appeared to act as one of the first upstream signals that control bud suppression (Holalu and Finlayson 2017). Plant hormone, brassinosteroids (BRs), which have various physiological effects like cell division and elongation were proved to be involved in the rice tillering control (Tong et al. 2009, 2012). The sugar as an early signal of triggering bud initiation has been suggested in another study (Mason et al. 2014).
To investigate the underlying molecular processes affiliated with regulation of SL-induced bud dormancy in rice, we established three different types of buds to simulate two different processes, decapitation-induced bud activation and SL-induced bud dormancy. RNA-seq approach was used to categorize and characterize the expression profile of differentially expressed genes (DEGs) within three different treatments, to find out the key genes and pathways related to SL-induced bud dormancy based on decapitation.
Materials and methods
Plant growth
The experiment was conducted in a net house at Nanjing Agriculture University (Jiangsu Province, China) during the rice-growing season, during 2015. An Indica cultivar, Yangdao 6, was used as a test crop in this study. The 20-day-old rice seedlings were transferred to plastic pots of 30 cm diameter and height. Each pot contained 15 kg of sieved soil, where four seedlings were transplanted. Nitrogen (1.6 g plot−1 as urea), phosphorus (0.8 g plot−1 as single superphosphate), and potassium (1.2 g plot−1 as KCl) were applied 3 days before seedling transplantation. Surface water was applied to irrigate these pots over the entire growing season of the plants.
Treatments
When the main stems began to differentiate their own panicles, the buds on the elongated upper internodes will convert to dormant, so we chose full heading stage to make sure that all the buds we observed are comparable. All rice plants were grown under normal rice growth condition until full heading stage. After this stage, these plants were divided into 3 treatment groups, viz. normal growth conditions were continued for one group of plants as a control (Co), the second group with removed panicle (RP). For the last group, panicle removal was followed by the application of 2 µM synthetic SLs (GR24) directly on bud located at the second node from panicle (node 2). As the buds on node 2 are always covered by leaf sheaths, so peeled out the sheaths from stem to pour the solution into the gap between stem and sheaths. To achieve uniformity among treatments in all other aspects, we supplied the same solution without GR24 to Co and RP treatments too.
RNA extraction, cDNA library construction and Illumina sequencing
Total RNA was extracted from the tiller buds on node-2 collected after 2 h of treatment, using E.Z.N.A.® Plant RNA Kit (Omega Bio-tek, USA), following the manufacturer’s instructions. To remove genomic DNA, samples were treated with DNase I and Oligo (dT) to isolate poly (A) RNA from total RNA. The mRNA was fragmented into small pieces using the fragmentation buffer, which were then used as templates and reverse transcribed into first-strand cDNA, while second-strand cDNA was synthesized using DNA polymerase I. Purified and resolved short fragments were treated with buffer EB for end reparation, after which a single nucleotide, adenine, was added and connected with adapters. Suitable fragments were selected for polymerase chain reaction (PCR) amplification. During the quality control steps, an Agilent 2000 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) and an ABI StepOnePlus Real-Time PCR System (Life Technologies Ltd., Carlsbad, CA, USA) were used to quantify and qualify the sample library, followed by sequencing of the library on an Illumina HiSeq 4000 (Illumina, CA, USA).
The resulted sequence data were submitted to National Center for Biotechnology Information, U.S. National Library of Medicine (NCBI; https://www.ncbi.nlm.nih.gov/) under the accession number SRP142674. The raw reads with adaptors, containing high number of unknown bases (N) and low-quality reads (the percentage of low-quality bases is over 50% in a read) were removed before downstream analysis. Bowtie (http://computing.bio.cam.ac.uk/local/doc/bowtie2.html#) and BWA (http://bio-bwa.sourceforge.net/bwa.shtml) were used to perform reference gene and genome mapping. DEGs between two comparison groups were identified using DEseq2 (http://bioconductor.org/packages/release/bioc/html/DESeq2.html), and subjected to further functional enrichment analyses using hierarchical clustering, classification, and pathway analyses. We used a false discovery rate (FDR) ≤ 1% and an absolute value of log2Ratio ≥ 1 as the threshold to judge the significance of gene expression difference in addition to probability ≥ 0.8 among three biological replicates.
Measurement of endogenous plant hormones
The extraction and purification of indole-3-acetic acid (IAA) and four types of CKs [trans-zeatin (tZ), trans-zeatin riboside (tZR), isopentenyladenine (iP) and iP riboside (iPR)] were carried out as described in the previous study (Dobrev and Kamınek 2002). The determination of IAA, tZ, tZR, iP and iPR was performed using a LC–MS/MS (Aglient 1290 and SCIEX-6500 trap) system as described in another previous study (Nakagawa et al. 2005).
Statistical analysis
Results were analyzed using SPSS 17.0. The data from each sampling event was analyzed separately. Means were tested with the least significant difference test and significance level was set at p ≤ 0.05.
Results
Bud length after treatment
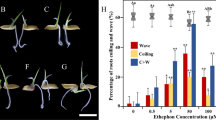
To demonstrate the supposed effectiveness of these treatments in controlling bud outgrowth, the bud length was measured within 48 h after execution of treatments. As shown in Fig. 1, the bud length on node 2 of control (Co) remained stable within 48 h, indicating the dormant condition of buds on node 2 in intact plants. The bud growth on node 2 was promoted in the removed panicle (RP) treatment, and a significant difference was noticed between Co and RP on bud length after 36 h of treatment. Non-significant increase in bud length had been found compared with Co, because of GR24 supply after decapitation (GR). But a considerable inhibition of bud growth was still observed as compared to RP. These results indicated that GR24 can inhibit the bud release function of RP significantly, but cannot rescue the buds intact with plants.
