Abstract
Key message
SbAP37 transcription factor contributes to a combination of abiotic stresses when applied simultaneously in rice. It modulates a plethora of proteins that might regulate the downstream pathways to impart salt stress tolerance.
Abstract
APETALA type of transcription factor was isolated from Sorghum bicolor (SbAP37), overexpressed in rice using a salt inducible abscisic acid 2 (ABA2) promoter through Agrobacterium tumefaciens following in planta method. Transgenics were confirmed by PCR amplification of SbAP37, hygromycin phosphotransferase (hptII) marker and ABA2 promoter and DNA blot analysis. Plants were exposed to 150 mM NaCl coupled with high day/night 36 ± 2/25 ± 2 °C temperatures and also drought stress by withholding water for 1-week separately at the booting stage. SbAP37 expression was 2.8- to 4.7-folds higher in transgenic leaf under salt, but 1.8- to 3.3-folds higher in roots under drought stress. Native gene expression analysis showed that it is expressed more in stem than in roots and leaves under 150 mM NaCl and 6% PEG stress. In the present study, proteomic analysis of transgenics exposed to 150 mM NaCl coupled with elevated temperatures was taken up using quadrupole time-of-flight (Q-TOF) mass spectrometry (MS). The leaf proteome revealed 11 down regulated, 26 upregulated, 101 common (shared), 193 newly synthesized proteins in transgenics besides 368 proteins in untransformed plants. Some of these protein sets appeared different and unique to combined stresses. Our results suggest that the SbAP37 has the potential to improve combined stress tolerance without causing undesirable phenotypic characters when used under the influence of ABA2 promoter.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa) is highly sensitive to salt and drought stresses among cereals. Salinity coupled with high temperature affects plants by closure of stomatal apertures and subsequently reduces photosynthetic rate. This situation increases the formation of reactive oxygen species (ROS) creating an oxidative stress. Saline soils cause ion (Na+ and Cl−) toxicity, low osmotic potential, and changes in nutrient uptake leading to major disturbances such as photosynthetic carbon gain and leaf growth rate (Munns 2005). In rice, exposure to drought coupled with high temperature conditions during the stages of panicle emergence and development results in delayed flowering time, reduced number of fertile spikelets and poor grain filling. Closure of stomata leads to restriction in CO2 uptake and photosynthetic electron transport chain becomes over-reduced leading to generation of ROS (Apel and Hirt 2004). In the field conditions, these stressors occur simultaneously with high day/night temperatures and high natural sunlight in tropics. Little attention has been paid how combination of stresses impact the proteome changes (Li et al. 2011). In wheat (Harrington and Alm 1988) and tobacco (Lei et al. 2005), some common elements exist in the cross tolerance to salt stress and heat shock. Some shared adaptation responses and some unique responses were also noticed earlier by Prasch and Sonnewald (2013) under combined abiotic stresses. On the other hand, Sewelam et al. (2014) reported that mechanisms such as production and detoxification of ROS are shared by multiple stresses.
Apetala2/ethylene responsive factor (AP2/ERF) class of transcription factors are a large family and associated with both biotic and abiotic stress tolerance besides development (Sharoni et al. 2011). Based on the presence of one or two AP2-DNA binding domains, the family is divided into four subfamilies, the AP2, dehydration-responsive element binding (DREB), ethylene responsive factor (ERF), ABI3/VP1 (RAV) and others. AP2 sub-family (Sakuma et al. 2002) encodes proteins with two AP2 domains and these proteins are implicated in various growth events (Krizek 2009; Licausi et al. 2013). Around 145 members of this family have been identified in Arabidopsis and 167 in rice (Nakano et al. 2006). The DREB, ERF, and RAV subfamily genes encode proteins with only one AP2 domain and members of these subfamilies are implicated in stress signaling network (Guo et al. 2005). Todaka et al. (2012) pointed out that abscisic acid (ABA)-dependent and ABA-independent osmotic stress signaling components modify constitutively expressed transcription factors. Transcription factors then activate several of the downstream stress related genes. AP2 transcription factors regulate stress-related signaling, although DREB types are involved in ABA-independent abiotic stress responses (Lin et al. 2007; Sharoni et al. 2011). Oh et al. (2009) functionally characterized AP37 (subgroup I), AP59 (subgroup II) and showed that they increase tolerance to drought when applied individually and improves grain yield in rice. Yang et al. (2016) demonstrated drought tolerance in Arabidopsis thaliana by overexpressing a novel AP2/ERF transcription factor from Stipa purpurea. Experiments by Oh et al. (2009) were carried out using a constitutive promoter in Japonica rice, but this has not been shown using stress/ABA inducible promoter. Therefore, a stress inducible promoter (ABA2) isolated from indica rice was used in the present study which can hopefully mitigate the stress better compared to constitutive promoter. Present study was undertaken to generate transgenic rice plants overexpressing SbAP37 isolated from Sorghum bicolor. The objective was to evaluate transgenic rice plants under a salt-inducible promoter ABA2 for tolerance to NaCl coupled with high day/night temperatures. Microarray experiments were performed to find out the global picture of the genes that are upregulated by AP37 when compared to the untransformed controls by Oh et al. (2009) under a single abiotic stress. However, proteomics is a better tool since transcriptional and post-transcriptional modifications occur which would not be revealed by genomic studies. In the field conditions, different abiotic stresses occur concurrently and proteomic changes associated with such combination stresses are rare (Li et al. 2014). But, it is crucial for us to learn the dynamics of proteome at the booting stage of the plants under combination of stresses to create climate resilient crops. Accordingly, transgenics were subjected to a combination of stresses concurrently. Therefore, to gain a global insight about the spectrum of proteins that are modulated by SbAP37, quadrupole time-of-flight (Q-TOF) analysis was performed in transgenics and untransformed controls (UC) grown under 150 mM NaCl combined with 36 ± 2/25 ± 2 °C day/night temperatures, respectively, and functionally categorized. A major chunk of these proteins represent molecular chaperones, histone/DNA synthesis/repair, carbohydrate and hormone metabolism, while some of them are different and unique to the combination of stresses.
Materials and methods
Isolation of SbAP37 gene from Sorghum
Total RNA was isolated from leaf tissues of Sorghum bicolor (L.) variety BTX623 using TRIZOL reagent (Invitrogen, USA). Single stranded cDNA was synthesized with reverse transcriptase, according to the manufacturer’s instructions (Thermo, Germany). Using NCBI databank, AP37 gene sequence (core nucleotide sequences of mRNA/cDNA) from rice was used to localize the SbAP37 gene in Sorghum bicolor genome by Blast-par tool (http://hpc.icrisat.cgiar.org/Pise/5.a/blast/blastpar-simple.html) and position of the gene was retrieved, which is on chromosome 3. From the BLAST (Altschul et al. 1990) search output, using chromosome 3 sequence, 3 kb upstream and 3 kb downstream of full length SbAP37 cDNA sequence was obtained from genome sequence using GENSCAN software (Burge and Karlin 1998) which gives only the coding sequence. Making use of the coding sequence, end to end primers were designed to amplify full length SbAP37 gene by polymerase chain reaction (PCR) as per the conditions mentioned in Supplementary Table 1. For amplification, 50 ng of cDNA, 10 pmol each of SbAP37 forward and reverse primers (sequences shown in Supplementary Table 1), 10 mM dNTP, 1.5 mM MgCl2 and 1 U Taq polymerase per 25 µl reaction volume was used. Amplified product was eluted, ligated into pTZ57R/T vector and sequenced. Using full length SbAP37 genomic sequence, it was characterized for number of exons, introns, and their length by Gene Structure Display Server (GSDS) tool.
