Abstract
Key message
HT-induced ROS burst in developing anther is closely related to the lowered CAT activity as the result of the markedly suppressed OsCATB transcript, thereby causing severe fertility injury for rice plants exposed to HT at meiosis stage.
Abstract
The reproductive stage of rice plants is highly sensitive to heat stress. In this paper, different rice cultivars were used to investigate the relationship of HT-induced floret sterility with reactive oxygen species (ROS) detoxification in rice anthers under well-controlled climatic conditions. Results showed that high temperature (HT) exposure significantly enhanced the ROS level and malondialdehyde (MDA) content in developing anther, and the increase in ROS amount in rice anther under HT exposure was closely associated with HT-induced decline in the activities of several antioxidant enzymes. For various antioxidant enzymes, SOD and CAT were more susceptible to the ROS burst in rice anther induced by HT exposure than APX and POD, in which SOD and CAT activity in developing anther decreased significantly by HT exposure, whereas APX activity was relatively stable among different temperature regimes. HT-induced decrease in CAT activity was attributable to the suppressed transcript of OsCATB. This occurrence was strongly responsible for HT-induced increase in ROS level and oxidative-damage in rice anther, thereby it finally caused significant reduction in pollen viability and floret fertility for the rice plants exposed to HT during meiosis. Exogenous application of 1000 µM salicylic acid (SA) may alleviate HT-induced reduction in pollen viability and floret fertility, concomitantly with the increased CAT activity and reduced ROS level in rice anther.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High temperature (HT) during rice-growing season is a major environmental factor that seriously limits rice yields in many agricultural regions (Teixeira et al. 2013). Normally, the reproductive growth stage of rice plants is highly sensitive to HT exposure (Prasad et al. 2006; Krishnan et al. 2011), and the male reproductive organ in rice florets is more vulnerable to HT injury than the female reproductive organ and functional leaves (Matsui and Omasa 2002; Endo et al. 2009). Previous studies suggested that HT exposure to about 32 °C during microspore development can induce pollen abortion and floret sterility without affecting vegetative development, whereas exposure to temperature exceeding 35 °C and persisting for more than 1 h during flowering markedly decrease pollen viability and increase floret sterility (Jagadish et al. 2007; Das et al. 2014). Furthermore, the response of pollen viability to HT exposure at the reproductive stage appears to be quantitative, with shorter durations at severe HT exposure having the same effect as longer durations at moderate HT exposure (Jagadish et al. 2007).
The generation of reactive oxygen species (ROS) is one of the key events that occur during the response of plants to stressful environmental changes. Plant exposure to unfavorable environments, such as extreme temperature, heavy metals, drought, air pollutants, nutrient deficiency, or salt stress, can increase the generation of superoxide radical \(\left( {{{\text{O}}_{\text{2}}}^{{ \cdot - }}} \right)\) and hydrogen peroxide (H2O2). If ROS are not promptly cleared, the organism will suffer oxidative stress, resulting in protein and nucleic acid damages, lipid peroxidation and even necrocytosis (Foyer and Noctor 2000; Jiang et al. 2007; Hasanuzzaman et al. 2014). According to Foyer and Noctor (2000), ROS only diffuse to an extremely short distance before reacting with cellular molecules, and excessive ROS generation in plant tissues can directly attack membrane lipids and trigger tissue specific programmed cell death (PCD), such as leaf senescence and abortion of microspores in the anther. Moreover, several studies on rice and wheat have provided the compelling evidence that there is an involvement of PCD and oxidative stress in pollen sterility on cytoplasmic male sterility (CMS) plants (Li et al. 2004; Wan et al. 2007).
To cope with heat stress, plants implement various mechanisms, including maintenance of membrane stability, ROS scavenging, and production of antioxidants. Antioxidant properties in plant tissues can provide protection against ROS (Gill and Tuteja 2010). One of the mechanisms actively used by plants to detoxify ROS is the activation of the antioxidant enzyme system, including superoxide dismutase (SOD, EC 1.15.1.1), peroxidase (POD, EC 1.11.1.1), catalase (CAT, EC 1.11.1.6), ascorbate peroxidase (APX, EC 1.11.1.11), glutathione reductase (GR, EC 1.6.4.2) and other antioxidant enzymes. Efficient antioxidant activity is essential to maintain the concentration of ROS at relatively low level. Among these enzymes, SOD catalyzes the conversion of \(\left( {{{\text{O}}_{\text{2}}}^{{ \cdot - }}} \right)\) to H2O2 and provides the first line of defense against oxidative stress, whereas other antioxidant enzymes (POD, CAT and APX) catalyze the conversion of H2O2 to H2O in different cell organelles (Asada 1997; Gill and Tuteja 2010). APX possesses high affinity to H2O2 and can effectively scavenge H2O2 in the chloroplast for photosynthetic organisms by ascorbic acid (AsA) reduction (Asada 1997). Comparatively, mitochondria and peroxisomes are major cellular sources of ROS in non-photosynthetic tissues of various plants, where CAT plays a crucial role in the removal of H2O2 (Willekens et al. 1997; Mhamdi et al. 2010). Several studies have confirmed that the developing anther tissues of CMS plant materialshad strikingly higher ROS level than those of their corresponding wild-types with normal male fertility (Jiang et al. 2007; Wan et al. 2007), and the interaction between CAT and oxidative stress is strongly responsible for the pollen abortion of CMS plant materials (Wan et al. 2007). Furthermore, a transient increase in the activities of antioxidant enzymes is possibly induced by various environmental stresses, such as drought, high temperature, chilling, salt stresses, heavy metals, and pathogen attack (Asada 1997; Gill and Tuteja 2010), whereas severe stresses may reduce the activities of SOD, CAT, and APX in plant tissues (Nguyen and Sutton 2009). According to Selote and Khanna-Chopra (2010), the adverse effect of drought stress on wheat yield and the photosynthetic rate in flag leaves varied greatly with wheat cultivars, and the wheat cultivars with stronger drought tolerance had significantly higher photosynthetic rate and higher activities of SOD, POD, and CATthan those with weaker drought tolerance under drought stress. Studies on rice plants also suggested that HT exposure increased ROS level and MDA content in rice leaves, and rice cultivars with HT-tolerance have relatively higher activities of antioxidant enzymes (SOD, POD, CAT, and APX) than those with heat-susceptible under HT exposure (Krishnan et al. 2011). However, previous findings about the contribution of various antioxidant enzymes to the ROS detoxification in different plant species and its relation to plant stressful tolerance are mostly obtained from the investigation with plant leaves and other tissues, little research has been done on the relationship of ROS burst and oxidative stress with HT-induced fertility injury for the developing anthers of rice florets. Our understandings are relatively poor for the effect HT exposure on ROS accumulation in the anther of rice florets and its relation to the HT-induced change in antioxidant enzyme activity and floret fertility for various rice cultivars differing in heat tolerance.
Salicylic acid (SA) belongs to a group of plant phenolics that are widely distributed in plants. As a hormone-like substance, SA plays an important role in the regulation of plant growth and development, such as transpiration, seed germination, fruit yield, glycolysis flowering, and heat production (Klessig and Malamy 1994). SA application at an appropriate concentration has been found to enhance the efficiency of antioxidant system in different plant species and to alleviate the oxidative stress for different plant organs (Choudhury and Panda 2004; Hayat et al. 2010). Studies on maize have revealed that SA may cause a decrease in net photosynthesis under normal growth conditions, but it can activate several antioxidant enzymes (POD and GR), which consequently enhance chilling tolerance in subsequent cold stress (Janda et al. 1999). In rice, SA has been reported to have an alleviating contribution to the adverse effect of HT exposure on pollen viability and fertility injury (Mohammed and Tarpley 2011). However, it remains unclear whether or not the SA-modulated upgrade in HT-tolerance is closely associated with HT-induced alteration in ROS level and CAT isozymes in the developing anthers of rice floret.