Effects of RP and GR treatments on the length of buds located at the second nodes from the panicle. Rice plants grown in normal condition till full heading stage and then were subjected to three treatments. Co: intact plants; RP: removed panicle; GR: removed panicle combined with GR24 apply. Vertical bars represent mean ± standard error (n = 40)
Analysis of IAA and CK contents in buds
Our previous studies have shown the interaction between auxin, CK and SLs on rice tiller regulation (Xu et al. 2014, 2015). Endogenous IAA and CK contents in buds located at node 2 were measured to investigate whether auxin and CK levels would change in bud, responding to different treatments (Table 1). In Co plants, the buds on node 2 stayed dormant so that the local IAA and four CK contents maintained stability. Compare to Co, the content of IAA in buds started to elevate 2 h after both RP and GR treatments. All forms of CK, in response to RP, showed higher contents than those in Co after 2 h of treatment. However, the tZ and iP levels in response to GR were noticed comparatively lower than those in RP, but still significantly higher than those in Co after 2 h of treatment. After 6 h of treatment, all forms of CK in response to GR showed lower contents than those of RP. These results suggest that IAA and CK contents in buds were promoted by panicle removal, but subsequent GR24 application could only reduce the CK amount in buds.
Analysis of RNA-seq libraries
In this study, we sequenced nine cDNA libraries, Co-1, Co-2, Co-3, RP-1, RP-2, RP-3, GR-1, GR-2, GR-3, taken from rice buds on node 2 from all three treatments, which resulted in 117.36 million raw reads. After elimination of low-quality reads and adaptor sequences, we obtained 115.11 million clean reads. All three biological replicates were in great agreement with respect to gene transcription levels (Table 2). Then, we pooled these clean reads and aligned them against the Nipponbare reference genome (IRGSP build 5.0). Out of total clean reads from nine samples, 61.43–62.46% were perfect matches, 21.16–22.67% had no more than two base mismatches, while 15.83–17.20% reads were unmapped. The square of the Pearson correlation coefficient (R2) varied 0.999–1 in Co, 0.997–0.999 in RP, and 0.995–0.998 in GR treatment, indicating good operational stability and reliability of DEG library sequencing (Supplementary Fig. 1).
DEG analysis and qRT-PCR verification
To understand and interpret the results of the RNA-seq experiment, the differential expression patterns of transcripts were analyzed. From three different treatment groups, differential expression analyses were performed between two compare groups: Co vs RP, and RP vs GR. The 6063 and 1529 transcripts were identified, respectively, with significant expression changes (Fig. 2a, p ≤ 0.05 and |log2| ≥ 1). Unsurprisingly, the different expression patterns of two compare groups revealed that the Co vs RP comparison has a bigger difference (4168 up-regulated transcripts and 1895 down-regulated transcripts). In contrast, for RP vs GR comparison, 1274 transcripts were up-regulated and 255 were down-regulated. Among these differentially expressed transcripts, 615 were expressed significantly in common (Fig. 2b), 116 and 51 transcripts were up- and down-regulated in both two comparisons, respectively. Among them, a total of 448 transcripts showed opposite expression profiles.
Overview of DEGs in Co vs RP and RP vs GR comparison. a Numbers of DEG in each comparison group. The number of DEGs was obtained from comparisons Co vs RP and RP vs GR. b Venn diagram representing the overlapping and non-overlapping DEGs among two comparison groups. Differentially regulated genes were identified by models at FDR ≤ 0.01, |log2| ≥ 1
A cluster analysis of transcript expression profile with functional enrichment was performed using familiar log ratio values for the transcript expression analysis. The cluster analysis was grouped into up-regulated and down-regulated transcripts separately. There is a total of 6978 DEGs in the two comparison groups, and the expression profile in the heatmap (Fig. 3, Online Resource 1) showed that more than half of the DEGs have an opposite expression. To verify the authenticity and reproducibility of RNA-seq results, 20 DEGs were randomly selected for qRT-PCR analysis, and the expression profiles of these selected genes were similar to the result of transcriptome analysis (Fig. 4).
Heatmap of the expression of DEGs in the two comparison groups. Each column represents a comparison group, each row represents a unigene. Differences in expression are shown in different colors. Data for unigene expression level are normalized to z-score. Red represents up-regulated expression and blue represents down-regulated expression
Quantitative expressional changes of 20 DEGs via RNA-seq and qRT-PCR. Both the FPKM value and qRT-PCR data have three biological replicates and bars represent SE. Total RNA was isolated from less than 0.1 g buds and nodes each time. β-Actin was used as a reference gene. The value obtained from the Co was arbitrarily set at 1.0. Expression of each genes response to RP and GR are represented relative to the Co
GO and KEGG classification of differential expression of genes (DEGs)
GO slim was used for the functional classification of DEGs and, resultantly, classified into three main categories viz. biological processes (BP), cellular components (CC) and molecular functions (MF). The major subcategories along with the analysis of all the transcripts among two compare groups are shown in Supplementary Fig. 2. In the biological processes’ category; cellular processes, metabolic processes and single-organism processes were the most highly represented groups, indicating that extensive metabolic activities were taking place during these processes. In the cellular components’ category, cell, cell part and organelle were prominently represented, while binding and catalytic activities predominated in molecular functions.
