Abstract
Hormones play an important role in regulating the growth of rice tiller buds. However, little is known about the hormonal changes that occur during tiller bud growth and the mechanism of hormonal regulation of tiller bud growth. Here, two rice cultivars, Yangdao 6 (Indica) and Nanjing 44 (Japonica), were used to investigate the changes in plant hormones during tiller bud growth and the mechanism that underlies the hormonal regulation of tiller bud growth. In the present study, panicles were removed after heading to stimulate the growth of dormant tiller buds located at the elongated upper internodes. At the same time, external abscisic acid (ABA), gibberellic acid (GA3) and α-naphthalene acetic acid (NAA) were applied. The results demonstrated that auxin and cytokinin (CTK) play important and different roles in the regulation of tiller bud growth. Auxin in the nodes inhibits tiller bud growth, while CTK is transferred to the tiller buds to promote growth. The inhibitory effects of GA3 and NAA on tiller bud growth are mainly due to the control of the indole-3-acetic acid (IAA) or CTK contents in plants. As opposed to auxin and CTK, the ABA contents in nodes and tiller buds remained unchanged before tiller bud growth after panicle removal. Meanwhile, external ABA application only slightly slowed the growth of the tiller buds, suggesting that ABA may not be a key regulator of tiller bud growth. These results indicate that auxin, CTK and ABA together likely play roles in the regulation of tiller bud growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa L.) is one of the most important food crops and is responsible for feeding more than half of the world’s population. The yield potential of rice can be dissected into four major components: grain weight, grain number per panicle, panicle number per plant and the ratio of filled grains. The tiller of rice, which determines the panicle number per plant, is an important agronomic trait for grain production and is also a model system for the study of branching in monocotyledonous plants (Li et al. 2003).
The rice tiller number is dynamic and adjustable (Kariali and Mohapatra 2007), and plant hormones play an important role in regulating tiller occurrence. Leopold (1949) indicated that shoot growth in grasses is regulated by the same type of auxin-induced apical dominance as in dicotyledons; removal or suppression of auxin activity could release the tiller (lateral buds) from apical control. It has been shown that the exogenous application of cytokinin (CTK) stimulates tiller bud growth in wheat (Langer et al. 1973). However, exogenous application of auxin inhibited the effect of CTK (Harrison and Kaufman 1982). In addition, the application of gibberellic acid 3 (GA3) inhibited the occurrence of rice tillers (Hong et al. 1998; Zhang 2006), and higher abscisic acid (ABA) contents in the stems and tillers decreased wheat tillers (Liang and Ma 1998), and the application of ethylene at an early tillering stage increased the number of tillers in sugarcane (Zhou et al. 2007). A recent study found a new terpenoid plant hormone that inhibits shoot branching and originates from carotenoids (Umehara et al. 2008).
Rice tiller growth occurs in a two-stage process: the formation of an axillary bud (tiller bud) and its subsequent growth. Previous research specifically studied the effects of exogenous hormones on tiller occurrence and the relationship between the percentage of tiller occurrence and the content endogenous hormones. However, the hormonal changes during tiller bud growth and the mechanism of hormonal regulation of tiller bud growth are still unclear.
Normally, rice tiller buds form on the elongated upper internodes and become arrested when the mother stems begin to differentiate their own panicles (Hanada 1993; Li 1979), but removing the panicle after heading stimulates the growth of the dormant tiller buds (Tomotsugu et al. 2007). In our study, we removed the panicle after full heading to stimulate the growth of dormant tiller buds located at elongated internodes and simultaneously applied α-naphthalene acetic acid (NAA), ABA and GA3 to control the growth of tiller buds. Our objectives were to investigate the changes in plant hormones during tiller bud growing and the mechanism that underlies the effect of hormonal regulation on tiller bud growth.