In this study, two indica rice cultivars, differing typically in heat tolerance, were selected to investigate the effect of HT exposure at meiosis stage on ROS generation and various antioxidant enzymes in developing anthers and also its relationship with HT-induced fertility injury under well-controlled climatic condition. Meanwhile, 16 rice cultivars with wide genetic backgrounds were further employed to examine the genotypic-dependent difference in ROS generation, pollen viability, floret fertility, and CAT isozymes in developing anthers as affected by HT exposure at meiosis stage. Moreover, the HT-induced alteration in the transcript expression of various CAT isoforms and its relation to ROS concentration in developing anther are analyzed for the rice plants exposed to different temperature regimes without or with exogenous SA application. Our objective is to clarify the possible interplay between HT-induced fertility injury and ROS detoxification in developing rice anther.
Materials and methods
Experimental cultivars and crop husbandry
The experiments were conducted during rice-growing seasons (April–October) from 2014 to 2017, using controlled-environment facilities at the experimental station of Zijingang campus (30°18′N, 120°04′E), Zhejiang University, Hangzhou, China. Two indica cultivars (Oryza sativa L.) differing in their tolerance of spikelet sterility to HT injury were used in 2014–2016. Xieqingzao (XQZ) was HT-susceptible cultivar and Qianjiang3 (QJ3) was HT-tolerant cultivar, in terms of a previous screening survey of grain-yield loss and spikelet fertility injury under HT exposure (Cao et al. 2015). Rice seeds were sown in a paddy field on 20 April, and 25-day-old seedlings were subsequently transplanted into 10 L plastic pots (two plants per pot) filled with the clay soil from the paddy field. The pots were placed in a greenhouse under natural light conditions and moderate growth temperatures (28 °C day/22 °C night) until the rice plants were exposed to different temperature treatments. Rice plants grown in different pots inside the greenhouse were also uniformly managed and usually irrigated every 2–3 days, depending on requirements. Pest and disease were intensively controlled to avoid yield loss.
To verify the interrelationship of ROS concentration and various CAT isoforms in developing anthers with HT-induced fertility injury, 16 rice cultivars with a wide range of genetic backgrounds and different heat tolerance were further employed to conduct an extensional experiment in 2017. Rice seeds were sown in a paddy field and 25-day-old seedlings were transplanted into 0.8 L plastic pots filled with the clayed soil from the paddy field (two plants per pot) filled with the clay soil from the paddy field. At the meiosis stage of each rice cultivar, rice plants grown in a greenhouse (28 °C day/22 °C night) were moved into phytotrons to conduct different temperature treatments.
Growth cabinets and temperature treatments
Experiment I
The effect of HT exposure on ROS concentration, pollen viability, floret fertility, and various antioxidant enzymes in developing anthers were investigated in 2014 and again in 2016. Different temperature regimes were performed using 3 phytotrons (Model PGV-36; Conviron, Winnipeg, Canada) at the meiosis stage of rice growth. The treatment temperatures in three phytotrons were designed at 35 °C day/27 °C night (HT1, high temperature regime), 38 °C day/30 °C night (HT2, extreme high temperature regime), and 28 °C day/22 °C night (NT, normal growth temperature), respectively. At the meiosis stage, rice plants grown in different pots were randomly classified into three groups, and the representative panicles with uniformity development were selected and tagged for each group. The pots moved into three phytotrons to impose different temperature treatments (HT1, HT2 and NT). The auricle distance between the auricle of the flag-leaf (last leaf) and that of the penultimate leaf was used as a nondestructive measurement to estimate the meiosis stage of rice growth (Cao et al. 2015). The daily temperature in three phytotrons was adjusted at 08:30 and 16:30. All other climatic conditions were identical in three phytotrons. The photoperiod was from 07:00 to 19:00, with light intensity 150–180 Jm− 2s− 1, relative humidity 75–85% and wind speed 0.5 ms− 1. After rice plants exposed to different temperature regimes (HT1, HT2, and NT) for three consecutive days, fresh anthers were picked out from the florets of tagged panicles with precision forceps. 10 fresh rice anthers were used to detect ROS distribution immediately, and the other anthers were transferred rapidly into tubes cooled in liquid nitrogen and stored at − 80 °C until further experimental use. Afterward, the pots were moved back to the greenhouse until rice harvest. At anthesis, 2–3 tagged panicles were used for the determination of pollen viability (5–8 florets being detected for each temperature regime). Grain weight and spikelet fertility were measured at rice grain maturity, each tagged panicle was separately sampled for the statistical replication.
Experiment II
The effect of HT duration on ROS concentration and CAT activity in rice anthers was further investigated using two phytotrons (Model PGV–36, Conviron, Winnipeg, Canada) in 2015. One phytotron was designed for high temperature (HT, 38 °C day/30 °C night), and the other was designed for normal temperature (NT, 28 °C day/22 °C night), respectively. The daily temperature adjustment and other climatic conditions were identical in two phytotrons as mentioned above. However, the pots for each phytotron were further classified into three groups to impose different duration of temperature treatment. At the meiosis stage, the pots were moved into phytotrons and the rice plants were then subjected to HT exposure for 1, 2 and 3 days, respectively. Rice anthers were sampled from the tagged panicle after the different treatments of consecutive durations (1, 2 and 3 days), with the rice anthers in the other phytotron (NT treatment) as control. Afterward, the rice plants were moved back to the greenhouse until anthesis to detect pollen viability.
Experiment III
To clarify the impact of salicylic acid (SA) on the pollen viability, ROS accumulation, and CAT activities in anther tissue for rice plants exposed to HT, an exogenous SA spraying experiment was conducted using rice cultivar (QJ3) in 2016. Rice plants in pots were managed under husbandry greenhouse until meiosis stage and then transferred into phytotron to impose HT exposure after SA spraying. The treatment of exogenous SA was conducted at meiosis stage and implemented with four different concentrations, 500, 1000, 2000 and 4000 µM, respectively, with 4 pots for each spraying concentration. The same spraying with distilled water was considered as the control (CK-HT). Rice plants were sprayed at 9:00 am for each time spraying, with 1.5–2.0 mL spraying dosage per panicle. The air temperature in the phytotron was maintained at 38 °C between 08:30 a.m. and 04:30 p.m. for HT exposure, and adjusted to 30 °C in the other time phase every day (14:30 to 08:30). HT exposure was started after SA spraying and lasted for 3 days. After 3 days of HT exposure, rice anthers were sampled for further analysis and then all the pots in phytotron were moved back to the husbandry greenhouse until grain maturity. Meanwhile, 3–4 pots of rice plants under the husbandry greenhouse (without HT exposure) were also sprayed with distilled water as CK-NT.
Experiment IV
To clarify the genotypic-dependent difference in the response of ROS concentration to HT exposure and its relation to HT-induced spikelet injury, 16 rice cultivars with a wide range of genetic backgrounds were applied to impose different temperature treatments using two phytotrons (Model PGV–36, Conviron, Winnipeg, Canada) in 2017. One phytotron was designed for high temperature (HT, 38 °C day/30 °C night), and the other was designed for normal temperature (NT, 28 °C day/22 °C night), respectively. The daily temperature adjustment and other climatic conditions were identical in two phytotrons as mentioned in Experiment I. At the meiosis stage, rice plants of each cultivar were classified into two groups and moved into phytotrons. Rice anthers were sampled after 2 and 3 days’ duration of different temperature treatments (HT and NT). Afterward, the rice plants were moved back to the greenhouse until grain maturity. Pollen viability and spikelet fertility were measured at flowering day and grain maturity, respectively.