To further understand the biological functions of DEGs in each compare group, the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database was used (Fig. 5). In the Co vs RP, a total of 3607 DEGs were assigned to 126 KEGG pathways. The most represented pathways included “Ribosome” (236, 6.54%), “Biosynthesis of secondary metabolites” (400, 11.09%), “Metabolic pathways” (753, 20.88%) and “Plant hormone signal transduction” (220, 6.1%). Unlike the Co vs RP, only 837 DEGs were assigned to 107 KEGG pathways in the RP vs GR. The most represented pathways included “Metabolic pathways” (260, 31.06%), “Biosynthesis of secondary metabolism” (186, 22.22%), “Plant hormone signal transduction” (90, 10.75%) and “Starch and sucrose metabolism” (40, 4.78%). Beyond “Metabolic pathways” and “Biosynthesis of secondary metabolism”, “Plant hormone signal transduction” was the most enriched pathways in both Co vs RP and RP vs GR comparison. That suggests that the plant hormone signal transduction may play an important role in both decapitation-induced bud release and SLs-induced bud inhibition.
KEGG pathway analysis of two comparison groups. “Rich factor” means that the ratio of the DEGs number and the number of genes have been annotated in this pathway. The greater of the Rich factor, the greater the degree of enrichment. a Enriched pathways of DEGs in buds located at the second node from panicle underlying Co vs RP; b enriched pathways of DEGs in buds located at the second node from panicle underlying RP vs GR
Differential expression of genes (DEGs) related to auxin and cytokinin signal transduction
Plant hormones play primary role in rice tiller bud regulation. The hormone signaling in bud may be caused by some of the differentially expressed local genes (Beveridge et al. 2009; Muller and Leyser 2011). In the present study, 263 DEGs, linked with plant hormone signal transduction, were identified in two compare groups, out of which the auxin and CK signaling pathways were further analyzed.
Among DEGs, 38 and 14 transcripts involved in auxin signal transduction were detected in the Co vs RP and RP vs GR comparison groups, respectively. We only found 8 transcripts encoding auxin response factor (OsARF) in the Co vs RP comparison, of which 6 were up-regulated (2.35- to 4.20-fold change, Fig. 6). Multiple early auxin signal transduction-related genes such as small auxin up RNA genes (SAURs) and auxin/indoleacetic acid (AUX/IAA) were also found to be involved in our results (Fig. 6). The Aux/IAA proteins are auxin inducible and function as the key regulators in auxin responses through interaction with ARF (Santner et al. 2009). Among 10 DEGs encoding OsIAAs, 9 were up-regulated (2.35- to 13.04-fold change) in the Co vs RP comparison group. While, only OsIAA1 and OsIAA13 were found in the RP vs GR comparison and both were down-regulated (OsIAA1: 0.49-fold change; OsIAA13: 0.36-fold change). Besides, a large quantity of transcripts, annotated as OsSAURs, were detected but did not show obvious expression regularity (Fig. 6). In Co vs RP comparison, 8 out of 14 DEGs encoding OsSAURs were up-regulated (2.47- to 36.32-fold change) and the other 6 were down-regulated (0.13- to 0.30-fold change). Similarly, half of 8 DEGs encoding OsSAURs was up-regulated (2.38- to 24.72-fold change) in RP vs GR comparison, the other half was down-regulated (0.14- to 0.49-fold change). However, 4 DEGs encoding OsSAURs (OsSAUR8, OsSAUR11, OsSAUR33 and OsSAUR55) were found in both compare groups, showing the opposite expression profiles.
DEGs associated with auxin signal transduction in rice bud growth regulation. Each column represents a unigene, each row represents a comparison group. Differences in expression are shown in different colors. Red represents up-regulated expression and green represents down-regulated expression. TIR1: transport inhibitor response 1; AUX/IAA: auxin/indoleacetic acid; ARF: auxin response factor; GH3: Gretchen Hagen 3; SAUR: small auxin up RNA gene. (Color figure online)
In the CK-responsive pathway, 24 and 5 transcripts were detected in the Co vs RP and RP vs GR comparison, respectively. Among these, 20 transcripts were up-regulated in the Co vs RP comparison. Rice histidine kinase (OHK), histidine-containing phosphotransfer protein (AHP), two-component response regulator ARR-B family (B-ARR) and two-component response regulator ARR-A family (A-ARR) were all found to be involved in this process (Fig. 7). Out of them, nine were found in Co vs RP comparison: two OHK (OHK2: down-regulated, 0.40-fold change; OHK4: up-regulated, 3.43-fold change), one AHP (OsAHP1, up-regulated, 2.36-fold change), ORR6 (down-regulated, 0.30-fold change) and five A-ARR genes (up-regulated, 2.5- to 9.6-fold change). Interestingly, we found only four downregulating DEGs encoding A-ARR in RP vs GR compare group (0.30- to 0.49-fold change). OsRR2, OsRR9 and OsRR10 were found in two comparisons and showed opposite expression profiles (Fig. 7).
DEGs associated with cytokinin signal transduction in rice bud growth regulation. Each column represents a unigene, each row represents a comparison group. Differences in expression are shown in different colors. Red represents up-regulated expression and green represents down-regulated expression. OHK: rice histidine kinase, AHP: histidine-containing phosphotransfer protein; B-ARR: two-component response regulator ARR-B family; A-ARR: two-component response regulator ARR-A family. (Color figure online)
Differential expression of genes (DEGs) related to secondary metabolism pathways
Plants use secondary metabolites to develop physical and chemical barriers against infection, as well as local and systemic signaling molecules for defense gene induction. Benzoxazinoids (BX) are secondary metabolites involved in bringing about inhibitory functions in various processes related to plant growth. In our study, several genes encoding the enzymes to modulate the last three steps of the BX biosynthesis were detected. As shown in Fig. 8, a total of 4 DEGs were mediated the DIBOA–DIBOA-glu–TRIBOA-glu pathway in Co vs RP comparison. Among them, three were down-regulated (0.10- to 0.20-fold change) and one up-regulated (5.26-fold change). However, seven genes were up-regulated (2.47- to 399-fold change) in the RP vs GR comparison group that mediate the last three steps of benzoxazinoid biosynthesis.