Materials and methods
Plant growth and treatments
The experiments were conducted at the experimental station of Nanjing Agriculture University, Jiangning County, Jiangsu Province, China in 2008. The soil type was Gleyed paddy soil with 16.1 g kg−1 organic matter, and the available amounts of nitrogen, phosphorus and potassium were 74.7, 10.36 and 82.6 mg kg−1, respectively. Nitrogen (90 kg urea ha−1 at basal levels and 90 kg urea ha−1 at panicle initiation), phosphorus (135 kg single superphosphate ha−1 at basal levels) and potassium (90 kg KCl ha−1 at basal levels and 90 kg KCl ha−1 at panicle initiation) were applied during the growing season.
Two rice cultivars, Nanjing 44 (a Japonica cultivar) and Yangdao 6 (an Indica cultivar), were grown in the paddy field. Seeds were sown on a seedbed on May 20, and they were raised and transplanted on June 25 at a hill spacing of 30.0 × 13.3 cm with two seedlings per hill. The plot dimensions were 4 × 4 m.
Each cultivar had five treatments: RP treatment (panicle removal after full heading), ABA treatment (application of ABA after panicle removal), GA treatment (application of GA3 after panicle removal), NAA treatment (application of NAA after panicle removal) and control treatment (intact plant). The concentrations of the three hormones are 50 mg l−1, and 5 ml of hormone solution was applied to each plant. The RP and control groups were sprayed with same quantity of water. ABA, GA3, and NAA were purchased from Sigma–Aldrich (USA). Each treatment had three replicates with a completely randomized block design.
Sampling and measurements
The panicles were removed and hormones were sprayed at 18:00 at the full heading stage. Plants were sampled from the day of treatment to 5 days later. After the treatments, samples were collected every day at 06:00.
Each day during the sampling dates, tiller buds located at the leaf axils of the second leaves from the top and the nodes where tiller buds grew were sampled. The samples were frozen in liquid nitrogen for 15 min and then stored at −40°C.
Three hills of whole plants for each plot were sampled on day five after treatment. Leaves and stems plus leaf sheaths were separated and put into different sample bags. All of the fresh samples were placed in a forced-air oven, incubated for 1 h at 105°C and dried at 75°C until a constant weight was obtained. The dried samples were milled to pass through a 1 mm screen and then stored in plastic bags for chemical analysis.
Tiller bud growth
At each sampling date, forty tiller buds were cut off, and their lengths were measured in one treatment. The weights of the tiller buds were taken on a sample group of 10 buds, and four sample groups were measured at each stage.
Measurements of endogenous hormones
The methods for extraction and purification of zeatin (Z) + zeatin riboside (ZR), isopentenyladenine (iP) + isopentenyladenine riboside (iPR), gibberellic acids (GAs, GA1 + GA4), indole-3-acetic acid (IAA) and ABA were essentially identical to those described by Yang et al. (2001). A sample of approximately 0.5 g (tiller buds about 0.2 g) was ground in a mortar (on ice) with 5 ml of 80% (v/v) methanol extraction medium containing 1 mmol l−1 butylated hydroxytoluene (BHT) as an antioxidant. The methanolic extracts were incubated at 4°C for 4 h and centrifuged at 10,000×g for 15 min at the same temperature. The supernatants were passed through Chromosep C18 columns (C18 Sep-Park Cartridge, Waters Corp, USA), which had been prewashed with 10 ml of 100% and 5 ml of 80% methanol. The hormone fractions were dried with N2 and dissolved in 1 ml of phosphate-buffered saline (PBS) containing 0.1% (v/v) Tween 20 and 0.1% (w/v) gelatin (pH 7.5) for analysis by enzyme-linked immunosorbent assay (ELISA).
The mouse monoclonal antigens and antibodies against Z + ZR, iP + iPR, GAs (GA1 + GA4), IAA and ABA and immunoglobulin G-horseradish peroxidase (IgG-HRP) used in the ELISA were produced at the Phytohormones Research Institute of China Agricultural University. The method for quantification of the Z + ZR, iP + iPR, GAs (GA1 + GA4), IAA and ABA contents by ELISA has been described previously (Yang et al. 2001). The recoveries of IAA, Z + ZR, iP + iPR, ABA and GAs were 79.2 ± 4.9, 86.5 ± 3.7, 87.9 ± 3.5, 82.3 ± 4.1 and 76.5 ± 4.6%, respectively.