Isolation of ABA2 promoter and vector construction
Using full length forward and reverse primers, ABA2 promoter was amplified from rice with PCR conditions as shown in Supplementary Table 1. It was cloned first into pTZ57R/T vector. Genomic DNA was isolated from the leaves of rice plants using CTAB extraction method (Doyle and Doyle 1990). The DNA pellet was dissolved in 100 µl of TE buffer and stored at −20 °C. PCR amplified product was eluted from the gel and ligated into pTZ57R/T vector, transferred into Top10 competent cells and recombinants were selected using blue-white screening with ampicillin as selection marker. The recombinant vector pTZ57R/T-AP37 was double digested with restriction enzymes KpnI and BamHI to release the gene insert and ligated into pRT100 vector with the same enzymes. The recombinant vector pTZ57R/T-ABA2 was double digested with restriction enzymes EcoRI and KpnI. SbAP37 gene with OsABA2 promoter and nopaline synthase (NOS) PolyA terminator were cloned into pCAMBIA1301 binary vector and transferred into Agrobacterium tumefaciens strain LBA4404 (Holsters et al. 1978) and the construct was used for in planta transformation.
Genetic transformation of rice
Seeds of indica rice variety TN1 were collected from the Directorate of Rice Research, Hyderabad. Seeds were dehusked and surface sterilized with 0.1% mercuric chloride for 4 min followed by 5–6 washes with autoclaved water. Seeds were soaked for 4 h in sterile water, blotted on an autoclaved tissue paper to remove excess water. Seedlings were pierced with a sterile needle at the scutellar portions and swirled in Agrobacterium culture having 0.6–0.8 OD for 20 min. Seeds were blotted again using sterile filter papers and transferred to co-cultivation plates containing Murashige and Skoog’s (MS) medium (Murashige and Skoog 1962) with 100 µM acetosyringone and incubated in dark for 2 days. Seedlings were washed with 250 mg l−1 cefotaxime for 15 min thrice, rinsed with autoclaved water and transferred to pots. After 20–30 days of acclimatization, putative transgenics were transferred to pots and maintained till maturity.
Molecular characterization of transgenics by PCR, DNA blot and segregational analysis
Screening of the transformants was carried out by GUS activity and PCR amplification of SbAP37, hptII genes and ABA2 promoter. Histochemical assay for GUS activity was performed according to (Jefferson et al. 1987). SbAP37 gene was detected using 21-mer primers designed to obtain a 729 bp amplicon with PCR profile as shown in Supplementary Table 1. The 750 bp region of hptII gene was amplified using 20-mer (forward) and 21-mer (reverse) oligonucleotide primers and the PCR conditions as mentioned in Supplementary Table 1. Presence of the introduced ABA2 promoter was detected using 18- to 21-mer primers designed to obtain 909 bp amplicon. Amplified products were assayed by electrophoresis on 1.2% agarose gels after staining with ethidium bromide. DNA blot analysis was carried out according to Sambrook and Russell (2001). PCR amplified fragments of coding sequences of SbAP37 gene was used as probe labeled with non-radioactive Alk-Phos direct system (Amersham Biosciences, UK). Labeling, hybridization and detection methods were performed according to the manufacturer’s instructions. Genomic DNA of different rice transformants was digested with KpnI and BamHI restriction enzymes and probed with SbAP37 gene sequence for southern blot. Copy number of the inserted gene was determined by digesting the genomic DNA samples with EcoRI and probed with SbAP37 gene sequence. For segregational analysis, both T1 and T2 seedlings were used. Mendelian inheritance was studied by finding out hygromycin (50 mg l−1) resistant and sensitive seedlings.
Combined effects of salt, high temperature, high natural sunlight and drought, high temperature, high light intensity stresses in T2 transgenic and UC plants
Plants were grown in the pot conditions during the months of February–May. Three plants per each independent transgenic line (PCR positives in case of transgenics) and UC were grown in the greenhouse until 60 days after transplantation. To each pot, 1 l of water was added or as it may be necessary. Both UC and transgenic lines were then exposed to 150 mM NaCl for 9 days. To each pot, 1 l of 150 mM NaCl solution was added on day 0 (equal volumes to transgenics and UC). At the time of exposure of the plants (booting stage) to salt or drought stresses, the temperature was 36 ± 2 °C (day) and 25 ± 2 °C (night) and the plants were also exposed to 12 h natural sunlight and 12 h dark conditions. Similarly, drought stress was imposed to UC and transgenic lines by withholding water for 9 consecutive days alongside same day/night temperatures and light intensity (natural sunlight). The recuperation step was performed after 9 days of exposure of all plants to stress conditions.
Reverse transcriptase (RT)-PCR and quantitative real time (qRT)-PCR analysis
Total RNA was isolated from transgenics and UC using TRIzol® reagent from 100 mg tissues. Total RNA (500 ng) was taken for first strand cDNA synthesis using Revert Aid single stranded synthesis kit (Thermo Kit #K1622 USA). The synthesized cDNA was used for RT-PCR amplification of the coding sequences of hptII and SbAP37 genes both in transgenics and UC using respective gene specific primers. Total RNA was isolated using TRIzol® reagent from 100 mg of tissues of transgenics exposed to both salt and drought stresses along with UC. β-Actin gene was used as an internal control to normalize the data. To amplify β-actin, forward and reverse primer sequences were used as shown in Supplementary Table 1. ABI 7500 real-time PCR system (Applied Biosystems, USA) was used with the thermal cycling conditions displayed in Supplementary Table 1. In every experiment, no template controls (NTC) were maintained. Relative quantification method (2−ΔΔCT) was used for quantitative evaluation of the variation between replicates (Schmittgen and Livak 2008). Three technical replicates for each biological replicate were used for qRT-PCR using SYBR Green PCR master mix (Applied Biosystems, Foster City, CA, USA) on a 7500 real time thermocycler (Applied Biosystems). Gene-specific primers were designed using primer 3 software.