DEGs associated with DIMBOA-glu biosynthesis in rice bud growth regulation. Each column represents a comparison group, each row represents a unigene. Differences in expression are shown in different colors. Red represents up-regulated expression and green represents down-regulated expression. DIBOA: 2,4-dihydroxy-1,4-benzoxazin-3-one; TRIBOA: 2,4,7-trihydroxy-1,4-benzoxazin-3-one; DIMBOA: 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one. (Color figure online)
DEGs involved in ‘flavonoids biosynthesis’ and ‘phenylpropanoid biosynthesis’ pathways were also largely founded in both of the two comparisons (Table 3 and Supplementary Figure S3). In Co vs RP comparison, 20 DEGs were annotated to flavonoids biosynthesis and 14 of them appeared to be down-regulating. While in RP vs GR comparison, 20 out of 22 DEGs annotated to flavonoids biosynthesis were up-regulated (Table 3). Flavonoids are synthesized from phenylpropanoid derivatives by condensation with malonyl-CoA (Ono et al. 2006). As shown in Supplementary Figure S3, 48 in 49 detected phenylpropanoid biosynthesis-related DEGs were up-regulated in RP vs GR comparison. These results suggest that phenylpropanoid and flavonoid biosynthesis enhancement response to GR may contribute to the bud growth inhibition of SLs.
Differential expression of genes (DEGs) related to cell cycle
Sixteen DEGs annotated to cell cycle control genes were found in the Co vs RP comparison group, and 15 of them were both up-regulated (2.19- to 10.91-fold change). Of them, seven were D-type cyclins (CycD) genes that mediate cell division at the G1-S-phase (Table 4). As CycD have been reported to be induced by brassinosteroid (BR) signal (Dewitte et al. 2007; Hu et al. 2000), a BR signal factor BRASSINAZOLE-RESISTANT1 (OsBZR1) was also shown up-regulated (2.09-fold change) in Co vs RP comparison (Table 4), while not even one cell cycle-related DEG was found in the RP vs GR comparison.
Discussion
Previous studies have shown that plant hormones were determinedly associated with bud dormancy and active transduction. Auxin and CKs play major roles in regulating the axillary bud outgrowth in many plant species. Auxin is involved in axillary bud inhibition, and exogenous CK application induces the expression of most of the type-A ARR genes (Brandstatter and Kieber 1998; D’Agostino et al. 2000; Imamura et al. 1999; Kiba et al. 1999; Taniguchi et al. 1998), subsequently controlling the cell division and shoot initiation.
In the RP-induced bud activation process, almost all the DEGs were up-regulated which covered the whole auxin signal transduction pathway, including ARF, AUX/IAA and SAUR. It indicated the swift auxin signal transduction in bud, responding to decapitation and showing its involvement in the RP-induced bud activation. The genes regulating cell enlargement and plant growth (AUX/IAA and SAUR) in auxin signal transduction process were mostly up-regulated. Activity of auxin synthesis gene OsYUC9, which was also found up-regulated in bud, might have yielded the higher auxin level in bud, 2 h after decapitation (Fig. 6). These results suggested an increased auxin level in bud and onset of auxin source to be exporting out auxin to stem after RP, which is consisted with the auxin transport canalization-based model in bud growth control. This proposal is also evidenced by the increased IAA levels in bud after RP treatment (Table 1). Unsurprisingly, all the DEGs in auxin signal transduction involved in AUX/IAA and SAUR were mostly down-regulated during the GR-induced bud dormancy process. However, not even one DEG was detected to be annotated the ARF, and the expression level of OsYUA9 did not appear reversed. Also, there was no significant difference in the IAA levels in buds between RP and GR treatment (Table 1). We hypothesize that SLs would not inhibit auxin biosynthesis in bud so that the transcription level of ARF did not change with exogenous SLs treatment; nevertheless, the ARF downstream genes (AUX/IAA, SAUR) may be mediated by SLs directly or through other ARF-independent pathways.
In addition to the auxin signal transduction in bud, the export of auxin from axillary bud is also required for bud release and outgrowth (Mason et al. 2014). As described above, DEGs involved in ‘Flavonoids biosynthesis’ and ‘Phenylpropanoid biosynthesis’ were especially notable and mostly down/up-regulated during RP-induced bud release and GR-induced bud dormancy, respectively. Flavonoids are known to inhibit auxin transport (Brown et al. 2001; Murphy et al. 2000; Peer et al. 2004; Peer and Murphy 2007), and the auxin canalization is mediated by cellular flavonoid levels. Some previous studies have also reported similar findings during dormancy bud release (Chao et al. 2016; Horvath et al. 2005; Min et al. 2017), indicating that decapitation decreased the cellular flavonoids levels, eventually, leading to promote bud growth by allowing auxin transport out of buds; whereas, SLs increased cellular flavonoid levels to inhibit bud growth. This hypothesis is consistent with previous studies that SLs inhibit the axillary bud growth by inhibiting auxin transport (Brewer et al. 2015; Crawford et al. 2010; Prusinkiewicz et al. 2009; Soundappan et al. 2015). In the category of phenylpropanoid biosynthesis, most of the genes involved in lignin biosynthesis were down-regulated in RP-induced active bud, and up-regulated in GR-induced dormant bud. It indicates that lignin biosynthesis is repressed during the bud dormancy and induced by SLs supply. The auxin transport was decreased in HCT-silenced (transport in hydroxycinnamoyl-CoA shikimate/quinate hydroxycinnamoyl transferase, a lignin biosynthetic gene) plants with flavonoid accumulation (Besseau et al. 2007). Above all, we hypothesized that flavonoid contents accomplish an important role in controlling bud initiation and dormancy transformation by regulating the auxin transport in response to decapitation and synthetic SLs apply.