Detection of pollen viability and spikelet fertility
Pollen viability was detected using 1% potassium iodide/iodine solution (KI-I2) with 6–10 florets (each floret representing a replicate) opened at 1–2 h before anthesis, according to the method described by Prasad et al. (2006). For each floret, 6 anthers were squashed in 1% KI–I2 solution and observed under a light microscope (Leica, DM4000B, Germany). Pollen viability was estimated as the ratio of number of stained pollen to total number of pollen grains. Spikelet fertility (seed setting rate) and grain weigh were measured at rice maturity according to the method described by Prasad et al. (2006).
Measurement of total ROS, \({{\text{O}}_{\text{2}}}^{{ \cdot - }}\) concentration, H2O2 and MDA contents in rice anther
Total ROS was detected according to the method described by Tiwari et al. (2002) with slight modification. After treatments, fresh anthers were carefully detached from the florets of tagged panicles and washed with sterile water to remove possible ROS released by cutting. The rice anthers were incubated in 10 µM 2,7-Dichlorodi-hydrofluorescein diacetate (DCFH-DA) dissolved in 0.1% dimethyl sulfoxide at 30 °C in dark for 30 min, and then washed with HBSS (pH 7.2) subsequently. The nonfluorescent probe (DCFH-DA) was used to react with ROS to form the highly fluorescent DCF, and the imaging fluorescence intensity was observed using a confocal microscope (LSM 780, ZEISS, Germany). Dye excitation was at 488 nm, and emitted light was detected at 522 nm. Ten anthers were analyzed for each treatment. The relative fluorescence fold change was quantified by measurement of fluorescence intensity with Quantity one software.
\({{\text{O}}_{\text{2}}}^{{ \cdot - }}\) concentration, H2O2 content and MDA amount were measured using the method of Hasanuzzaman et al. (2014). Protein content was detected according to Bradford (1976) using bovine serum albumin (BSA) as a standard.
Determinations of antioxidant enzyme activity and Native-PAGE identification of their isozymes for these antioxidant enzymes
The activities of SOD, POD, and CAT were assayed according to the procedures of Hasanuzzaman et al. (2014). The activities of APX and GR were measured as described by Ozgur et al. (2014). Rice anthers were ground in liquid nitrogen to a fine powder and then homogenized in 1000 µL50 mM Tris–HCl (pH 7.8) containing 1% PVP (w/v), 1 mM PMSF, 0.1% Triton-X100 (w/v) and 0.1 mM EDTA. The extraction was performed at 4 °C. After centrifugation at 15,000g for 20 min, the supernatant solution was used as the preparation for total soluble protein, individual enzyme activity and Native-PAGE analysis. Total soluble protein content of enzyme extracts was assayed in accordance with the method of Bradford (1976). The absorbance data collections for enzymatic activity were carried out on a Shimadzu UV–vis 2450 spectrophotometer (Shimadzu, Japan). Triplicate measurements were performed for each enzyme.
Enzyme extracts containing equal amounts of protein were subjected to Native-PAGE to identify the different isozymes for SOD, POD,CAT, APX and GR. Native-PAGE/ activity staining of SOD and APX were performed on 10% (w/v, resolving gel) acrylamide slab gels as described previously (Ozgur et al. 2014). For the separation of POD, GR, and CAT isozymes, 7.5% (w/v, resolving gel) acrylamide slab gels were used, and the gels were stained using the according to the procedures of Ozgur et al. (2014). The band intensities of different isozymes were quantified using densitometry (Bio-Rad Quantity one software, Bio-Rad, USA).
Total RNA extraction, cDNA preparation and quantitative PCR
For RNA isolation, rice anthers from different florets were ground in liquid nitrogen. The RNA extractions were carried out using Trizol reagent kit (Invitrogen, Carlsbad, CA, USA) as the protocol provided by the manufacturer. Two micrograms total RNA was used as starting material for the cDNA preparation. First strand cDNA synthesis were performed using the PrimeScript™ RT reagent Kit with DNA Eraser (TaKaRa, Japan) and the cDNAs were used as templates for reverse real-time quantitative PCR (RT-qPCR) using SYBR Green real-time PCR Master Mix reagent Kit (Toyobo, Osaka, Japan). Fluorescent signals and data analyses were carried out with the Bio-Rad CFX96 real-time system (Bio-Rad, USA). All gene-specific primer pairs used in this study were listed in Supplemental Table 1. The amplification of various CAT genes were normalized by ACTIN1 expression and their relative expression levels were determined by the 2(− ΔΔCT) method (Schmittgen and Livak 2008). The mean values and standard errors were measured from triplicate independent biological replicates.
Data analysis
Statistical analysis was performed using a statistic software version SPSS 18.0 (Chicago, IL, USA). The data were submitted to variance analysis and the means were tested by least significant difference (LSD) at 0.05 probability.
Results
Relationship of HT-induced decline in pollen viability and floret fertility with ROS burst in developing anther
As shown in Fig. 1, HT exposure caused significant reduction in floret fertility and pollen viability for two rice cultivars, with the larger decrease for HT2 regime relative to HT1 one (Fig. 1a–c). However, the extent of HT-induced decrease in floret fertility and pollen viability was cultivar-dependent. The HT-susceptible cultivar (XQZ) showed relatively lower floret fertility and pollen viability than the HT-tolerant cultivar (QJ3) under the same HT regimes (HT1 and HT2) (Fig. 1b, c). Microscopic observations of rice anthers stained by green fluorescence indicted that the intensity of green fluorescence in rice anther enhanced markedly under HT exposure (Fig. 1d, e). This result suggested that HT exposure increased ROS concentration in rice anther. Just like HT-induced reduction in floret fertility and pollen viability, the effect of HT exposure on ROS intensity in rice anther was greatly variable, depending on the severe degree of HT exposure and also the susceptibility of rice cultivars to HT exposure. For instance, the elevated extent of ROS concentration in rice anther under severe HT (HT2 regime) was significantly higher than that under the moderate HT (HT1 regime), and the larger increase in ROS concentration under the same HT regimes (HT1 and HT2) was also observed for XQZ relative to QJ3 (Fig. 1d, e).