Proline estimation, chlorophyll fluorescence and photosynthetic leaf gas exchange
Proline content was determined at 520 nm as described by Bates et al. (1973) from leaf samples. Chlorophyll and carotenoid contents in the leaf discs were determined as per the procedure of Arnon et al. (1974). Leaves from 60-day-old UC and transgenic rice plants were used for chlorophyll fluorescence analysis (Maxwell and Johnson 2000). Readings were taken before the combined treatments of salt and high temperature, drought and high temperature, and after recovery from stress. Photosystem II (PSII) activity in the intact leaves was determined by measuring chlorophyll a fluorescence using Handy plant efficiency analyzer (Hansatech Instruments Ltd., Norfolk, United Kingdom). Transients were exposed to 1 s illumination with an array of three light emitting diodes providing a maximum light intensity of 3000 µmol m−1 s−1 and a homogenous irradiation over a 4 mm diameter leaf area. Variation in PSII activity was measured as the ratio of variable to maximum fluorescence (F v /F m). The minimal (F 0) and maximal (F m ) chlorophyll a fluorescence emissions were assessed in leaves after 2 h of dark adaptation and the maximum quantum efficiency of PSII was calculated as \({F_{\text{v}}}/{F_{\text{m}}}\,=\,\left( {{F_{\text{m}}}~ - ~{F_{\text{0}}}} \right)/{F_{\text{m}}}\).
Protein estimation and analysis by quadrupole-time-of-flight (Q-TOF) mass spectrometry
For performing 2-dimensional (2-D) gel electrophoresis, proteins were extracted using protein extraction buffer (50 mM Na2HPO4 and 50 mM NaH2PO4, pH 7.0). Crude extract was centrifuged at 12,000×g for 10 min at 4 °C and used for the separation by 2-D gel electrophoretic method. Total protein was estimated by Bradford method (Bradford 1976). Twenty µl of crude protein isolated from leaves of UC and transgenic line 5 after exposing them to 150 mM NaCl combined with high temperature stress. Proteins were digested using trypsin with in-solution method. After digestion, samples were vacuum-dried and dissolved in 50 µl of 0.1% formic acid. One µl of each samples (transgenic line 5 and UC) was separated on the NanoAcquity BEH C18 column (Cat. no: 186003543) connected to nanoUPLC system (WATERS make) for 150 min with 50% gradient of water, 0.1% formic acid (buffer A) and 0.1% acetonitrile. The nano liquid chromatography (LC) system (WATERS make) separated the peptides which were analysed by mass spectrometry (MS and MS/MS) for fragmentation on SYNAPT G2 nano LC coupled Q-TOF (WATERS make) with electrospray ionization source. Changes in protein abundance between the UC and transgenic line were noted. For reproducibility of results, proteomic experiments were repeated. Data were analysed for protein identification using the Protein Lynx Global Server (PLGS) v4.1 against the UNIPROT database of Oryza sativa (http://www.uniprot.org/).
Statistical analysis
Experiments were repeated and the data represent average values taken from three biological replicates from each independent line unless otherwise mentioned. Physiological data were analyzed by two-way ANOVA, taking P < 0.05 and P < 0.01 as significant according to Tukey’s post hoc test.
Results
Isolation, characterization of SbAP37 gene and construct preparation
The length of SbAP37 cDNA (Fig. S1) coding sequence is 729 bp (Accession Number JF714972) which encodes a putative polypeptide of 243 amino acids with a predicted molecular weight of 24.94 kDa. SbAP37 genomic sequence has a single exon. Phylogenetic analysis revealed that SbAP37 has 81, 80 and 79% homology with Zea mays, Oryza sativa and Malus domestica, respectively, at the amino acid levels (Fig. 1). ABA2 promoter measuring 909 bp was isolated from indica rice variety BPT5204 (Fig. S2). Vector construct was prepared with OsABA2 promoter in pCAMBIA1301 binary vector (Fig. S3). To release the gene SbAP37, pCAMBIA1301 was digested with KpnI and BamHI restriction enzymes and shown in Supplementary Fig. S4.
Molecular characterization of transgenics
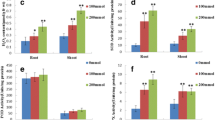
Genetic transformation frequency was 56% in rice by in planta method. Eleven independent transformants were regenerated and transferred to greenhouse for acclimatization. All transformants showed GUS expression, but not the UC. Also, 729 bp amplification of SbAP37 gene was noticed in all transgenics but such amplification was not noticed in UC (Supplementary Fig. S5A). OsABA2 promoter and hptII gene amplifications with bands of 909 bp (Supplementary Fig. S5B) and 750 bp (Supplementary Fig. S5C) respectively, were also observed. While T1 progenies segregated in 3 (tolerant):1 (susceptible), T2 progenies segregated in 1:2:1 ratio. Transgenics were confirmed for gene insertion through PCR amplification and DNA blotting techniques. Gene integration was confirmed by presence of signals on the DNA blot generated by autoradiogram after exposing the blot to film which showed positive hybridization at 729 bp region for each putative transgenic but not in UC (Fig. 2a). Out of 11 transgenics (Supplementary Fig. S6), only five independent lines displayed single copy gene insertions and hence, these lines were used in subsequent experiments. DNA isolated from transgenics when digested with EcoRI restriction enzyme and probed with SbAP37 gene sequence showed single copy insertions (Fig. 2b). Reverse transcriptase-PCR results showed higher transcript levels in transgenics, while UC did not exhibit any amplification (Fig. 3). In salt treated transgenics, gene expression by qRT-PCR revealed 2.8- to 4.7-folds higher (except in line 4) in leaves (Fig. 4a) when compared with UC. Since partial gene sequence of SbAP37 was used for qRT-PCR, some expression of AP37 is noticed in UC rice plants. This could be due to sequence homology of the gene among the two species. In drought stressed roots, 1.8- to 3.3-folds higher expressions were recorded than the UC roots (Fig. 4b). Again, transgene expression level was lower in the line 4 (1.8-folds) than that of others, but no phenotypic variation was noticed. The expression of transgene under normal conditions (without any stress) in different tissues is represented in Fig. 4c. To find out the native gene expression in S. bicolor, qRT-PCR experiment was performed in root, stem and leaf tissue of untreated plants (control), treated with 100, 150, 200 mM NaCl, 3 and 6% PEG. AP37 was highly expressed in the stem especially at 150 mM NaCl stress compared to root and leaf (Fig. 5a). Under drought stress conditions also (6% PEG), stem showed higher expression of AP37 in comparison to root and leaf (Fig. 5b).