CKs have been known to promote bud release from dormancy in intact plants (Dun et al. 2012; Xu et al. 2015). DEGs annotated to CK signal transductions, AHP, B-ARR and A-ARR, were mostly up-regulated that contributed to the RP-induced bud release. These suggest that CK signal transduction is involved in the RP-induced bud initiation. Interestingly, only 4 A-ARR genes were suppressed in response to synthetic SLs application. The A-ARR have been reported as CK-mediated genes that are required for bud release but not for bud transcriptional response to CK in Arabidopsis (Bangerth 1994; Muller et al. 2015; Pillay and Railton 1983). Meanwhile, SLs did not rescue the decapitation-induced bud outgrowth in arr3,4,5,6,7,15 mutants (Muller et al. 2015). It implies that the A-ARR may be the key factor in CK signal transduction contributing to SLs-induced bud inhibition. SLs can suppress the A-ARR expression independent of CK signal transduction.
As described above, differential expression patterns of BX biosynthesis-related genes were detected in bud release and rescue processes. So, the DIMBOA (2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one)-glucoside contents in buds may be brought down in RP-induced bud release process and accumulated in SLs-induced dormancy process. The DIMBOA-glucoside is a predominant form of DIMBOA in phloem sap, which is used by ZmCKX1 as an electron acceptor, subsequently, enhancing the cytokinin oxidase/dehydrogenase (CKX) activity (Frébortová et al. 2004). CKX enzymes are responsible for catalysis of cytokinin degradation reaction in plants. The decreased/enhanced DIMBOA-glucoside biosynthesis is likely to be modifying the CKX activity, ultimately, leading to altered CK contents in buds. Hence, it is an ordinary way for decapitation and SLs supply to mediate CKX activity by controlling BX biosynthesis, thus regulating the CK levels in buds. This hypothesis is generally consistent with the CK content change in buds.
Several studies indicated that bud dormancy initiation results in the up-regulation of genes responsible for the G1- to S-phase transition (Devitt and Stafstrom 1995; Horvath et al. 2002). D-type cyclins (CYCD) function as the mediators of internal and environmental stimuli to drive cell division at the G1–S-phase, which were also regulated by brassinosteroid (BR) (Dewitte et al. 2007; Hu et al. 2000). In the present study, several CycD were up-regulated in RP-induced active buds, and no significant differential expression was observed in SL-induced dormant bud. Consistently, a BR signal factor BRASSINAZOLE-RESISTANT1 (OsBZR1) was found to be up-regulated only in RP-induced active buds (Bai et al. 2007; Qiao et al. 2017; Zhu et al. 2015). These results imply that only BR-induced cell division (CYCD) is involved in the RP-induced bud activation, while the SLs bud inhibition function is independent of it.
Taken together, the bud on node 2 was initiated and onset to grow out after removing the panicle from intact rice plant at full heading stage. The auxin and CK signal transduction were both enhanced in buds, which represents a higher auxin and CK level. At the same time, phenylpropanoid and flavonoid biosynthesis were weakened, which is necessary for a stronger auxin transport (Brown et al. 2001; Murphy et al. 2000; Peer et al. 2004; Peer and Murphy 2007). These results were consistent with the auxin canalization model on bud growth regulation (Domagalska and Leyser 2011). More auxin biosynthesis and enhanced auxin transport in buds imply a new auxin source formed and onset to efflux auxin to stem for further outgrowth. The increased expression of OsYUC9 confirmed the local auxin biosynthesis in buds which is necessary as an auxin source. Moreover, we did not find any DEGs that encode auxin PIN auxin efflux facilitator proteins, suggesting that the auxin biosynthesis is ahead of auxin canalization formation. The decreased BX biosynthesis may result in the reduction of CKX activity that allowed CK accumulation in bud for bud growth (Frébortová et al. 2004). Panicle removal accompanied with synthetic SLs application reversed the auxin signal transduction, except ARF in buds (AUX/IAA, ARF, SAUR). It is strange that there is no significant change in auxin biosynthesis, also, phenylpropanoid and flavonoid biosynthesis were enhanced that may inhibit auxin transport. We hypothesize that SLs can mediate auxin response genes (AUX/IAA, SAUR) independent of ARF and auxin contents, meanwhile, can negatively regulate the auxin transport without changing the auxin biosynthesis in buds. Without the change of other CK response genes, A-ARR were decreased in bud response to additional SLs supply. It indicates that the A-ARR may act as a key CK-independent signal, downstreaming the SLs in regulating bud growth. CKX activity could also be enhanced by SLs, following the DIMBOA-glu biosynthesis strengthening. Beside those, BR-induced cell division (CYCD) is induced by the removal of panicle but no response to SLs. In a word, SL can inhibit bud growth by reducing the auxin early response genes independent of ARF, and suppressing type-A ARR expression in buds.
Author contribution statement
MZ and SW were responsible for the overall design and conduct of experiments. MZ and YW prepared plant tissues and performed RNA extraction and sequencing. MZ and JX conducted the sequencing data analysis. MZ, MI and YD took the lead on manuscript development. All authors read and approved the final manuscript.