Measurements of nitrogen and non-structural carbohydrates in plants
Total plant nitrogen (N) was determined by the Kjeldahl method. Dry plant samples (0.5 g) were digested with 3 g catalyst (3:1 K2SO4:CuSO4), 10 ml of H2SO4 and 2 ml of H2O2 for at least 6 h at 375°C. The N content was measured according to Bao (2007).
Non-structural carbohydrates (NSC) were extracted with perchloric acid and measured by the anthrone solution colorimetric method (Wang 2006). Dry plant samples (0.2 g) were extracted with 5 ml of 36 mol l−1 perchloric acid and centrifuged at 4,000×g for 10 min. The residue was then extracted with 5 ml of 18 mol l−1 perchloric acid. The supernatants were combined, and water was added to 100 ml. For the anthrone colormetric method, 0.1 ml of the combined supernatant was boiled in 5 ml of anthrone-H2SO4 solution (0.15 g anthrone in 100 ml of 70% H2SO4) for 20 min at 80°C, and then the absorption at 620 nm was measured.
Statistical analysis
The results were analyzed for variance using SPSS 16.0 for Windows. Data from each sampling set were analyzed separately. The means were tested by the least significant difference method at P = 0.05 (LSD 0.05).
Results
Tiller bud development from dormancy or germination
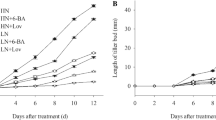
Panicle removal quickly released the dormancy of tiller buds and stimulated their germination. Thirty-six hours after panicle removal, the length and fresh weight of the tiller buds were significantly higher in the RP plots than in the control treatments (Fig. 1). The lengths of tiller buds at 108 h after panicle removal were 3.16 cm for Nanjing 44 and 3.80 cm for Yangdao 6, respectively, which were 4.9 and 6.6 times greater than those of the control, respectively. The application of ABA, GA3 and NAA had different effects on the growth of tiller buds after panicle removal. The application of GA3 or NAA completely reversed the effect of panicle removal on tiller bud growth. There were no differences in the lengths and fresh weights of tiller buds among the GA, NAA and control treatments 108 h after panicle removal. However, the ABA application could not completely offset the effect of panicle removal on tiller bud growth. The lengths and fresh weights of the tiller buds in the ABA treatment showed increasing trends that were similar to the RP treatment, although the magnitudes of the former treatment were significantly less than the latter 36 h after panicle removal (Fig. 1).
Changes in the fresh weight (a: Yangdao 6; b: Nanjing 44) and length (c: Yangdao 6; d: Nanjing 44) of tiller buds under different treatments. RP panicle removal after full heading, ABA application of ABA after panicle removal, GA application of GA3 after panicle removal, NAA application of NAA after panicle removal, control: intact plant. Vertical bars represent the standard error of the mean (length of tiller buds n = 10, fresh weight of buds n = 4)
Phytohormone contents in nodes
The IAA content in nodes decreased notably by 65.5 and 56.2% in Yangdao 6 and Nanjing 44, respectively, compared with the control treatment at 12 h after treatment. GA3 application after panicle removal increased the IAA content in the nodes, but the value did not return to that of the control plants. The application of ABA or NAA had no obvious effects on the IAA contents in nodes (Fig. 2a, b).
Hormonal changes in the second node from the top of Yangdao 6 (in boxes a, c, e, g, and i) and Nanjing 44 (in boxes b, d, f, h, and j) under different treatments. RP panicle removal after full heading, ABA application of ABA after panicle removal, GA application of GA3 after panicle removal, NAA application of NAA after panicle removal, control: intact plant. Vertical bars represent the standard error of the mean (n = 4)
Panicle removal decreased the ABA content in the nodes of both cultivars. Compared with the RP treatment, GA3 or NAA application increased ABA content in the nodes, but the enhancement was lower than that in the control plants. ABA application transiently increased the ABA content in the nodes. After reaching a peak at 12 h after treatment, the ABA content in the nodes gradually decreased. There was no significant difference in the ABA contents in nodes between the ABA and control treatments of the two cultivars at 60 and 36 h after treatment (Fig. 2c, d).