a qRT-PCR analysis of SbAP37 expression levels in root, internode and leaves exposed to salt. b SbAP37 expression in root, internodes and leaves exposed to drought (control untransformed plant, 1–5 transgenic lines). c SbAP37 gene expression in roots, internodes and leaves under normal (without any stress) conditions
Total chlorophyll, carotenoid contents, PSII activity and proline accumulation
Under 150 mM NaCl and drought stress, total chlorophyll (Fig. 6a, b) and carotenoid contents (Fig. 6c, d) were higher in transgenics (P ˃ 0.05) in comparison with UC. Before salt treatment, there was no considerable change in PSII activity between the transgenic and UC. On the other hand, transgenics recorded approximately 30% higher photosynthetic efficiency than the UC under 150 mM NaCl stress (Fig. 7a). Under drought stress, photosynthetic efficiency was 20% better after 9 days of treatment but decreased once the stress is relieved (by 15th day, Fig. 7b) in both UC and transgenic plants. After 9 days of stress, PSII activity decreased both under salt and drought stress conditions compared to corresponding UC. Chlorophyll degradation was faster in UC compared to transgenics. Consistent with this, levels of transgene expression were higher in leaves grown under salt, but under drought, levels were lower in leaf compared to root. Levels of proline in different transgenics varied from 35.7 to 279.1 µg/100 mg of fresh tissue after 9 days of 150 mM NaCl treatment but decreased once the stress is relieved by 15th day (Fig. 8a). The highest amount of proline was noticed in transgenic line 5, consistent with gene expression level. Proline levels did not differ much in UC without (22.6 µg) and with salt stress (19.1 µg/100 mg of fresh tissue). Under drought stress, transgenics exhibited 35.8–285.6 µg of proline per 100 mg of fresh tissue after 9 days of drought, but decreased by 15th day after relieving (Fig. 8b).
Combined effects salt, high temperature and light intensity tolerance at reproductive/booting stage
Morphological features like plant height and leaf shape looked similar in both transgenics and UC devoid of any stress. Plants were exposed concurrently to 150 mM NaCl and 36 ± 2/25 ± 2 °C day/night temperatures, respectively, under natural Sun light intensities. Internodes, leaves and culms were brittle in UC, but not in transgenics. In a separate experiment, plants were exposed to drought combined with similar temperatures and natural light intensities during the panicle initiation or booting stage. In UC, visual symptoms of salt and drought-induced damage like severe leaf rolling and wilting were noticed with a concomitant loss of chlorophyll in 9 days after exposure. Leaves eventually turned brown indicating degradation of chlorophylls. In contrast, leaves were erect, leaf rolling was not recorded but, delayed leaf browning (after 15-day) was noticed in transgenics. While transgenics recovered faster upon rewatering, UC recovered slowly. Transgenics displayed significant increase (P < 0.005) in root biomass (Fig. 9) in 150 mM NaCl plus high temperature stress when compared to UC (P < 0.005).
Proteomic analysis
After concurrently exposing the UC and transgenic line 5–150 mM NaCl stress with 36 ± 2/25 ± 2 °C day/night temperatures and natural light for 9 days, proteins from leaf tissues of transgenic line 5 and UC (exposed to similar stress conditions) were separated by 2-D gels (Fig. 10a, b, respectively). To find out the spectrum of proteins that are modulated by the overexpression of SbAP37, Q-TOF analysis was performed in the transgenics and UC. Transgenic line 5 was taken for Q-TOF analysis since the gene expression level was higher in this when compared with other lines. A total of 331 proteins were modulated in the transgenic and are functionally annotated. Out of them, 11 downregulated (2- to 4-folds), 26 upregulated (1- to 70-folds), 101 common (0- to 1-fold) (shared between transgenic and UC) and 193 newly synthesized proteins were observed (Supplementary Table 2). A graphic representation of all the proteins is shown in Fig. 11 and the proposed mode of action of AP37 in the Fig. 12. In UC grown under identical stress conditions, 368 proteins were detected. These proteins are grouped into different categories based on the function (Supplementary Table 2). A large number of them belong to DNA/RNA metabolism followed by transporters, cell processes/growth/development, carbohydrate, lipid/energy metabolism, chaperones, signal transduction and hormone metabolism. Some of these proteins are also associated with grain filling. The remaining proteins belong to cell wall biosynthesis/degradation, transcription/protein metabolism, photosynthesis, nitrogen/amino acid metabolism, plant defense, unknown proteins and others.
Detection of down-, up-, common, newly synthesized and unique proteins
Among the downregulated proteins, three are associated with photosynthesis, 2 DNA molecular chaperones and 2 histone/DNA syntheses/repair and others. MOR1, a microtubule-associated protein, essential for organization and function of cortical microtubules is down-regulated by fourfold. Three of the upregulated proteins belong to molecular chaperones, 3 carbohydrate metabolism, 3 auxin responsive factors and others. Surprisingly, one of the auxin response factors (ARF4) was upregulated by 70-folds. Proteins (101) that are shared/common between transgenic and untransformed plants were functionally annotated and include DNA/RNA metabolism, molecular chaperones, transcription/protein metabolism, growth, transporters and others. Among the newly synthesized proteins, 20 belong to cell processes/growth and development, 23 molecular chaperones, 19 DNA/RNA metabolism, 17 carbohydrate/lipid/energy metabolism, 16 hormone metabolism, 15 growth and development, 14 ubiquitin, 13 signal transduction, 12 transporters (3 of them potassium transporters), 11 chlorophyll/photosynthesis, 11 cell wall synthesis/degradation proteins, 11 transcription/protein metabolism, 5 cytoskeleton/cell structure and 6 others (Supplementary Table 2). In the UC, proteins associated with DNA/RNA metabolism, chaperones, transporters, carbohydrate/lipid/energy metabolism, ubiquitination, cell processes/growth, hormone metabolism, cell wall synthesis/degradation, signal transduction were recorded. Some different sets/unique proteins like formins (associated with cell wall), chloroplast specific chaperones, genomic stability protein (in transgenic), COBRA-like, YABBAY, and Tubby-like F box proteins (in UC), viral proteins were detected which are not usually observed in plants when stress was applied individually. Thus, there appear some unique sets of proteins associated with combined abiotic stresses.
Discussion
Isolation of SbAP37 gene and stress inducible OsABA2 promoter
Out of the 139 APETALA (AP2) type of genes predicted in rice, Oh et al. (2009) identified 42 genes that are induced by one or more stress (salt, drought, low temperature, ABA) conditions. They functionally characterized AP37 and AP59 which are induced upon exposure to drought and salt stress. Further, in AP37 transgenics, the yield was higher compared to untransformed controls. This has led us to use it under concurrent imposition of salt and high temperature stress conditions before the booting stage. SbAP37 appears evolutionarily closer to other AP37 genes isolated from different members of the family Poaceae. We report here an efficient and reproducible method for genetic transformation of rice through in planta Agrobacterium-mediated method. Earlier, Supartana et al. (2005) reported in planta transformation of rice with ~40% frequency, while 56% is observed in the present study. Naseri et al. (2012) successfully attempted in planta transformation method in rice without any phenotypic abnormalities. Transgenics with single copy insertions were taken for stress tolerance studies since position of the inserted gene (positional-effect) in the genome and also gene copy number influence the expression of it as pointed out by Rai et al. (2007). Yi et al. (2010) studied six different promoters that are drought-inducible and pointed out that a variety of them are useful for crop biotechnology. Overexpression of ABA biosynthetic pathway gene using a stress-inducible promoter increased the drought resistance in Petunia (Estrada-Melo et al. 2015). OsABA2 promoter showed the presence of stress and ABA-responsive cis-acting elements, indicating that the promoter drives transcription under stress (Rai et al. 2009) and encodes zeaxanthin epoxidase in rice and plays a role in ABA biosynthesis (Agrawal et al. 2001). OsABA2 was identified as the key and promising promoter among Rab16A, HP1 and OsABA2 with regard to low constitutive transgene expression under normal conditions, but high induction under salt and drought stress (Rai et al. 2009). This would reduce energy burden on the plants under normal conditions. Besides, high induction of AP37 was recorded in response to ABA, salt and drought treatments (Oh et al. 2009) indicating that the signal transduction pathway is ABA-dependent and inducible.