References
Arite T et al (2007) DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J 51:1019–1029. https://doi.org/10.1111/j.1365-313X.2007.03210.x
Bai MY, Zhang LY, Gampala SS, Zhu SW, Song WY, Chong K, Wang ZY (2007) Functions of OsBZR1 and 14-3-3 proteins in brassinosteroid signaling in rice. Proc Natl Acad Sci USA 104:13839–13844. https://doi.org/10.1073/pnas.0706386104
Balla J, Kalousek P, Reinohl V, Friml J, Prochazka S (2011) Competitive canalization of PIN-dependent auxin flow from axillary buds controls pea bud outgrowth. Plant J 65:571–577. https://doi.org/10.1111/j.1365-313X.2010.04443.x
Bangerth F (1994) Response of cytokinin concentration in the xylem exudate of bean (Phaseolus vulgaris L.) plants to decapitation and auxin treatment, and relationship to apical dominance. Planta 194:439–442. https://doi.org/10.1007/BF00197546
Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O (2006) The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Biol 16:553–563. https://doi.org/10.1016/j.cub.2006.01.058
Besseau S, Hoffmann L, Geoffroy P, Lapierre C, Pollet B, Legrand M (2007) Flavonoid accumulation in Arabidopsis repressed in lignin synthesis affects auxin transport and plant growth. Plant Cell 19:148–162. https://doi.org/10.1105/tpc.106.044495
Beveridge CA, Kyozuka J (2010) New genes in the strigolactone-related shoot branching pathway. Curr Opin Plant Biol 13:34–39. https://doi.org/10.1016/j.pbi.2009.10.003
Beveridge CA, Dun EA, Rameau C (2009) Pea Has its tendrils in branching discoveries spanning a century from auxin to strigolactones. Plant Physiol 151:985–990. https://doi.org/10.1104/pp.109.143909
Booker J (2003) Auxin acts in xylem-associated or medullary cells to mediate apical dominance. Plant Cell 15:495–507. https://doi.org/10.1105/tpc.007542
Brandstatter I, Kieber JJ (1998) Two genes with similarity to bacterial response regulators are rapidly and specifically induced by cytokinin in arabidopsis. Plant Cell 10:1009–1019. https://doi.org/10.1105/tpc.10.6.1009
Braun N et al (2012) The pea TCP transcription factor PsBRC1 acts downstream of Strigolactones to control shoot branching. Plant Physiol 158:225–238. https://doi.org/10.1104/pp.111.182725
Brewer PB, Dun EA, Ferguson BJ, Rameau C, Beveridge CA (2009) Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol 150:482–493. https://doi.org/10.1104/pp.108.134783
Brewer PB, Dun EA, Gui R, Mason MG, Beveridge CA (2015) Strigolactone inhibition of branching independent of polar auxin transport. Plant Physiol 168:1820–1829. https://doi.org/10.1104/pp.15.00014
Brown DE et al (2001) Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol 126:524–535. https://doi.org/10.1104/pp.126.2.524
Chao WS, Dogramaci M, Horvath DP, Anderson JV, Foley ME (2016) Phytohormone balance and stress-related cellular responses are involved in the transition from bud to shoot growth in leafy spurge. BMC Plant Biol 16:47. https://doi.org/10.1186/s12870-016-0735-2
Crawford S et al (2010) Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 137:2905–2913. https://doi.org/10.1242/dev.051987
D’Agostino IB, Deruère J, Kieber JJ (2000) Characterization of the response of the Arabidopsis response regulator gene family to cytokinin. Plant Physiol 124:1706–1717. https://doi.org/10.1104/pp.124.4.1706
Devitt ML, Stafstrom JP (1995) Cell cycle regulation during growth-dormancy cycles in pea axillary buds. Plant Mol Biol 29:255–265. https://doi.org/10.1007/BF00043650
Dewitte W et al (2007) Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses. Proc Natl Acad Sci USA 104:14537–14542. https://doi.org/10.1073/pnas.0704166104
Dobrev PI, Kamınek M (2002) Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed mode solid-phase extraction. J Chromatogr A 205:21–29. https://doi.org/10.1016/S0021-9673(02)00024-9
Domagalska MA, Leyser O (2011) Signal integration in the control of shoot branching. Nat Rev Mol Cell Biol 12:211–221. https://doi.org/10.1038/nrm3088
Dun EA, de Saint Germain A, Rameau C, Beveridge CA (2012) Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol 158:487–498. https://doi.org/10.1104/pp.111.186783
Dun EA, de Saint Germain A, Rameau C, Beveridge CA (2013) Dynamics of strigolactone function and shoot branching responses in Pisum sativum. Mol Plant 6:128–140. https://doi.org/10.1093/mp/sss131
Frébortová J et al (2004) Catalytic reaction of cytokinin dehydrogenase preference for quinones as electron acceptors. Biochem J 380:121–130. https://doi.org/10.1042/bj20031813
Gomez-Roldan V et al (2008) Strigolactone inhibition of shoot branching. Nature 455:189–194. https://doi.org/10.1038/nature07271
Hanada K (1993) Tiller. In: Matsuo THK (ed) Science of the rice plant, vol 1. Food and Agriculture Policy Research Center, Tokyo, pp 222–229
Hayward A, Stirnberg P, Beveridge C, Leyser O (2009) Interactions between auxin and strigolactone in shoot branching control. Plant Physiol 151:400–412. https://doi.org/10.1104/pp.109.137646
Holalu SV, Finlayson SA (2017) The ratio of red light to far red light alters Arabidopsis axillary bud growth and abscisic acid signalling before stem auxin changes. J Exp Bot 68:943–952. https://doi.org/10.1093/jxb/erw479