Panicle removal and ABA or NAA application did not obviously affect the GA1+4 content in the nodes. On the contrary, the application of GA3 significantly increased the GA1+4 content in the node transiently, which reached a peak at 12 h after treatment and then decreased gradually. There were no significant differences between the GA1+4 contents in all treatments at 36 and 60 h after treatment in Yangdao 6 and Nanjing 44 (Fig. 2e, f).
Unlike IAA, the Z + ZR content increased notably in the nodes of both Yangdao 6 and Nanjing 44 after panicle removal and reached a peak at 36 h after treatment and then decreased gradually. The value, however, was higher than that in the control plants at 108 h after treatment. ABA application also increased the Z + ZR content in nodes significantly compared with the control plants, but the Z + ZR content in nodes of the ABA treatment were lower than that in the RP treatment. The application of GA3 or NAA after removal of the panicle completely reversed the stimulation of panicle removal on the Z + ZR content in nodes (Fig. 2g, h). The changes in the iP + iPR content with different treatments showed a similar trend to Z + ZR (Fig. 2i, j).
Phytohormone contents in tiller buds
Panicle removal had different effects on the IAA contents in tiller buds of the two cultivars. Thirty-six hours after panicle removal, the IAA content in tiller buds increased notably in Yangdao 6 and remained unchanged in Nanjing 44 compared with the control plants. The application of ABA after panicle removal had no significant effect on the IAA content in tiller buds. The application of GA3 and NAA after panicle removal decreased the IAA content in tiller buds in Yangdao 6 compared with the RP treatment, and there was no significant effect on the IAA content in tiller buds of Nanjing 44 (Fig. 3a, b).
Hormonal changes in tiller buds located at the second node from the top of Yangdao 6 (boxes a, c, e, g, and i) and Nanjing 44 (boxes b, d, f, h, and j) under different treatments. RP panicle removal after full heading, ABA application of ABA after panicle removal, GA application of GA3 after panicle removal, NAA application of NAA after panicle removal, control: intact plant. Vertical bars represent the standard error of the mean (n = 4)
Panicle removal decreased the ABA contents in the tiller buds of both cultivars, and the ABA content in tiller buds of the RP treatment was significantly lower than that of control plants from 36 h after treatment. Application of GA3 or NAA reversed the negative effect of panicle removal on the ABA content in tiller buds during entire treatment period. There were no significant differences in the ABA contents in tiller buds from the GA3, NAA and control treatments (Fig. 3c, d).
Compared with the RP treatment, the application of ABA increased the ABA contents in tiller buds, but the levels remained lower than in the control plants from 36 h after treatment (Fig. 3c, d). Neither panicle removal nor the application of growth regulators had an obvious effect on the GA1+4 content in tiller buds (Fig. 3e, f).
The Z + ZR content increased notably in tiller buds of both Yangdao 6 and Nanjing 44 after panicle removal and reached a peak at 36 h after treatment, after which the Z + ZR content remained stable, and the value was higher than that in the CK plants at 108 h after treatment. The ABA treatment also increased the Z + ZR content in tiller buds compared with the control plants, but compared with the RP treatment, the Z + ZR content in tiller buds of the ABA treatment was significantly lower than that of Yangdao 6. The application of GA3 or NAA completely inhibited the increase in the Z + ZR content in tiller buds after panicle removal. The Z + ZR contents in tiller buds of these two treatments were not significantly different compared with the control plants at 108 h after treatments (Fig. 3g, h). The changes in the iP + iPR content under different treatments showed a similar trend to the Z + ZR content (Fig. 3i, j).