Chlorophyll fluorescence, carotenoid and proline contents
Slightly higher chlorophyll and carotenoid contents in transgenic rice lines indicate minimal damage to photosynthetic apparatus when compared with UC plants. Reduction in degradation of chlorophyll was reported previously in transgenic sorghum grown under abiotic stress conditions (Reddy et al. 2015). The maintenance of higher PSII activity in transgenics may be due to protection of chlorophyll from degradation under stress. Molecular chaperones upregulated in the present study under salt stress perhaps may protect the proteins associated with chlorophyll biosynthesis. Further, Gomathi and Rakkiyapan (2011) reported that high carotenoid content enhances adaptation of sugarcane plants to salinity by scavenging ROS. Our results suggest while in UC, chlorophyll degradation is promoted by several proteins under multiple stresses, its synthesis is promoted in transgenics.
The exact function of AP37 during salt or drought stress is not known yet. We propose the following mechanistic explanation for the action of the same. Its overexpression results in the tolerance to salt stress and identification of proteins related to the metabolism of oxylipins such as jasmonic acid (JA) and ABA in the present study. Zeaxanthin epoxidase (ZEP), lipoxygenase (LOX) associated with ABA biosynthesis and 12-oxophytodienoic acid (OPDA) associated with JA synthesis have been shown to be triggered during the present study. Miyamoto et al. (2013) found out that JA tightly regulates the expression of basic helix-loop-helix transcription factor RERJ1during drought. Jisha et al. (2015) reported a cross talk between ABA and JA signalling mediated by OsHLH148/OsJaz1 interaction. This interaction perhaps may result in the upregulation of transcripts encoding ethylene responsive factors (ERF) like AP59 or AP37 associated with abiotic stress tolerance as depicted in Fig. 12. JA and ABA biosynthetic pathway proteins were newly synthesized in transgenics indicating that both of them are important to regulate stress. Superoxide dismutase (SOD) removes superoxide radicals generated in chloroplasts during stress and thus may protect chloroplasts from oxidative damage. It has been observed in the present study that chloroplastic SOD protein is upregulated under salt stress in transgenics. SOD is implicated in countering the oxidative damage and protecting transgenics such as tobacco against abiotic stresses (Negi et al. 2015). Under salt/drought stresses, ABA is accumulated and it helps to sponge H2O2 by upregulating the CatB expression in rice (Ye et al. 2011). CatB has been observed indeed as a shared protein between transgenic and untransformed plants in this study. Thus, ABA2 stress inducible promoter driven activity of SbAP37 confers tolerance to both salt and drought stresses by modulating JA and ABA and the downstream proteins in the signaling pathway.
Tissue specific expression of AP37 and proline accumulation during stress
qRT-PCR experiments revealed higher levels of SbAP37 gene expression in salt treated transgenic rice leaves, but such an expression was observed in roots under drought stress when compared with other tissues. Thus, distinct, tissue specific expressions of genes are not uncommon under stress as also observed in other plants (Basu and Roychoudhury 2014; Wang et al. 2015). Proline is an important osmoprotectant and implicated in protecting plants against abiotic stress (Kavi Kishor and Sreenivasulu 2014). Higher accumulation of proline in transgenic rice may help in osmotic adjustment and in protecting antioxidant enzyme activities under stress (Reddy et al. 2015).
Down-, up-regulated proteins in transgenics and common proteins
Oh et al. (2009) overexpressed AP37 gene in rice and identified ten genes that are upregulated in transgenics using microarray. But, a global picture of proteome would help to understand interaction circuits and co-expression hubs better between various components in cells especially when exposed to multiple stresses concurrently (Weston et al. 2008; Barkla et al. 2013). Such an identification of proteins and gaining an insight into their functions is important to dissect out the combined abiotic stress complexity (Barkla et al. 2013). In the present study, three of the photosynthesis related proteins (EXECUTER1, plastocyanin protein and a probable NAD kinase2) are downregulated in transgenics indicating that photosynthesis is highly sensitive to the combined salt and high temperature stresses. Proteins involved in chlorophyll biosynthesis are generally downregulated while proteins related to light-dependent reactions are upregulated under stress (Gupta and Huang 2014). Downregulation of proteins related to photosynthesis may ultimately lead to impaired grain filling and less yield under combined stresses. Three molecular chaperons were upregulated followed by three AUXIN RESPONSE FACTORS (ARFs) in transgenics. ARF transcription factor family regulates gene expression in response to auxin indicating that ARFs directly/indirectly may be associated with increased root biomass as observed in the present study (Fig. 12). In UC, several ARFs were noticed indicating the importance of IAA pathway under multiple stress conditions.
Newly synthesized proteins
AP2/ERFs are involved in the control of responses to many environmental stimuli (Licausi et al. 2013). Major groups of newly synthesized proteins (DNA/RNA metabolism, chaperones, transporters, carbohydrate, lipid and energy metabolism, ubiquitins and cell processes) in transgenic line are discussed below.
DNA and RNA impairment is common under such combined multiple stress application. In the present study, two DNA topoisomerases were newly induced (OsTOP6A3 or OsSPO11C and OsTOP6B). They were identified in indica rice earlier by Jain et al. (2006) and their overexpressions revealed a function in salinity and dehydration adaptation. Some of the helicases are responsive to abiotic stress in rice (Macovei et al. 2012). DEAD-box helicases play a role in stabilizing growth under stress probably by regulating stress-induced pathways (Tuteja et al. 2012). Several chaperones were either upregulated or newly synthesized indicating their crucial role for protecting the macromolecules. Surprisingly, no heat shock protein (HSPs) and heat shock factor (HSF) were detected in transgenics though they are common at high temperature stress, but in UC, HSFB4A was observed. Contrarily, Jagadish et al. (2011) reported upregulation of HSFs and proteins under combined stresses compared with either heat or water deficit alone in rice. Three out of 11 transporters belong to high affinity potassium transporters (HAK) and indicate that K+ ion acquisition under stress plays major role in osmotic adjustment and ion homeostasis (Wang et al. 2013). In the UC, out of 36 porters, 9 K+ porters were noticed implying their critical role under stress. Collectively, present results suggest that these porters are essential for ion homeostasis, protein trafficking and survival of plants. Sugars are essential primary metabolites under stress and non-stress conditions (Krasensky and Jonak 2012) and regulate stress-related genes (Rosa et al. 2009). While Tao et al. (2011) reported that vacuole localized β-glucosidase contributes to drought in Arabidopsis by decreased water loss via transpiration, Xu et al. (2012) demonstrated that β-glucosidase homolog (vacuolar located) possesses glucose-conjugated ABA hydrolysing activity which is vital for dehydration and NaCl stress. In the UC, 30 proteins related to carbohydrate metabolism were noticed and mostly associated with energy metabolism/grain filling. Plant hexokinases (HXK) are involved in sugar sensing and signalling and development (Xiao et al. 2000). The degradation of misfolded proteins is important under stress and is carried out by the 26S proteasome (Zhang et al. 2015). The synthesis of new proteins such as 26S proteasome subunit and NPL4 like indicates effective degradation of such misfolded proteins in rice transgenics. Detection of nine ubiquitin associated proteins in the UC implies that these are crucial players in this complex game.