Horvath DP, Chao WS, Anderson JV (2002) Molecular analysis of signals controlling dormancy and growth in underground adventitious buds of leafy spurge. Plant Physiol 128:1439–1446. https://doi.org/10.1104/pp.010885
Horvath DP, Soto-Suárez M, Chao WS, Jia Y, Anderson JV (2005) Transcriptome analysis of paradormancy release in root buds of leafy spurge (Euphorbia esula). Weed Sci 53:795–810. https://doi.org/10.1614/WS-05-066R1.1
Hu Y, Bao F, Li JY (2000) Promotive effect of brassinosteroids on cell division involves a distinct CycD3-induction pathway in Arabidopsis. Plant J 24:693–701. https://doi.org/10.1046/j.1365-313x.2000.00915.x
Imamura A et al (1999) Compilation and characterization of Arabidopsis thaliana response regulators implicated in His-Asp phosphorelay signal transduction. Plant Cell Physiol 40:733–742. https://doi.org/10.1093/oxfordjournals.pcp.a029600
Ishikawa S, Maekawa M, Arite T, Onishi K, Takamure I, Kyozuka J (2005) Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol 46:79–86. https://doi.org/10.1093/pcp/pci022
Jiang L et al (2013) DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature 504:401–405. https://doi.org/10.1038/nature12870
Johnson X, Brcich T, Dun EA, Goussot M, Haurogne K, Beveridge CA, Rameau C (2006) Branching genes are conserved across species. Genes controlling a novel signal in pea are coregulated by other long-distance signals. Plant Physiol 142:1014–1026. https://doi.org/10.1104/pp.106.087676
Kiba T, Taniguchi M, Imamura A, Ueguchi C, Mizuno T, Sugiyama T (1999) Differential expression of genes for response regulators in response to cytokinins and nitrate in Arabidopsis thaliana. Plant Cell Physiol 40:767–771. https://doi.org/10.1093/oxfordjournals.pcp.a029604
Liu Y, Wang Q, Ding Y, Li G, Xu J, Wang S (2011) Effects of external ABA, GA3 and NAA on the tiller bud outgrowth of rice is related to changes in endogenous hormones. Plant Growth Regul 65:247–254. https://doi.org/10.1007/s10725-011-9594-x
Luo L et al (2018) Strigolactones affect the translocation of nitrogen in rice. Plant Sci. https://doi.org/10.1016/j.plantsci.2018.02.020
Mader JC, Emery RJN, Turnbull CGN (2003) Spatial and temporal changes in multiple hormone groups during lateral bud release shortly following apex decapitation of chickpea (Cicer arietinum) seedlings. Physiol Plant 119:295–308. https://doi.org/10.1034/j.1399-3054.2003.00179.x
Mason MG, Ross JJ, Babst BA, Wienclaw BN, Beveridge CA (2014) Sugar demand, not auxin, is the initial regulator of apical dominance. Proc Natl Acad Sci USA 111:6092–6097. https://doi.org/10.1073/pnas.1322045111
Min Z, Zhao X, Li R, Yang B, Liu M, Fang Y (2017) Comparative transcriptome analysis provides insight into differentially expressed genes related to bud dormancy in grapevine (Vitis vinifera). Sci Hortic 225:213–220. https://doi.org/10.1016/j.scienta.2017.06.033
Muller D, Leyser O (2011) Auxin, cytokinin and the control of shoot branching. Ann Bot 107:1203–1212. https://doi.org/10.1093/aob/mcr069
Muller D et al (2015) Cytokinin is required for escape but not release from auxin mediated apical dominance. Plant J 82:874–886. https://doi.org/10.1111/tpj.12862
Murphy A, Peer W, Taiz A L (2000) Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta 211:315–324. doi.https://doi.org/10.1007/s004250000300
Nakagawa H et al (2005) Overexpression of a petunia zinc-finger gene alters cytokinin metabolism and plant forms. Plant J 41:512–523. https://doi.org/10.1111/j.1365-313X.2004.02316.x
Nordstrom A, Tarkowsk T, Tarkowska D, Norbaek R, Åstot C, Dolezal D, Sandberg G (2004) Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor pf potential importance for auxin cytokinin regulated development. Proc Natl Acad Sci USA 101:8039–8044. https://doi.org/10.1073/pnas.0402504101
Oikawa T, Kyozuka J (2009) Two-step regulation of LAX PANICLE1 protein accumulation in axillary meristem formation in rice. Plant Cell 21:1095–1108. https://doi.org/10.1105/tpc.108.065425
Ono E et al (2006) Localization of a flavonoid biosynthetic polyphenol oxidase in vacuoles. Plant J 45:133–143. https://doi.org/10.1111/j.1365-313X.2005.02625.x
Peer WA, Murphy AS (2007) Flavonoids and auxin transport: modulators or regulators? Trends Plant Sci 12:556–563. https://doi.org/10.1016/j.tplants.2007.10.003
Peer WA, Bandyopadhyay A, Blakeslee JJ, Makam SN, Chen RJ, Masson PH, Murphy AS (2004) Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 16:1898–1911. https://doi.org/10.1105/tpc.021501
Petrasek J, Friml J (2009) Auxin transport routes in plant development. Development 136:2675–2688. https://doi.org/10.1242/dev.030353
Pillay I, Railton ID (1983) Complete release of axillary buds from apical dominance in intact, light-grown seedlings of Pisum sativum L. following a single application of cytokinin. Plant Physiol 71:972–974. https://doi.org/10.1104/pp.71.4.972
Prusinkiewicz P, Crawford S, Smith R, Ljung K, Bennett T, Ongaro V, Leyser O (2009) Control of bud activation by an auxin transport switch. Proc Natl Acad Sci USA 106:17431–17436. doi.https://doi.org/10.1073/pnas.0906696106