N and NSC contents in plants
Panicle removal increased the N content in leaves but had no significant effect on the N contents in stems and leaf sheaths. In contrast, panicle removal significantly increased the NSC content in the leaves and stem sheath. The application of growth regulators had no significant effect on the N and NSC contents in the leaves and stem sheath (Fig. 4).
N and NSC contents in leaves and stem sheaths at 108 h after different treatments. RP panicle removal after full heading, ABA application of ABA after panicle removal, GA application of GA3 after panicle removal, NAA application of NAA after panicle removal, control: intact plant. Vertical bars represent the standard error of the mean (n = 3)
Discussion
The correlation between endogenous hormones and tiller bud growth
Tillers are grain-bearing branches in monocotyledonous plants. Normally, tiller buds formed on the elongated upper internodes become arrested when the mother stems begin to differentiate their own panicles (Hanada 1993; Li 1979), but those buds exhibit significant elongation after decapitation (Tomotsugu et al. 2007). In this study, the length and fresh weight of dormant tiller buds increased greatly after panicle removal (Fig. 1). Thus, panicle removal releases tiller buds from dormancy and stimulates their growth. Before the length and fresh weight of the tiller buds increased after panicle removal, the Z + ZR and iP + iPR contents in both the nodes and buds increased significantly (Figs. 2g–j, 3g–j). On the contrary, the IAA content in the nodes decreased significantly (Fig. 2a, b), while the IAA content in the buds did not change notably after panicle removal (Fig. 3a, b). The results of our study indicate that, similarly to other plants (Bangerth et al. 2000; Li et al. 1995), auxin acts in the stem to inhibit tiller bud growth, and, in opposition, CTK is transferred to the buds to promote the growth of tiller buds. In addition to these two hormones, ABA was also thought to be a possible auxin-induced second messenger that directly represses axillary bud outgrowth (Tucker 1978), and additive repression has been observed when apical auxin and basal ABA treatments were combined in Ipomoea (Cline and Oh 2006). In the present study, before tiller bud growth after panicle removal, the ABA contents in the nodes and buds did not significantly change. The decrease in ABA content in tiller buds and the growth of tiller buds occurred simultaneously (Fig. 3c, d), and these changes occurred later than the changes in IAA in the nodes. These results indicate that ABA may not be the decisive factor for tiller bud growth.
Effects of applied hormones on tiller bud growth
Auxin significantly inhibits lateral bud outgrowth following decapitation when applied to the stem stumps of many species. NAA, a synthetic auxin, is generally more potent than IAA for the inhibition of lateral bud outgrowth (Cline 1996). In the present study, NAA application completely reversed the promoting effect of panicle removal on tiller bud growth; the fresh weight and length of tiller buds did not increase notably during the entire treatment period (Fig. 1). Some research has suggested that basipolar auxin may control CTK production in roots and that it is possibly delivered to lateral buds, consequently regulating the growth of lateral buds (Bangerth et al. 2000; Li et al. 1995). In this study, we found that NAA inhibits the increase in the Z + ZR and iP + iPR contents in tiller buds and the tiller nodes induced by panicle removal. This occurrence may be the main factor responsible for the inhibitory effect of NAA on the growth of tiller buds induced by panicle removal.
The application of GA3 inhibits tiller bud growth of barley, and the application of chlormequat chloride (CCC), which inhibits GA biosynthesis, promotes tillering of spring barely (Ma and Smith 1992; Woodward and Marshall 1988). In this study, GA3 application also completely reversed the effect of panicle removal on tiller bud growth. Zhang (2006) proposed that GA3 promotes the transfer of nutritive substances to the main stem to control tiller occurrence; however, our study shows that GA3 application does not significantly affect the N and NSC contents in plants (Fig. 4), indicating that GA3 may not promote the transfer of nutritive substances to the main stem to control tiller occurrence. Our experiments showed that the application of GA3 increases the IAA content in the nodes compared with the RP treatments and inhibits the increase in the Z + ZR and iP + iPR contents in tiller buds and nodes induced by panicle removal. We suggest that GA3 may increase the IAA content in plants to control CTK production, which thus inhibits the growth of tiller buds in rice. As opposed to GA3 and NAA application, ABA application did not inhibit the growth of tiller buds completely, but it slowed the growth rate of tiller buds (Fig. 1). Combined with the changes in endogenous ABA levels, this result confirms the hypothesis that ABA may not be the decisive factor for tiller bud growth (Liu et al. 2009).