Effects of different temperature treatments on the pollen viability, floret fertility and ROS burst in developing anther. a–c Differences in pollen viability (a and b) and spikelet fertility (c) of QJ3 (heat-tolerant) and XQZ (heat-susceptible) exposed to different temperature treatments lasted for 3 days. NT, HT1 and HT2 indicated normal temperature (28 °C day/22 °C night), high temperature 1 (35 °C day/27 °C night) and high temperature 2 (38 °C day/30 °C night) treatment, respectively. d ROS distribution in rice anthers stained by 10µM DCFH-DA after 3 days of different temperature treatments. Bar 250 µm. GF, green fluorescence; DIC, differential interference contrast bright field; Merged, overlap of GF and DIC. e Green fluorescent intensity of ROS accumulation calculated by Quantity One software. Fold change indicate relative green fluorescence (intensity of green fluorescence in QJ3 rice anthers under normal temperature treatment was considered as 1). f, g and h The \({{\text{O}}_{\text{2}}}^{{ \cdot - }}\) generation rate (f), content of H2O2 (g), and content of MDA (f) in anthers of rice exposure to different temperature treatments. Error bars represent SD values of at least three replicates. Within the same cultivar, the values followed by the same letter are not significantly different (P < 0.05)
We further measured the genotypic-dependent difference in \({{\text{O}}_{\text{2}}}^{{ \cdot - }}\) concentration, H2O2 content and MDA accumulation among different temperature regimes. HT exposure increased \({{\text{O}}_{\text{2}}}^{{ \cdot - }}\) concentration, H2O2 accumulation and MDA content in rice anther (Fig. 1f–h), which was well consistent with the phenomenon observed using green fluorescein staining (Fig. 1d, e). Furthermore, HT-induced increase in \({{\text{O}}_{\text{2}}}^{{ \cdot - }}\) concentration and H2O2 content in rice anthers was also detected for all the rice cultivars with a wide range of genetic backgrounds, and the HT-susceptible cultivars generally had more substantial levels of \({{\text{O}}_{\text{2}}}^{{ \cdot - }}\) concentration and H2O2 content than the HT-tolerant cultivars (Table 1). For 16 rice cultivars, both spikelet fertility and pollen viability were statistically and negatively correlated with \({{\text{O}}_{\text{2}}}^{{ \cdot - }}\) concentration and H2O2 content in rice anther under HT exposure, although no significant correlation was observed between pollen viability and \({{\text{O}}_{\text{2}}}^{{ \cdot - }}\) concentration, and also between spikelet fertility and \({{\text{O}}_{\text{2}}}^{{ \cdot - }}\) concentration in rice anthers under NT regime (Fig. 2). Therefore, we inferred that higher ROS concentration in developing anther under HT exposure was strongly responsible for HT-induced injury in floret fertility and pollen viability, because the oxidative damage and lipid peroxidation in developing anther might be initiated by rapid ROS burst (Gill and Tuteja 2010).
Correlation analysis between fertility parameter (spikelet fertility and pollen viability) and ROS content (\({{\text{O}}_{\text{2}}}^{{ \cdot - }}\) concentration and H2O2 content). Black Square and white square indicate high temperature (38 °C day/30 °C night) and normal temperature (28 °C day/22 °C night) treatments, respectively. ** Represent significant at 0.01
Effect of HT exposure on the activities of antioxidant enzymes in developing anther for different rice cultivars
The activities of several key antioxidant enzymes, including SOD, POD, APX, GR and CAT, were assayed to compare the response of different antioxidant enzymes to HT exposure for two rice cultivars (Fig. 3). The results showed that the activities of SOD and POD in developing anther were notably depressed by HT exposure (Fig. 3a–d), whereas no obvious changes were detected for the activities of AXP and GR in response to HT exposure (Fig. 3e–h). Interestingly, two rice cultivars (XQZ and QJ3) appeared to be similar in the relative amount of various isoforms for these enzymes (SOD, POD, AXP, and GR) and also their response to HT exposure.
Effects of different temperature treatments on the activities of antioxidant enzymes in rice anthers. SOD isoenzyme patterns (a), POD isoenzyme patterns (c), APX isoenzyme patterns (e), GR isoenzyme patterns (g) and CAT isoenzyme patterns (i) in anthers of QJ3 (heat-tolerant) and XQZ (heat-susceptible) exposure to different temperature treatments lasted for 3 days. NT, HT1 and HT2 indicate normal temperature (28 °C day/22 °C night), high temperature 1 (35 °C day/27 °C night) and high temperature 2 (38 °C day/30 °C night) treatments, respectively. Total activities of SOD (b), POD (d), APX (f), GR (h) and CAT (j) in QJ3 and XQZ rice anthers. Error bars represent SD values of at least three replicates. Within the same cultivar, the values followed by the same letter are not significantly different (P < 0.05)
HT exposure had a marked influence on the CAT activity and its various isozymes in developing rice anther (Fig. 3i, j). In comparison of NT, the total CAT activity in rice anthers under the HT2 regime decreased by 79.9% for HT-tolerant cultivar (QJ3) and by 90.8% for HT-susceptible cultivar (XQZ), respectively. However, the effect of HT exposure on total CAT activity was largely variable, depending on the severity degree of HT exposure and also the susceptibility of rice cultivars to HT stress. For a comparison of different rice cultivars imposed to the same HT regime, CAT activity in the rice anthers of XQZ was relatively lower than that of QJ3, which was well coincided with the relatively higher ROS level in the anther of XQZ compared to QJ3 under HT exposure (Fig. 3i, j). Furthermore, the expression amounts of CAT protein in the rice anthers of HT-susceptible cultivar decreased more profoundly than those of HT-tolerant cultivar after 3 day’s HT exposure (HT, 38 °C day/30 °C night) (Fig. 4). Correspondingly, HT-sensitive cultivars generally had lower intensity of CAT protein bands than HT-tolerant cultivars under HT exposure (Fig. 4). These results implied that HT-induced decrease in CAT activity in rice anthers was closely and negatively associated with HT-induced increases in \({{\text{O}}_{\text{2}}}^{{ \cdot - }}\) concentration and H2O2 content in rice anther for different rice cultivars with a wide range of genetic backgrounds. Thus, we suggested that HT-induced decrease in CAT activity was strongly responsible for the ROS accumulation and oxidative damage in rice anther under HT exposure.
To clarify the relationship of HT-induced alteration in CAT activity with ROS accumulation in developing anther, we further investigated the response of CAT activity and ROS level to the duration of HT exposure (Fig. 5). The result showed that CAT activity in developing anther differed obviously among different duration of HT exposure. The shorter duration of HT exposure increased the CAT activity in rice anther, but the opposite was true for the longer duration of HT exposure at meiosis stage (Fig. 5a). Furthermore, the varying extent of CAT activity affected by the same duration of HT exposure also was greatly variable, depending on rice cultivars. For HT-susceptible cultivar (XQZ), the CAT activity in developing anther decreased by 72.1% after 2 days’ HT exposure, whereas for HT-tolerant cultivar (QJ3), the CAT activity of developing anther decreased only by 13.4% after 2 days’ HT exposure, although the markedly lowered CAT activity was observed for both rice cultivars after 3 days’ HT exposure (Fig. 5a, b).
Effects of high temperature duration on the CAT activity and ROS accumulation in rice anthers. a CAT isozymes in response to HT exposure duration for the rice anthers of QJ3 (heat-tolerant) and XQZ (heat-susceptible). NT represents normal temperature (28 °C day/22 °C night). HT-1d, HT-2d, and HT-3d indict HT exposure for 1, 2 and 3 days, respectively. b Total activity of CAT. c and d Differences in pollen viability exposed to HT duration treatments. e ROS distribution in rice anthers stained by 10 µM DCFH-DA after different days of high temperature treatments. Bar 250 µm. GF green fluorescence, DIC differential interference contrast bright field, Merged overlap of GF and DIC. f Green fluorescent intensity of ROS accumulation calculated by Quantity One software. Fold change indicate relative green fluorescence (intensity of green fluorescence in QJ3 rice anthers under NT was 1). g The content of MDA in anthers of QJ3 (heat-tolerant) and XQZ (heat-sensitive) exposed to high temperature for different days. Error bars represent SD values of at least three replicates. Within the same cultivar, the values followed by the same letter are not significantly different (P < 0.05)
The ROS level in developing anther and the pollen viability of rice floret were closely associated with the duration of HT exposure (Fig. 5c–f). In comparison of 1 day’s duration of HT exposure, the extent of increase in ROS level induced by HT exposure was more profound for the longer duration of HT exposure (HT-2d and HT-3d) (Fig. 5c–f). Meanwhile, MDA content in developing anther increased steadily with the prolonged duration of HT exposure, regardless of rice cultivars (Fig. 5g). This result indicated that a serious oxidative stress in developing anther was caused by abnormal increase in ROS concentration under the longer duration of HT exposure. Notably, the increased levels of ROS concentration and MDA content in rice anther were observed only by 1 day’s duration of HT exposure for HT-susceptible cultivar (XQZ) (Fig. 5e–g), while CAT activity in rice anther also enhanced evidently after the same duration of HT exposure (HT-1d) (Fig. 5a, b). This phenomenon implied that the shorter duration of HT exposure may lead to a transient increase in CAT activity in rice anther. However, the rising extent of CAT activity induced by the shorter duration of HT exposure possibly was not sufficient to scavenge the rapid ROS generation and oxidative damage in rice anther after the same duration of HT exposure, thereby the lowered pollen viability was detected for 1 day’s duration of HT exposure.