Cell processes, cell wall synthesis-related proteins
Histone modification level is generally related with gene activation and repression in stress-responsive processes (Kim et al. 2012). Zhao et al. (2013) pointed out that hyperacetylated histones H3K9 and H4K5 are essential at the gene promoter region for the transcription of the osmotic stress-induced ZmDREB2A. Zhou et al. (2013) showed that CYCLINH;1 interacts with and activates CDKDs and thus regulate water stress responses and blue light-induced stomatal opening. Zhao et al. (2014) reported histone modification and cell cycle gene expression under stress. Further, it has been found that CYCLIN-DEPENDENT KINASE G2 regulates salinity stress response and salt mediated flowering in Arabidopsis (Ma et al. 2015). Thus, abiotic stresses impact plant cell cycle progression, CDK activity and growth. In response to salt and heat stresses, changes in cell wall structure are common (Deinlein et al. 2014). Decreased pectin in soybean (An et al. 2014), and cellulose concentration in maize were pronounced in the salt-sensitive lines under salt stress (Muszynska et al. 2014). Zhao et al. (2013) showed that COMT and CCoAOMT proteins of lignin biosynthetic pathway are responsive to salt stress. In the present study, LAC9, CAD8C, 4CL5, 4-coumarate CoA ligase like5 (4CLL5) were newly synthesized perhaps giving mechanical strength to the leaf cell walls which is consistent with erect leaves that are not rolled in transgenics. Besides lignin biosynthetic pathway genes, a stress-inducible β-glucosidase gene in rice (Opassiri et al. 2007) and nine cellulose synthase genes have been shown highly expressed under combined stresses (Guerriero et al. 2014). Our results support their observations and suggest that lignin and cellulose biosynthesis is pivotal that could be targeted to improve both abiotic stress and biomass.
Signal transduction and unique proteins
Several serine threonine protein (SAP) kinases have been found regulated in this study. Kobayashi et al. (2004) discovered that SAPK8, SAPK9 and SAPK10 are activated by ABA. Zhang et al. (2012) observed that SAPK9 is induced under high temperature in rice panicle. It appears that all the four SAPKs (WNK1, WNK6, WNK9 and SAPK9) are activated by ABA and essential for salt coupled high temperature stress. Among the other transcription factors, NAC16 and 19 have been found derepressed. Several NAC transcription factors (root specific NAC10, and NAC5) enhanced abiotic stress tolerance in transgenic rice (Jeong et al. 2010; Song et al. 2011). Taken together, these transcription factors appear to be vital for multiple stress tolerance. Strikingly, circadian rhythms/biological clock associated proteins were seen only in UC, but not in transgenics. Formin (FHs) proteins are implicated in the organization of actin microfilaments required for many cellular processes (Banno and Chua 2000). In the UC, COBRA-like proteins associated with culm brittleness and YABBY proteins were detected. These proteins were not reported earlier under individual stresses in plants. Therefore, they appear to be unique and plants adapt diverse mechanisms for survival under abiotic stresses when applied concurrently during booting stage.
To surmise, that proteins modulated by SbAP37 in transgenics provide invaluable information for understanding the combination stresses and indicate the existence of a very wide cross talk. Both ABA and JA appear to modulate AP37 under abiotic stress conditions. This work facilitates the design of engineering strategies for development of crop plants for combined stress tolerance during reproductive stage.
Author contribution statement
MP and PBK conceived and designed the experiments; MP performed the experiments; MP, BK, PDL and PBK analyzed the data. MP, BK, PDL and PBK wrote the paper.
References
Agrawal GK, Yamazaki M, Kobayashi M, Hirochika R, Miyao A, Hirochika H (2001) Screening of the rice viviparous mutants generated by endogenous retrotransposon Tos17 insertion. Tagging of a zeaxanthin epoxidase gene and a novel OsTATC gene. Plant Physiol 125:1248–1257
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
An P, Li X, Zheng Y, Matsuura A, Abe J, Eneji AE, Tanimoto E, Inanaga S (2014) Effects of NaCl on root growth and cell wall composition of two soybean cultivars with contrasting salt tolerance. J Agron Crop Sci 200:212–218
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Ann Rev Plant Biol 55:373–599
Arnon DI, McSwain BD, Tsujimoto HY, Wada K (1974) Photochemical activity and components of membrane preparation from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochem Biophys Acta 357:231–245
Banno H, Chua NH (2000) Characterization of the Arabidopsis formin-like protein AFH1 and its interacting protein. Plant Cell Physiol 41:617–626
Barkla B, Castellanos-Cervantes T, de Le´on JL, Matros A, Mock HP, Perez-Alfocea F, Salekdeh GH, Witzel K, Zörb C (2013) Elucidation of salt stress defense and tolerance mechanisms of crop plants using proteomics-current achievements and perspectives. Proteomics 13:1885–1900
Basu B, Roychoudhury A (2014) Expression profiling of abiotic stress-inducible genes in response to multiple stresses in rice (Oryza sativa L.) varieties with contrasting level of stress tolerance. BioMed Res Int. doi:10.1155/2014/706890 (Article ID 706890)
Bates L, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Burge CB, Karlin S (1998) Finding the genes in genomic DNA. Curr Op Struct Biol 8:346–354
Deinlein U, Stephan AB, Horie T, Luo W, Xu G, Schroeder JI (2014) Plant salt-tolerance mechanisms. Trends Plant Sci 19:371–379
Doyle JJ, Doyle JL (1990) A rapid total DNA preparation procedure for fresh plant tissue. Focus 12:13–15
Estrada-Melo A, Ma C, Reid MS, Jiang CZ (2015) Overexpression of an ABA biosynthesis gene using a stress-inducible promoter enhances drought resistance in petunia. Hort Res 2:15013. doi:10.1038/hortres.2015.13