It is widely agreed that lateral bud outgrowth depends on the balance of hormones rather than the absolute level of any individual hormone (Emery et al. 1998; Sae and Hitoshi 2001). The relative levels of IAA and CTK are important for the early growth of axillary buds, while at later stages, there is a significant negative correlation between the level of ABA and the growth of axillary buds (Gong and Li 2005). Research on transgenic plants has verified the importance of the relative concentrations of IAA and CTK on the outgrowth of axillary buds (Medford et al. 1989; Romano et al. 1991; Romano et al. 1993; Sitbon et al. 1992).
In our research, before the length and fresh weight of the tiller buds increased after panicle removal, the Z + ZR and iP + iPR contents in both the nodes and buds and the IAA content in nodes increased significantly, and GA3 and NAA inhibited the growth of rice tiller buds by regulating the IAA or CTK contents in plants. This result indicates that auxin and CTK may play key roles in regulating the growth of rice tiller buds. In contrast to auxin and CTK, the ABA content in the nodes and buds remained unchanged before tiller bud growth after panicle removal, and the application of ABA did not completely inhibit the growth of tiller buds. Therefore, ABA may not be a key regulator of tiller bud growth, though it affects the growth velocity of tiller buds. The results of the present study indicate that auxin, cytokinin and ABA together take part in the regulation of tiller bud growth.
Abbreviations
- CTK:
-
Ctyokinin
- NAA:
-
α-Naphthalene acetic acid
- ABA:
-
Abscisic acid
- GAs:
-
Gibberellic acids
- Z + ZR:
-
Zeatin + zeatin riboside
- iP + iPR:
-
Isopentenyladenine + isopentenyladenine riboside
- IAA:
-
Indole-3-acetic acid
- NSC:
-
Non-structural carbohydrates
- N:
-
Nitrogen
References
Bangerth F, Li CJ, Gruber J (2000) Mutual interaction of auxin and cytokinins in regulating correlative dominance. Plant Growth Regul 32:205–217
Bao SD (2007) Soil and agricultual chemistry analysis. China Agricultural Press, Beijing (in Chinese)
Cline M (1996) Exogenous auxin effects on lateral bud outgrowth in decapitated shoots. Ann Bot 78:256–266
Cline M, Oh C (2006) A Reappraisal of the role of abscisic acid and its interaction with auxin in apical dominance. Ann Bot 98:891–897
Emery R, Longnecker N, Atkins C (1998) Branch development in Lupinus angustifolius L.II. Relationship with endogenous ABA, IAA and cytokinins in axillary and main stem buds. J Exp Bot 49:555–562
Gong P, Li D (2005) Genetic control of plant shoot branching. Mol Plant Breed 3:151–162 (in Chinese with English abstract)
Hanada K (1993) Tillers. In: Matsuo T, Hoshikawa K (eds) Science of the rice plant, vol 1. Morphology. Food and Agriculture Policy Research Center, Tokyo, pp 222–229
Harrison M, Kaufman P (1982) Does ethylene play a role in the release of lateral buds(tillers) from apical dominance in oats? Plant Physiol 70:811–814
Hong X, Jiang P, Zheng Z, Lu C, Wang C (1998) Relationships between employ GA3 during tillering stage and promote the panicle bearing tiller rate. J Zhejiang Agric Sci 1:3–5 (in Chinese with English abstract)
Kariali E, Mohapatra P (2007) Hormonal regulation of tiller dynamics in differentially-tillering rice cultivars. Plant Growth Regul 53:215–223
Langer R, Prasad P, Laude H (1973) Effects of kinetin on tiller bud elongation in wheat (Triticum aestivum L.). Ann Bot 37:565–571
Leopold A (1949) The control of tillering in grasses by auxin. Am J Bot 36:437–440
Li Y (1979) Morphology and anatomy of grass family crops. Shanghai Science and Technology Press, Shanghai, pp 138–142