Effect of HT exposure on the transcriptional expression of various CAT genes in developing anther for different rice cultivars
In rice, CATs are generally encoded by a small multi-gene family consisting of three isozyme genes, namely OsCATA, OsCATB, and OsCATC (Iwamoto et al. 2000). To verify the interplay between CAT expression and ROS concentration in developing anthers under HT exposure at meiosis stage, HT-induced alteration in the transcriptional expression of various CAT genes in rice anther were investigated by quantitative real-time reverse transcription (qRT)-PCR (Fig. 6). The result showed that HT exposure had a considerable impact on the transcriptional expression of various CAT genes in developing anthers, although the pattern and extent of various CAT genes in response to HT exposure were greatly variable and also dependent on rice genotypes. For instance, OsCATB transcript was notably repressed by HT exposure (HT1 and HT2), whereas OsCATA expression was significantly down-regulated only by HT2 regime, with the up-regulated transcript for OsCATA under HT1 regime (Fig. 6a, b). In comparison of HT-tolerant cultivar (QJ3), HT-susceptible cultivar (XQZ) had relatively higher amount of OsCATB transcript under NT regime, but the transcript of OsCATB in the anther of XQZ decreased sharply to an extremely low level under HT2 regime (Fig. 6a, b). Interestingly, the temperature effect on the transcripts of OsCATA and OsCATB was greatly variable among different duration of HT exposure (Fig. 6c, d). For HT-susceptible cultivar (XQZ), OsCATB transcript was evidently depressed after 2 days’ duration of HT exposure, while OsCATA transcript was substantially depressed only by 3 days’ duration of HT, with a moderate increase in OsCATA transcript for 2 days’ duration of HT exposure (Fig. 6c, d). This result implied that the transcriptional expression of OsCATB in developing anther tended to be more susceptible to HT exposure than that of OsCATA.
Effects of HT exposure on the transcriptional expression of various CAT genes in developing anther. a and b Expression of CATA and CATB in anthers of rice exposed to different temperature treatments lasted for 3 days. NT, HT1 and HT2 indicate normal temperature (28 °C day/22 °C night), high temperature 1 (35 °C day/ 27 °C night) and high temperature 2 (38 °C day/30 °C night) treatment, respectively. c and d Expression of CATA and CATB in anthers in response to exposure duration of HT2 treatment (38 °C day/ 30 °C night). NT represents normal temperature (28 °C day/22 °C night). HT-1d, HT-2d, and HT-3d indicate the exposure duration of HT2 treated for 1, 2 and 3 days, respectively). Error bars represent SD values of at least three replicates. Within the same cultivar, the values followed by the same letter are not significantly different (P < 0.05). e Tissue-specific expression of CATA, CATB and CATC in QJ3
We further examined the tissue-specific expression of three CAT genes (OsCATA, OsCATB and OsCATC) in rice plants using QJ3 under NT growth (Fig. 6e). OsCATA expressed constitutively in most tissues of rice plants, including stem, leave, anther, and seed. In contrast, OsCATB expressed preferentially in anther and weakly in leaf and stem, whereas OsCATC expressed exclusively in leaf and sheath, with an extremely low expression or no detectable levels in anther and seed. This result clearly indicated that OsCATB was one of dominant isoforms expressed highly in the anther tissue of rice plants. Considering their difference in the susceptibility of two CAT genes (OsCATA and OsCATB) at transcriptional level in response to HT exposure, we inferred that HT-induced decline in CAT activity in rice anther was mainly attributed to the rapid suppression of OsCATB transcript induced by HT exposure, which was among the regulatory switch steps leading to the elevated ROS concentration and reduced pollen viability for rice plants subjected to HT exposure at meiosis.
Linking the exogenous SA-mediated alleviation for the heat injury of spikelet fertility and pollen viability with ROS generation and CAT expression in developing anther
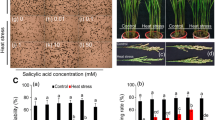
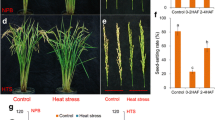
The alleviating effect of exogenous SA application on HT-induced injury was greatly variable, depending on SA concentration (Fig. 7). In comparison of CK-HT (without SA spraying before the implement of 3 days’ HT exposure), the stained pollen percentage and pollen viability in rice florets were obviously enhanced by the SA spraying of low concentration (500 and 1000 µM), whereas an insignificant difference and even opposite trend were observed between the SA treatments and CK-HT for the SA spraying of high concentration (2000 and 4000 µM) (Fig. 7a, b). This result indicated that the HT-induced injury for pollen viability may be partly alleviated by the SA spraying of appropriate concentration, although this alleviating extent was not as great as the impact of HT exposure on spikelet fertility and pollen viability, as reflected by more considerable difference in pollen viability between CK-NT and CK-HT (Fig. 7a, b). Interestingly, under HT exposure, the SA-modulated regulation to pollen viability and spikelet fertility was closely associated with ROS concentration, MDA content and CAT activity in developing anthers (Fig. 7). For instance, the SA spraying of 1000 µM concentration resulted in a reduction of ROS concentration and MDA content in rice anthers, which was well coincident with the corresponding alleviation for the lowered pollen viability under HT exposure by 1000 µM SA concentration. On the other hand, the SA spraying of higher concentration (2000 and 4000 µM) contributed slightly to the alleviation of HT-induced pollen injury, in which higher ROS concentration and lower pollen viability were found for the treatment of high SA concentration (4000 µM) relative to CK-HT (Fig. 7a–e). This phenomenon indicated clearly that the exogenous SA-mediated alleviation for HT-induced fertility injury was closely associated with ROS detoxification and also relief of oxidative stress in developing anthers.
Impact of salicylic acid (SA) on the pollen viability, ROS accumulation and CAT activities in anther for rice plants exposed to high temperature. a and b Effects of different concentrations of exogenous SA on pollen fertility exposure to high temperature. CK-NT represent QJ3 plants sprayed with distilled water under normal temperature. CK-HT, 500-HT, 1000-HT, 2000-HT and 4000-HT represent plants exposed to high temperature (38 °C day/30 °C night) and sprayed with distilled water, 500 µM SA, 1000 µM SA, 2000 µM SA and 4000 µM SA, respectively. c ROS distribution in rice anthers stained by 10 µM DCFH-DA after spraying of exogenous SA treatments. Bar 250 µm. GF green fluorescence, DIC differential interference contrast bright field, Merged overlap of GF and DIC. d Green fluorescent intensity of ROS accumulation calculated by Quantity One software. Fold change indicated relative green fluorescence (green fluorescence intensity of CK-NT treatment was 1). e The content of MDA in anthers of QJ3 exposure to heat stress with different concentrations of exogenous SA. f and g CAT isoenzyme patterns and total activities in anthers of QJ3 exposure to heat stress with different concentrations of exogenous SA. h and i Expression of CATA and CATB in rice anther of QJ3 exposure to heat stress with different concentrations of exogenous SA. Error bars represent SD values of at least three replicates. Within the same cultivar, the values followed by the same letter are not significantly different (P < 0.05)
Under HT exposure, exogenous SA treatment evidently affected CAT activity in developing anther (Fig. 7f, g). the spraying of 1000 µM SA concentration significantly enhanced CAT activity compared with the control group without SA spraying under HT exposure (CK-HT) (Fig. 7g), which agreed with the lowering levels of ROS concentration and MDA content for the same spraying concentration (1000 µM) (Fig. 7d, e). This result suggested that CAT contributed to the SA-mediated alleviation for the heat injury of spikelet fertility and pollen viability by regulating ROS level in developing anther. For the response of two CAT genes (OsCATA and OsCATB) to SA spraying, the transcript level of OsCATB was evidently enhanced by SA treatment at a low concentration (500 µM), while OsCATA transcript did not vary substantially under the same SA concentration (500 µM). This result implied that the transcript of OsCATB was more easily induced by the SA spraying of low concentration than that of OsCATA. However, OsCATB transcript was detectable at a relatively lower level than OsCATA transcript for the SA spraying of high concentration (4000 µM). Such diversity possibly played a complementary role in scavenging ROS accumulation at various ROS concentrations.