Gomathi R, Rakkiyapan P (2011) Comparative lipid peroxidation, leaf membrane thermostability, and antioxidant system in four sugarcane genotypes differing in salt tolerance. Int J Plant Physiol Biochem 3:67–74
Guerriero G, Legay S, Hausman JF (2014) Alfalfa cellulose synthase gene expression under abiotic stress: a Hitchhiker’s guide to RT-qPCR normalization. PLoS One 9(8):e103808
Guo A, He K, Liu D, Bai S, Gu X, Wei L, Luo J (2005) DATF: a database of Arabidopsis transcription factors. Bioinformatics 21:2568–2569
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genom. doi:10.1155/2014/701596 (Article ID 701596)
Harrington HM, Alm DM (1988) Interaction of heat and salt shock in cultured tobacco cells. Plant Physiol 88:618–625
Holsters M, de Waele D, Depicker A, Messens E, van Montagu M, Schell J, Holsters M, de Waele D, Depicker A, Messens E, van Montagu M, Schell J (1978) Transfection and transformation of Agrobacterium tumefaciens. Mol Gen Genet MGG 163(2):181–187
Jagadish SVK, Muthurajan R, Rang ZW, Malo R, Heuer S, Bennett J, Craufurd PQ (2011) Spikelet proteomic response to combined water deficit and heat stress in rice (Oryza sativa cv. N22). Rice 4:1–11
Jain M, Tyagi AK, Khurana JP (2006) Overexpression of putative topoisomerase 6 genes from rice confers stress tolerance in transgenic Arabidopsis plants. FEBS J 273:5245–5260
Jefferson RA (1987) Assaying chimeric genes in plants: The GUS gene fusion system. Plant Mol Biol Rep 5:387–405
Jeong JS, Kim YS, Baek K, Jung H, Ha SH, Choi YD, Kim M, Reuzeau C, Kim J (2010) Root-specific expression of OsNAC10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiol 153:185–197
Jisha V, Dampanaboina L, Vadassery J, Mithöfer A, Kappara S, Ramanan R (2015) Overexpression of an AP2/ERF type transcription factor OsEREBP1 confers biotic and abiotic stress tolerance in rice. PLoS One 10(6):e0127831. doi:10.1371/journal.pone.0127831
Kavi Kishor PB, Sreenivasulu N (2014) Is proline accumulation per se correlated with stress tolerance or is proline homoeostasis a more critical issue? Plant Cell Environ 37:300–311
Kim JM, To TK, Ishida J, Matsui A, Kimura H, Seki M (2012) Transition of chromatin status during the process of recovery from drought stress in Arabidopsis thaliana. Plant Cell Physiol 53:847–856
Kobayashi Y, Yamamoto S, Minami H, Kagaya Y, Hattori T (2004) Differential activation of the rice sucrose nonfermenting1-related protein kinase2 family by hyperosmotic stress and abscisic acid. Plant Cell 16:1163–1177
Krasensky J, Jonak C (2012) Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot 63:1593–1608
Krizek B (2009) AINTEGUMENTA and AINTEGUMENTA-LIKE6 act redundantly to regulate Arabidopsis floral growth and patterning. Plant Physiol 150:1916–1929
Lei Y, Song S, Fu J (2005) Possible involvement of antioxidant enzymes in the cross tolerance of the germination/growth of wheat seeds to salinity and heat stress. J Integr Plant Biol 47:1211–1219
Li W, Zhang C, Lu Q, Wen X, Lu C (2011) The combined effect of salt stress and heat shock on proteome profiling in Suaeda salsa. J Plant Physiol 168:1743–1752
Li X, Cai J, Liu F, Dai T, Cao W, Jiang D (2014) Physiological, proteomic and transcriptional responses of wheat to combination of drought or waterlogging with late spring low temperature. Funct Plant Biol 41:690–703
Licausi F, Ohme-Takagi M, Perata P (2013) APETALA2/ethylene responsive factor (AP2/ERF) transcription factors: mediators of stress responses and developmental programs. New Phytol 199:639–649
Lin R, Zhao W, Meng X, Wang M, Peng Y (2007) Rice gene OsNAC19 encodes a novel NAC-domain transcription factor and responds to infection by Magnaporthe grisea. Plant Sci 172:120–130
Ma X, Qiao Z, Chen D, Yang W, Zhou R, Zhang W, Wang M (2015) CYCLIN-DEPENDENT KINASE G2 regulates salinity stress response and salt mediated flowering in Arabidopsis thaliana. Plant Mol Biol 88:287–289
Macovei A, Vaid N, Tula S, Tuteja N (2012) A new DEAD-box helicase ATP-binding protein (OsABP) from rice is responsive to abiotic stress. Plant Sign Behav 7:1138–1143
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
Miyamoto K, Shimizu T, Mochizuki S, Nishizawa Y, Minami E, Nojiri H, Yamane H, Okada K (2013) Stress-induced expression of the transcription factor RERJ1 is tightly regulated in response to jasmonic acid accumulation in rice. Protoplasma 250:241–249
Munns R (2005) Genes and salt tolerance: bringing them together. New Phytol 167:645–663
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Muszynska A, Jarocka K, Kurczynska EU (2014) Plasma membrane and cell wall properties of an aspen hybrid (Populus tremula × tremuloides) parenchyma cells under the influence of salt stress. Acta Physiol Plant 36:1155–1165
Nakano T, Suzuki K, Fujimura T, Shinshi H (2006) Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol 140:411–432
Naseri G, Sohani MM, Pourmassalehgou A, Allahi S (2012) In planta transformation of rice (Oryza sativa) using thaumatin-like protein gene for enhancing resistance to sheath blight. Afr J Biotech 11:7885–7893
Negi NP, Shrivastava DC, Sharma V, Sarin NB (2015) Overexpression of CuZnSOD from Arachis hypogaea alleviates salinity and drought stress in tobacco. Plant Cell Rep 34:1109–1126
Oh SJ, Kim YS, Kwon CW, Park HK, Jeong JS, Kim JK (2009) Overexpression of the transcription factor AP37 in rice improves grain yield under drought conditions. Plant Physiol 150:1368–1379
Opassiri R, Pomthong B, Akiyama T, Nakphaichit M, Onkoksoong T, Cairns MK, Cairns JRK (2007) A stress-induced rice (Oryza sativa L.) β-glucosidase represents a new subfamily of glycosyl hydrolase family 5 containing a fascin-like domain. Biochem J 408:241–249
Prasch CM, Sonnewald U (2013) Simultaneous application of heat, drought, and virus to Arabidopsis plants reveals significant shifts in signaling networks. Plant Physiol 162:1849–1866
Rai M, Datta K, Parkhi V, Tan J, Oliva N, Chawla HS, Datta SK (2007) Variable T-DNA linkage configuration affects inheritance of carotenogenic transgenes and carotenoid accumulation in transgenic indica rice. Plant Cell Rep 26:1221–1231