Qiao S et al (2017) The RLA1/SMOS1 transcription factor functions with OsBZR1 to regulate brassinosteroid signaling and rice architecture. Plant Cell 29:292–309. https://doi.org/10.1105/tpc.16.00611
Reddy SK, Holalu SV, Casal JJ, Finlayson SA (2013) Abscisic acid regulates axillary bud outgrowth responses to the ratio of red to far-red light. Plant Physiol 163:1047–1058. https://doi.org/10.1104/pp.113.221895
Riou-Khamlichi C, Huntley R, Jacqmard. A, Murray JAH (1999) Cytokinin activation of Arabidopsis Cell division through a D-type cyclin. Science 283:1541–1544
Santner A, Calderon-Villalobos LI, Estelle M (2009) Plant hormones are versatile chemical regulators of plant growth. Nat Chem Biol 5:301–307. https://doi.org/10.1038/nchembio.165
Shinohara N, Taylor C, Leyser O (2013) Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biol 11:e1001474. https://doi.org/10.1371/journal.pbio.1001474
Song X et al (2017) IPA1 functions as a downstream transcription factor repressed by D53 in strigolactone signaling in rice. Cell Res 27:1128–1141. https://doi.org/10.1038/cr.2017.102
Soundappan I et al (2015) SMAX1-LIKE/D53 family members enable distinct MAX2-dependent responses to strigolactones and karrikins in Arabidopsis. Plant Cell 27:3143–3159. https://doi.org/10.1105/tpc.15.00562
Sun H et al (2014) Strigolactones are involved in phosphate- and nitrate-deficiency-induced root development and auxin transport in rice. J Exp Bot 65:6735–6746. https://doi.org/10.1093/jxb/eru029
Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H (2006) Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J 45:1028–1036. https://doi.org/10.1111/j.1365-313X.2006.02656.x
Taniguchi M, Kiba T, Sakakibara H, Ueguchi C, Mizuno T, Sugiyama T (1998) Expression of Arabidopsis response regulator homologs is induced by cytokinins and nitrate. FEBS Lett 429:259–262
Thimann K, Skoog F (1933) Studies on the growth hormone of plants: III. The inhibiting action of the growth substance on bud development. Proc Natl Acad Sci USA 19:714–716
Tong H et al (2009) DWARF AND LOW-TILLERING, a new member of the GRAS family, plays positive roles in brassinosteroid signaling in rice. Plant J 58:803–816. https://doi.org/10.1111/j.1365-313X.2009.03825.x
Tong H et al (2012) DWARF AND LOW-TILLERING acts as a direct downstream target of a GSK3/SHAGGY-like kinase to mediate brassinosteroid responses in rice. Plant Cell 24:2562–2577. https://doi.org/10.1105/tpc.112.097394
Umehara M, Hanada A, Magome H, Takeda-Kamiya N, Yamaguchi S (2010) Contribution of strigolactones to the inhibition of tiller bud outgrowth under phosphate deficiency in rice. Plant Cell Physiol 51:1118–1126. https://doi.org/10.1093/pcp/pcq084
Wang J, Lu K, Nie H, Zeng Q, Wu B, Qian J, Fang Z (2018) Rice nitrate transporter OsNPF7.2 positively regulates tiller number and grain yield. Rice 11:12. https://doi.org/10.1186/s12284-018-0205-6
Waters MT, Brewer PB, Bussell JD, Smith SM, Beveridge CA (2012) The Arabidopsis ortholog of rice DWARF27 acts upstream of MAX1 in the control of plant development by strigolactones. Plant Physiol 159:1073–1085. https://doi.org/10.1104/pp.112.196253
Xu J, Ding C, Ding Y, Tang S, Zha M, Luo B, Wang S (2014) A proteomic approach to analyze differential regulation of proteins during bud outgrowth under apical dominance based on the auxin transport canalization model in rice (Oryza sativa L.). J Plant Growth Regul 34:122–136. https://doi.org/10.1007/s00344-014-9450-0
Xu J, Zha M, Li Y, Ding Y, Chen L, Ding C, Wang S (2015) The interaction between nitrogen availability and auxin, cytokinin, and strigolactone in the control of shoot branching in rice (Oryza sativa L.). Plant Cell Rep 34:1647–1662. https://doi.org/10.1007/s00299-015-1815-8
Yao C, Finlayson SA (2015) Abscisic acid is a general negative regulator of arabidopsis axillary bud growth. Plant Physiol 169:611–626. https://doi.org/10.1104/pp.15.00682
Zhou F et al (2013) D14-SCF(D3)-dependent degradation of D53 regulates strigolactone signalling. Nature 504:406–410. https://doi.org/10.1038/nature12878
Zhu X et al (2015) Brassinosteroids promote development of rice pollen grains and seeds by triggering expression of Carbon Starved Anther, a MYB domain protein. Plant J 82:570–581. https://doi.org/10.1111/tpj.12820
Funding
This work was supported by the Ministry of National Science and Technology of China (Project no. 31371569), the Science and Technology Department of China (Project no. 2013BAD07B09), and the Science and Technology Department of Jiangsu Province (Project no. BE2014393).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of the interest
The authors have no conflicts of the interest to declare.
Additional information
Communicated by Renate Schmidt.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zha, M., Imran, M., Wang, Y. et al. Transcriptome analysis revealed the interaction among strigolactones, auxin, and cytokinin in controlling the shoot branching of rice. Plant Cell Rep 38, 279–293 (2019). https://doi.org/10.1007/s00299-018-2361-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-018-2361-y