Discussion
Meiosis in the developing anthers of rice florets is one of the most crucial stages for spikelet fertility and is extremely sensitive to stressful environments (Matsui and Omasa 2002; Endo et al. 2009; Krishnan et al. 2011). A short episode (2–3 days) of HT stress (> 35 °C, daytime maximum temperature) at the meiosis stage can induce floret sterility and thus limit grain yield (Krishnan et al. 2011; Das et al. 2014). In contrast with HT-induced fertility injury at the flowering stage, an increase in floret sterility under HT exposure at meiosis stage mainly results from abnormal floret development and pollen production obstacle in the anther tissue, thereby HT exposure at meiosis stage severely impaired pollen viability and pollen abortion (Endo et al. 2009; Krishnan et al. 2011). In this paper, the effects of HT injury on pollen viability and floret fertility were investigated at meiosis stage, with different degrees and durations of HT exposure. Our results revealed that the pollen viability of XQZ (HT-susceptible cultivar) considerably decreased after 1 day of exposure to 38 °C at meiosis stage (Fig. 5c, d). In addition, the extent of HT-induced injury for pollen viability and floret fertility increased with the elevation of HT degree and also the prolong of HT duration (Figs. 1, 5). This result was basically concurrent with several previous studies on HT exposure during anthesis (flowering stage), which concluded that the impact of HT exposure on spikelet fertility wasmarkedly variable depending on HT severity and also rice cultivars (Jagadish et al. 2007). Moreover, HT-susceptible cultivars show higher sensitivity of pollen viability to HT exposure compared with HT-tolerant cultivars in a manner independence of leaf photosynthesis (Prasad et al. 2006; Krishnan et al. 2011; Zhang et al. 2015). In the present work, dyes (DCFH-DA) staining was used to examine the interplay between ROS accumulation in developing anther and pollen fertility for rice plants imposed to HT stress at meiosis stage. This technique helps not only in detecting ROS accumulation, but also in observing its localization in the anthers. Our observation strongly supported several previous findings that HT exposure enhanced ROS production and MDA content in tomato anthers (Frank et al. 2009). Our present results demonstrated that the HT-susceptible cultivar showed relatively higher ROS concentration in the anther of rice florets than the HT-tolerant cultivar under the same HT regimes (Fig. 1; Table 1). Moreover, ROS level in rice anthers significantly increased with the degree and duration of HT exposure, which was well coincidence with the decreasing pollen viability and increasing floret sterility induced by HT exposure during meiosis (Figs. 1, 5). In previous studies, ROS accumulation in plant organ decreased membrane thermo-stability and increased lipid peroxidation in cell membranes (Krishnan et al. 2011; Hasanuzzaman et al. 2014). The CMS line had significantly higher ROS concentration in rice anthers than its corresponding maintainer line (Li et al. 2004; Wan et al. 2007); ROS may be a signal for aberrant PCD during anther development for CMS, thus leading to floret sterility (Wan et al. 2007). Hence, HT-induced ROS accumulation in the developing anther of rice florets is strongly responsible for the decreasing pollen viability and increasing floret sterility rate under HT exposure during meiosis, because excessive ROS level may cause the occurrence of oxidative damage and lipid peroxidation in rice anther tissues (Gill and Tuteja 2010). According to Nguyen and Sutton (2009), the interplay between PCD and oxidative stress in anthers may be a cause of pollen sterility in drought-stressed rice. In our present result, HT exposure during meiosis significantly increased MDA content in the developing anther of rice florets (Figs. 1h, 5g), this phenomenon also provide a supporting evidence for our conclusion.
Extensive studies had demonstrated that the maintenance of high antioxidant ability was closely associated with the heat tolerance of various plant species and their different organs (Hasanuzzaman et al. 2014; Zhang et al. 2015). The overexpression of ROS scavenging enzymes, such as SOD, CAT, and APX, resulted in abiotic stress tolerance in various crop plantsbecause of efficient ROS scavenging capacity (Krishnan et al. 2011; Hasanuzzaman et al. 2014). In this paper, HT exposure during meiosis markedly affected the protein expressions and activities of SOD, CAT and POD in developing anthers, but no obvious change in the response of their protein bands and enzymatic activities to HT exposure was observed for APX and GR (Fig. 3). For different rice cultivars, QJ3 (HT-tolerant cultivar) did not differ obviously from XQZ (HT-susceptible cultivar) in the band intensities and activities of APX and GR under the same HT regime (Fig. 3). These results implied that the activities of APX and GR in rice anthers were relatively less susceptible to HT exposure during meiosis than those of CAT and SOD in rice anthers. In previous reports, the ascorbate–glutathione cycle (AsA–GSH cycle) was thought to perform a fundamental critical function in the redox protection of different plant organs and many cellular compartments, particularly for ROS detoxification in the chloroplasts of photosynthetic organisms in plants (Asada 1997). In non-photosynthetic tissues of various plants, mitochondria and peroxisomes were the major cellular sources of ROS. Recently, Mhamdi et al. (2010) revealed that the function of APX in scavenging H2O2 in plants might be partly compensated by CAT in mitochondria and peroxisomes. Considering the relatively stable activities of APX and GR in rice anthers responsive to HT exposure as presented here (Fig. 3), we inferred that the AsA–GSH cycle did not play a predominant role in the regulation and detoxification of HT-induced ROS burst among the antioxidant enzyme system in developing rice anther. Compared with APX, CAT catalyzes H2O2 into H2O in an energy-efficient manner (Gill and Tuteja 2010). In addition, CATexhibits one of the highest turnover rates among all the antioxidant enzymes, and it plays an indispensable role in the detoxification of excess H2O2 levels under stressful environments (Willekens et al. 1997; Mhamdi et al. 2010). In previous studies, a significant increase in CAT activity after heat stress has been reported in Sinapis alba L. seedlings (James et al. 1998). However, no significant difference in the CAT activities of rice flag leaves was observed between heat-treated and control plants in response to HT exposure (Zhang et al. 2015). In grapevine, the CAT activities in leaf tissues markedly decreased after heat stress (Wang et al. 2010). Our present results revealed that the impact of HT exposure on the protein expression and enzymatic activity of CAT in developing rice anther was highly variable depending on the severity and duration of stressful HT, in addition to the differential behavior under the same HT regime between the HT-tolerant cultivar (QJ3) and HT-susceptible cultivar (XQZ) (Figs. 3, 5). In general, the shorter duration of HT exposure (1 day) increased the CAT activity in rice anther, whereas the longer duration of HT exposure (2–3 days) during meiosis (Fig. 5a, b) yielded the opposite effect. By contrast, CAT activity in the anther of XQZ was relatively lower than that of QJ3 under normal growth (NT), and severe HT exposure (HT2 regime) considerably decreased CAT activity in XQZ relative to QJ3, in terms of the altering extent between HT2 and NT (Fig. 3i). This result indicates that CAT activity in HT-susceptible cultivar (XQZ) is more sensitive to HT exposure than that in HT-tolerant cultivar (QJ3), which was well consistent with the cultivar-dependent alteration in ROS level and MDA content in developing anthers (Fig. 1; Table 1). In a previous study, CAT activity in a sterile male line was distinctly lower than that in the maintainer, and this change trend was negatively related to ROS level in rice anthers (Govindaraj 1986). Our present results provided strong evidence that CAT activity in rice anthers is closely associated with pollen viability and floret fertility in rice plants. Considering the effect of HT exposure on CAT activity in rice anthers and its relation to ROS level and floret fertility in this work, we inferred that the HT-induced decrease in CAT activity is strongly responsible for ROS detoxification in rice anthers when rice plantsare imposed to HT stress during meiosis. Under HT exposure, HT-induced ROS generation exceeds the antioxidant endurance ability because of a severe decrease in CAT activity; the decreased CAT activity leads to oxidative stress, which subsequentlydiminishes pollen viability and increases floret sterility. Furthermore, the contribution of CAT activity to ROS detoxification in developing anthers and its relation to floret fertility and pollen viability could be further confirmed by an exogenous SA spraying experiment, in which the SA-induced increase in CAT activity alleviated heat injuryto spikelet fertility and pollen viability by regulating the ROS level in developing anthers (Fig. 7).