Li C, Guevera E, Herrera J, Bangerth F (1995) Effect of apex excision and replacement by 1-naphthylacetic acid on cytokinin concentration and apical dominance in pea plants. Physiol Plant 94:465–469
Li X, Qian Q, Fu Z, Wang Y, Xiong G, Zeng D, Wang X, Liu X, Teng S, Hiroshi F, Yuan M, Luo D, Han B, Li J (2003) Control of tillering in rice. Nature 422:618–621
Liang Z, Ma X (1998) Studies on the effects of endogenous hormones on tiller development process of winter whea. Acta Agron Sin 24:788–792 (in Chinese with English abstract)
Liu Y, Wang Q, Ding YF, Liu ZH, Li GH, Wang SH (2009) Endogenous phytohormone changes in the release of dormant tillering bud in rice. Acta Agron Sin 35:356–362 (in Chinese with English abstract)
Ma B, Smith D (1992) Chlormequat and ethephon timing and grain production of spring barley. Agron J 84:934–939
Medford JI, Horgan R, El-Sawi Z, Klee H (1989) Alterations of endogenous cytokinins in transgenic plants using a chimeric isopentenyl transferase gene. Plant Cell 1:403–413
Romano C, Hein M, Klee H (1991) Inactivation of auxin in tobacco transformed with the indoleacetic acid-ly-sine synthetase gene of Pseudomonas savastano. Genes Dev 5:438–446
Romano C, Cooper M, Klee H (1993) Uncoupling auxin and ethylene effects in transgenic tobacco and Arabidopsis plants. Plant Cell 5:181–189
Sae S, Hitoshi M (2001) Control of outgrowth and dormancy in axillary buds. Plant Physiol 127:1405–1413
Sitbon F, Hennion S, Sundberg B, Little C, Olsson O, Sandberg G (1992) Transgenic tobacco plants coexpressing the Agrobacterium tumefaciens iaaM and iaaH genes display altered growth and indoleacetic-acid metabolism. Plant Physiol 99:1062–1069
Tomotsugu A, Hirotaka I, Kenji O, Masahiko M, Masatoshi N, Mikiko K, Hitoshi S, Junko K (2007) DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J 51:1019–1029
Tucker D (1978) Apical dominance in the tomato: the possible roles of auxin and abscisic acid. Plant Sci Let 12:273–278
Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, Kyozuka J, Yamaguchi S (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455:195–200
Wang X (2006) Principles and techniques of plant physiological biochemical experiment, 2nd edn. Higher Education Press, Beijing (in Chinese)
Woodward E, Marshall C (1988) Effects of plant-growth regulators and nutrient supply on tiller bud outgrowth in Barley (Hordeum Distichum L). Ann Bot 61:347–354
Yang J, Zhang J, Wang Z, Zhu Q, Wang W (2001) Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant Physiol 127:315–323
Zhang Z (2006) Research of chemical regulation on promote the panicle bearing tiller rate of rice. Fujian Sci Tech Rice Wheat 2:10–13 (in Chinese)
Zhou C, Li Y, Yang L (2007) Effect of ethephon sprayed at early tillering stage on the activities of peroxidase, IAA oxidase and acid invertase in sugarcane in correlation to tillering. Guihaia 27:649–652 (in Chinese with English abstract)
Acknowledgments
This work was supported by the Ministry of National Science and Technology of China (Project No. 2006BAD02A03-2), Doctor Subject Foundation of the Ministry of Education of China (Project No.20100097110032), and the National Natural Science Foundation of China (Project No.31071364).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Wang, Q., Ding, Y. et al. Effects of external ABA, GA3 and NAA on the tiller bud outgrowth of rice is related to changes in endogenous hormones. Plant Growth Regul 65, 247–254 (2011). https://doi.org/10.1007/s10725-011-9594-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-011-9594-x