Rai M, He C, Wu R (2009) Comparative functional analysis of three abiotic stress-inducible promoters in transgenic rice. Transgenic Res 18:787–799
Reddy PS, Jogeswar G, Rasineni GK, Maheswari M, Reddy AR, Varshney RK, Kavi Kishor PB (2015) Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor (L.) Moench]. Plant Physiol Biochem 94:104–113
Rosa M, Prado C, Podazza G, Interdonato R, Gonzalez JA, Hilal M, Prado FE (2009) Soluble sugars-metabolism, sensing and abiotic stress. Plant Sign Behav 4:388–393
Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, Yamaguchi-Shinozaki K (2002) DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold inducible gene expression. Biochem Biophys Res Comm 290:998–1009
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Sewelam N, Oshima Y, Mitsuda N, Ohme-Takagi M (2014) A step towards understanding plant responses to multiple environmental stresses: a genome-wide study. Plant Cell Environ 37:2024–2035
Sharoni AM, Nuruzzaman M, Satoh K, Shimizu T, Kondoh H, Sasaya T, Choi IR, Omura T, Kikuchi S (2011) Gene structures, classification and expression models of the AP2/EREBP transcription factor family in rice. Plant Cell Physiol 52:344–360
Song SY, Chen Y, Chen J, Dai XY, Zhang WH (2011) Physiological mechanisms underlying OsNAC5-dependent tolerance of rice plants to abiotic stress. Planta 234:331–345
Supartana P, Shimizu T, Shioiri H, Nogawa M, Nozue M, Kojima M (2005) Development of simple and efficient in planta transformation method for rice (Oryza sativa L.) using Agrobacterium tumefaciens. J Biosci Bioeng 4:391–397
Tao WP, Hao L, Hong Jie H, Lei W, Chun-Peng S (2011) A vacuole localized β-glucosidase contributes to drought tolerance in Arabidopsis. Chin Sci Bull 56:3538–3546
Todaka D, Shinozaki K, Yamaguchi-Shinozaki K (2012) Recent advances in the dissection of drought stress regulatory networks and strategies for development of drought-tolerant transgenic rice plants. Front Plant Sci 6:84. doi:10.3389/fpls.2015.00084
Tuteja N, Gill SS, Tiburcio AF, Tuteja R (2012) Helicases in improving abiotic stress tolerance in crop plants. In: Tuteja N, Singh S, Tuteja R (eds) Improving crop resistance to abiotic stress, vol 1 and 2. Wiley, Hoboken. doi:10.1002/9783527632930.ch10
Wang M, Zheng Q, Shen Q, Guo S (2013) The critical role of potassium in plant stress response. Int J Mol Sci 14:7370–7390
Wang Y, Chai C, Valliyodan B, Maupin C, Annen B, Nguyen HT (2015) Genome-wide analysis and expression profiling of the PIN auxin transporter gene family in soybean (Glycine max). BMC Genom 16:951. doi:10.1186/s12864-015-2149-1
Weston DJ, Gunter LE, Rogers A, Wullschleger SD (2008) Connecting genes, coexpression modules, and molecular signatures to environmental stress phenotypes in plants. BMC Syst Biol 2:16. doi:10.1186/1752-0509-2-16
Xiao W, Sheen J, Jang JC (2000) The role of hexokinase in plant sugar signal transduction and growth and development. Plant Mol Biol 44:451–461
Xu ZY, Lee KH, Dong T, Jeong JC, Jin JB, Kanno Y, Kim DH, Kim SY, Seo M, Bressan RA, Yun DJ, Hwang I (2012) A vacuolar β-glucosidase homolog that possesses glucose-conjugated abscisic acid hydrolyzing activity plays an important role in osmotic stress responses in Arabidopsis. Plant Cell 24:2184–2199
Yang Y, Dong C, Li X, Du J, Qian M, Sun X, Yang Y (2016) A novel Ap2/ERF transcription factor from Stipa purpurea leads to enhanced drought tolerance in Arabidopsis thaliana. Plant Cell Rep 5:2227–2239
Ye N, Guohui Zhu G, Liu Y, Li Y, Zhang J (2011) ABA controls H2O2 accumulation through the induction of OsCATB in rice leaves under water stress. Plant Cell Physiol 52:689–698
Yi N, Kim YS, Jeong MH, Oh SJ, Jeong JS, Park SH, Jung H, Choi Y, Kim JK (2010) Functional analysis of six drought-inducible promoters in transgenic rice plants throughout all stages of plant growth. Planta 232:743–754
Zhang X, Li J, Liu A, Zou J, Zhou X, Jianhua Xiang J, Rerksiri W, Peng Y, Xiong X, Chen X (2012) Expression profile in rice panicle: insights into heat response mechanism at reproductive stage. PLoS One 7(11):e49652. doi:10.1371/journal.pone.0049652
Zhang ZY, Li JH, Liu HH, Chong K, Xu YY (2015) Roles of ubiquitination-mediated protein degradation in plant responses to abiotic stresses. Env Exp Bot 114:92–103
Zhao L, Wang P, Yan S, Gao F, Li H, Hou H, Zhang Q, Tan J, Li L (2013) Promoter-associated histone acetylation is involved in the osmotic stress-induced transcriptional regulation of the maize ZmDREB2A gene. Physiol Plant 151:459–467
Zhao L, Wang P, Hou H, Zhang H, Wang Y, Yan S, Huang Y, Li H, Tan J, Hu A, Gao F, Zhang Q, Li Y, Zhou H, Zhang W, Li L (2014) Transcriptional regulation of cell cycle genes in response to abiotic stresses correlates with dynamic changes in histone modifications in maize. PLoS One 9(8):e106070
Zhou XF, Jin YH, Yoo CY, Lin XL, Kim WY, Yun DJ, Bressan RA, Hasegawa PM, Jin JB (2013) CYCLIN H; 1 regulates drought stress responses and blue light-induced stomatal opening by inhibiting reactive oxygen species accumulation in Arabidopsis. Plant Physiol 162:1030–1041
Acknowledgements
This work was supported by the UGC, New Delhi (No.F.4-2/2006(BSR)/18-1(3)/2011) and CSIR, New Delhi (No.21(0934)/12/EMR-II), and we are thankful for financial assistance of the projects. MP is thankful to the UGC for providing UGC-RGNF fellowship (F. 14-2(SC)/2010(SA-III). PBK is thankful to the UGC and CSIR, New Delhi for sanctioning UGC-BSR and CSIR-Emeritus fellowships.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have read and approved the manuscript. Authors declare no conflict of interest.
Additional information
Communicated by Nese Sreenivasulu.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Parveda, M., Kiran, B., Punita, D.L. et al. Overexpression of SbAP37 in rice alleviates concurrent imposition of combination stresses and modulates different sets of leaf protein profiles. Plant Cell Rep 36, 773–786 (2017). https://doi.org/10.1007/s00299-017-2134-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-017-2134-z