Previous studies proposed that each CAT isoform performs a unique function in scavenging H2O2 in different tissues or species, because of isoform specificity in substrate binding affinity, preferential organ/tissue expression, and development stage (Iwamoto et al. 2000; Mhamdi et al. 2010). In rice, the small CAT family comprises three isoforms, namely OsCATA, OsCATB and OsCATC (Iwamoto et al. 2000; Horváth et al. 2002). Our results showed that OsCATB is one of the dominant isoforms that are highly expressed in the anther tissue of rice plants (Fig. 6e). Moreover, OsCATB differed evidently from OsCATA in the response of their transcript expression to HT exposure (Fig. 6a–d) and in the SA spraying experiment of different concentrations (Fig. 7h, i), in which the transcriptional amount of OsCATB in developing anthers appeared to be more susceptible to HT exposure than that of OsCATA (Fig. 6a–d). Furthermore, the upregulation or downregulation of OsCATB transcript was closely associated with the change of CAT activity in developing anthers among different treatments (Figs. 3, 5). This observation implies HT-induced regulation of CAT activity in developing anthers at the transcription level. Hence, HT-induced decline in CAT activity in rice anthers can be mainly attributable to the rapid suppression of OsCATB transcript induced by HT exposure, which was among the regulatory switch steps leading to the elevated ROS accumulation and reduced pollen viability in rice plants subjected to HT exposure during meiosis.
Sulfosalicylic acid, a derivative of SA, can effectively remove ROS and increase the heat tolerance of several plants (Mohammed and Tarpley 2011). In plant leaves, SA enhances HT-tolerance (James et al. 1998; Wang et al. 2010), in which Ca2+ homeostasis and antioxidant systems are thought to be involved (Klessig and Malamy 1994; Hayat et al. 2010). Previous studies revealed that SA treatment could enhance the activities of several antioxidant enzymes (CAT, POD, and SOD) in the leaves of drought stressed L. esculentumor and salinity stressed B. juncea (Hayat et al. 2010). Krantev et al. (2008) reported that the exogenous application of SA increased the activities of antioxidant enzymes (CAT and SOD) with a concomitant decrease in the activity of APX in maize plants. According to Panda and Patra (2007), seedpriming with low concentrations of SA prior to sowing decreased ROS accumulation induced by cadmium (Cd) exposure and also enhanced the activities of various antioxidant enzymes (CAT, SOD, and GR) in Oryza sativa, thereby protecting rice seedlings from oxidative burst. Similar findings were previously observed on barley (Metwally et al. 2003). However, other reports suggested that exogenous SA treatments induced an increase in H2O2 levels and caused a decline in the activity of antioxidant enzymes (CAT and SOD) in plant tissues. These outcomes are partly supported by the results of Choudhury and Panda (2004), who concluded that the activities of CAT and SOD in rice seedlings were significantly decreased by soaking rice seeds in SA. In our present results, plants sprayed with 1000 µM SA showed significantly higher CAT activity in rice anthers compared with the control group when subjected to HT (CK-HT) (Fig. 7g), which was well coincided with the lowering levels of ROS accumulation and MDA content in rice anthers at the same spraying concentration (1000 µM). This phenomenon suggests that SA application alleviates heat injury of pollen viability because of the enhanced antioxidant capacity in developing anthers, as reflected by the SA-mediated change in OsCATB transcript and CAT activity (Fig. 7i). However, OsCATB transcript was detectable at a relatively low level with spraying at a high concentration (4000 µM), accompanied by the markedly lowered CAT activity in developing anthers (Fig. 7). This result indicates that SA application of higher concentration (4000 µM) decreases the antioxidant capacity of developing anthers. Thus, ROS injury could be not effectively alleviated by higher SA concentration. Interestingly, the SA spraying of 2000 µM concentration showed relatively higher levels of CAT activity and OsCATB transcript in developing anthers than CK-HT (Fig. 7g, i), while the insignificant difference in pollen viability and ROS amount was found between the two treatments under HT exposure (Fig. 7a–d). This phenomenon could be explained by the possibility that the exogenous application of SA under certain concentration (2000 µM) play a dual role on ROS homeostasis in developing anther. In this case, the contribution of SA-induced increase in CAT activity to ROS scavenging may be completely offset by the additional ROS generation under relatively higher SA concentration (2000 µM), thereby the SA spraying of 2000 µM concentration have little impact on pollen viability and ROS amount in developing anther. Indeed, several previous studies have demonstrated that excessive SA application can trigger PCD thought the stimulation of ROS generation (Rao and Davis 1999; Matsumura et al. 2003). In Arabidopsis, Kawaiyamada et al. (2004) have found that SA may motivate the expression of AtBI-1 elicitor and accelerate plant cell death via the increasing ROS generation. Further research is necessary to investigate the mechanism by which SA alleviates HT-induced ROS burst and fertility injury by regulating CAT activity under HT exposure in developing anther.
Author contribution statement
FC, QZ and LZ conceived and designed research. QZ, LZ, JL, ZC and FH conducted experiments. XD and GP analyzed data. FC and QZ wrote the manuscript.
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- CMS:
-
Cytoplasmic male sterility
- GR:
-
Glutathione reductase
- HT:
-
High temperature
- MDA:
-
Malondialdehyde
- NT:
-
Normal temperature
- PCD:
-
Programmed cell death
- POD:
-
Peroxidase
- ROS:
-
Reactive oxygen species
- SA:
-
Salicylic acid
- SOD:
-
Superoxide dismutase
References
Asada K (1997) The role of ascorbate peroxidase and monodehydro ascorbte reductase in H2O2 scavenging in plants. Cold Spring Harb Monogr Arch 34:715–735
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cao Z, Zhao Q, Huang F, Wei K, Zaidi S, Zhou W, Cheng F (2015) Effects of high temperature at anthesis on spikelet fertility and grain weight in relation to floral positions within a panicle of rice (Oryza sativa L.). Crop Pasture Sci 66:922–929
Choudhury S, Panda SK (2004) Role of salicylic acid in regulating cadmium induced oxidative stress in Oryza sativa L. roots. Bulg J Plant Physiol 30:95–110
Das S, Krishnan P, Nayak M, Ramakrishnan B (2014) High temperature stress effects on pollens of rice (Oryza sativa L.) genotypes. Environ Exp Bot 101:36–46
Endo M, Tsuchiya T, Hamada K, Kawamura S, Yano K, Ohshima M, Higashitani A, Watanabe M, Kawagishi-Kobaya M (2009) High temperatures cause male sterility in rice plants with transcriptional alterations during pollen development. Plant Cell Physiol 50:1911–1922
Foyer CH, Noctor G (2000) Oxygen processing in photosynthesis: regulation and signaling. New Phytol 146:359–388
Frank G, Pressman E, Ophir R, Althan L, Shaked R, Freedman M, Shen S, Firon N (2009) Transcriptional profiling of maturing tomato microspores reveals the involvement of heat shock proteins, ROS scavengers, hormones, and sugars in the heat stress response. J Exp Bot 60:3891–3908
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Govindaraj K (1986) Biochemical characterization of normal and male sterile anthers in rice (Oryza sativa L.). Indian J Genet Plant Breed 1:13–15
Hasanuzzaman M, Nahar K, Alam MM, Fujita M (2014) Modulation of antioxidant machinery and the methylglyoxal detoxification system in selenium-supplemented Brassica napus seedlings confers tolerance to high temperature. Biol Trace Elem Res 161:297–307
Hayat Q, Hayat S, Irfan M, Ahmad A (2010) Effect of exogenous salicylic acid under changing environment: a review. Environ Exp Bot 68:14–25
Horváth E, Janda T, Szalai G, Páldi E (2002) In vitro salicylic acid inhibition of catalase activity in maize: differences between the isozymes and a possible role in the induction of chilling tolerance. Plant Sci 163:1129–1135
Iwamoto M, Higo H, Higo K (2000) Differential diurnal expression of rice catalase genes: the 5′-flanking region of CatA is not sufficient for circadian control. Plant Sci 151:39–46
Jagadish SVK, Craufurd PQ, Wheeler TR (2007) High temperature stress and spikelet fertility in rice (Oryza sativa L.). J Exp Bot 58:1627–1635
James DF, Foyer CH, Scott IM (1998) Parallel changes in H2O2 and catalase during thermo-tolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol 116:1351–1357
Janda T, Szalai G, Tari I, Paldi E (1999) Hydroponic treatment with salicylic acid decreases the effect of chilling injury in maize (Zea mays L.) plants. Planta 208:175–180
Jiang P, Zhang Y, Zhu W, Zhu H, Wang X (2007) Metabolism of reactive oxygen species in cotton cytoplasmic male sterility and its restoration. Plant Cell Rep 26:1627–1634
Kawaiyamada M, Ohori Y, Uchimiya H (2004) Dissection of Arabidopsis Bax inhibitor-1 suppressing Bax-, hydrogen peroxide-, and salicylic acid-induced cell death. Plant Cell 16:21–32
Klessig DF, Malamy J et al (1994) The salicylic acid signal plants. Plant Mol Biol 26:439–1458
Krantev A, Yordanova R, Janda T, Szalai G, Popova L (2008) Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J Plant Physiol 165:920–931
Krishnan P, Ramakrishnan B, Reddy KR, Reddy VR (2011) High-temperature effects on rice growth, yield, and grain quality. Adv Agron 111:87–206
Li S, Wan C, Kong J, Zhang Z, Li Y, Zhu Y (2004) Programmed cell death during microgenesis in a Honglian CMS line of rice is correlated with oxidative stress in mitochondria. Funct Plant Biol 31:369–376
Matsui T, Omasa K (2002) Rice (Oryza sativa L.) cultivars tolerant to high temperature at flowering: anther characteristics. Ann Bot 89:683–687
Matsumura H, Nirasawa S, Kiba A, Urasaki N, Saitoh HM, Kawai-Yamada M, Uchimiya H, Terauchi R (2003) Overexpression of Bax inhibitor suppresses the fungal elicitor-induced cell death in rice (Oryza sativa L.) cells. Plant J Cell Molecular Biol 33:425–434
Metwally A, Finkemeier I, Georgi M, Dietz KJ (2003) Salicylic acid alleviates the cadmium toxicitybarley seedlings. Plant Physiol 1321:272–281
Mhamdi A, Queval G, Chaouch S, Vanderauwera S, Breusegem FV, Noctor G (2010) Catalase function in plants: a focus on Arabiopsis mutants as stress-mimic models. J Exp Bot 61:4197–4220
Mohammed AR, Tarpley L (2011) Effects of night temperature, spikelet position and salicylic acid on yield and yield-related parameters of rice (Oryza sativa L.) plants. J Agron Crop Sci 33:117–123
Nguyen GN, Sutton BG (2009) Water deficit reduced fertility of young microspores resulting in a decline of viable mature pollen and grain set in rice. J Agron Crop Sci 195:11–18
Ozgur R, Turkan I, Uzilday B, Sekmen AH (2014) Endoplasmic reticulum stress triggers ROS signaling, changes the redox state, and regulates the antioxidant defence of Arabidopsis thaliana. J Exp Bot 65:1377–1390
Panda SK, Patra HK (2007) Effect of salicylic acid potentiates cadmium-induced oxidative damage in Oryza sativa L. leaves. Acta Physiol Plant 29:567–575
Prasad PVV, Boote KJ, Allen JLH, Sheehy JE, Thomas JMG (2006) Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. Field Crop Res 95:398–411
Rao MY, Davis KR (1999) Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: the role of salicylic acid. Plant J 17:603–614
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protocols 3:1101–1108
Selote DS, Khanna-Chopra R (2010) Antioxidant response of wheat roots to drought acclimation. Protoplasma 245:153–163
Teixeira EI, Fischer G, Velthuizen HV, Walter C, Ewert F (2013) Global hot-spots of heat stress on agricultural crops due to climate change. Agric Forest Meteorol 170:206–215
Tiwari BS, Belengh B, Levine A (2002) Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiol 128:1271–1281
Wan CX, Li SQ, Wen L, Kong J, Wang K, Zhu YG (2007) Damage of oxidative stress on mitochondria during microspores development in Honglian CMS line of rice. Plant Cell Rep 26:373–382
Wang LJ, Fan L, Loescher W, Duan W, Liu GJ, Cheng JS, Luo HB, Li SH (2010) Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biol 10:34
Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C (1997) Catalase is a sink for H2O2 and is indispensable for stress defense in C3 plants. Embo J 14:4806–4816
Zhang C, Fu G, Yang X, Yang Y, Zhao X, Chen T, Zhang X, Jin Q, Tao L (2015) Heat stress effects are stronger on spikelets than on flag leaves in rice due to differences in dissipation capacity. J Agron Crop Sci 202:394–408
Acknowledgements
The authors are indebted to National Key Research and Development Plan of China (No. 2016YFD0300502), National Natural Science Foundation of China (No. 31571602) and Zhejiang Provincial Natural Science Foundation of China (LZ15C130001) for its financial support to this research project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Kang Chong.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, Q., Zhou, L., Liu, J. et al. Involvement of CAT in the detoxification of HT-induced ROS burst in rice anther and its relation to pollen fertility. Plant Cell Rep 37, 741–757 (2018). https://doi.org/10.1007/s00299-018-2264-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-018-2264-y