Abstract
Key message
We described identification, expression, subcellular localization, and functions of genes that encode fatty acid desaturase enzymes in Perilla frutescens var. frutescens.
Abstract
Perilla (Perilla frutescens var. frutescens) seeds contain approximately 40 % of oil, of which α-linolenic acid (18:3) comprise more than 60 % in seed oil and 56 % of total fatty acids (FAs) in leaf, respectively. In perilla, endoplasmic reticulum (ER)-localized and chloroplast-localized ω-3 FA desaturase genes (PfrFAD3 and PfrFAD7, respectively) have already been reported, however, microsomal oleate 12-desaturase gene (PfrFAD2) has not yet. Here, four perilla FA desaturase genes, PfrFAD2-1, PfrFAD2-2, PfrFAD3-2 and PfrFAD7-2, were newly identified and characterized using random amplification of complementary DNA ends and sequence data from RNAseq analysis, respectively. According to the data of transcriptome and gene cloning, perilla expresses two PfrFAD2 and PfrFAD3 genes, respectively, coding for proteins that possess three histidine boxes, transmembrane domains, and an ER retrieval motif at its C-terminal, and two chloroplast-localized ω-3 FA desaturase genes, PfrFAD7-1 and PfrFAD7-2. Arabidopsis protoplasts transformed with perilla genes fused to green fluorescence protein gene demonstrated that PfrFAD2-1 and PfrFAD3-2 were localized in the ER, and PfrFAD7-1 and PfrFAD7-2 were localized in the chloroplasts. PfrFAD2 and perilla ω-3 FA desaturases were functional in budding yeast (Saccharomyces cerevisiae) indicated by the presence of 18:2 and 16:2 in yeast harboring the PfrFAD2 gene. 18:2 supplementation of yeast harboring ω-3 FA desaturase gene led to the production of 18:3. Therefore, perilla expresses two functional FAD2 and FAD3 genes, and two chloroplast-localized ω-3 FA desaturase genes, which support an evidence that P. frutescens cultivar is allotetraploid plant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Perilla (Perilla frutescens var. frutescens) is an annual plant of the Lamiaceae family and is mainly cultivated in northeast Asia. Perilla seed oil has been used for food, medicinal use and industrial uses such as ink and varnish. Perilla seed oil comprises approximately 40 % of perilla seed mass and its content of α-linolenic acid (18:3) is slightly higher than that of flaxseed oil and chia seed oil, accounting for more than 60 % of total FAs in seed oil (Shin and Kim 1994; Ciftci et al. 2012). Polyunsaturated fatty acids (PUFAs), such as linoleic acid (18:2) and 18:3, comprise approximately 80 % of perilla seed oil and have demonstrated health benefits (Asif 2011). Perilla leaves have also been ingested as food. Lipid content of perilla leaf is very low but the PUFA proportion in lipids of perilla leaf is as much as 70 %. As the benefits of perilla seed oil for human health have become apparent, there has been a consequent increase in the production of Perilla.
Vegetable oil and membrane lipid of plants contain relatively large quantities of PUFAs which are synthesized by membrane-bound fatty acid desaturases. 18:2 is converted from oleic acid (18:1) by the catalytic reaction of a microsomal oleate 12-desaturase that is commonly referred to as FA desaturase 2 (FAD2; Okuley et al. 1994). Subsequently, 18:3 is synthesized from 18:2 by the catalytic reaction of a microsomal linoleate 15-desaturase known as FA desaturase 3 (FAD3; Browse et al. 1993). These reactions occur at the sn-2 positions of phospholipids such as phosphatidylcholine (PC), phosphatidylethanolamine, and phosphatidylinositol, except phosphatidylglycerol (PG) that is synthesized from diacylglycerol (DAG) originating from phosphatidic acid (PA) via the eukaryotic pathway for the synthesis of glycerolipids in the endoplasmic reticulum (ER; Ohlrogge and Browse 1995). In contrast, PA is converted into PG or DAG via a prokaryotic pathway in plastid inner membranes. Galactolipids, such as monogalactosyldiacylglycerol and digalactosyldiacylglycerol, and sulfolipids, such as sulfoquinovosyldiacylglycerol, are synthesized from DAG in plastids (Joyard et al. 1993). 18:1 and 16:0 are predominantly incorporated at the sn-1 and sn-2 positions of plastid lipids, respectively, whereas 16:0 incorporated PG is converted into 16:1Δ3t by FAD4 and 16:0 incorporated galactolipid or sulfolipid is converted into 16:1Δ7 by FAD5. Subsequently, 16:1Δ7 and 18:1 incorporate all plastid lipids and are converted into 16:2Δ7,10 and 18:2 by FAD6; subsequently, they are converted into 16:3Δ7,10,13 and 18:3 by FAD7 and FAD8, respectively (Ohlrogge and Browse 1995).
FAD2 and FAD3 genes were first identified from Arabidopsis thaliana using T-DNA tagging methods and map-based chromosome walking (Okuley et al. 1994; Arondel et al. 1992). In addition, FAD7 and FAD8 genes were cloned from Arabidopsis according to their homology with Arabidopsis FAD3 (Iba et al. 1993; Yadav et al. 1993; Gibson et al. 1994). FAD2 genes have one intron located in the 5′-untranslated region (UTR; Okuley et al. 1994), three histidine boxes (H boxes) that are essential for FA desaturase activity (Shanklin et al. 1994; Kurdrid et al. 2005), transmembrane domains (TMDs) that are the characteristics of membrane-bound proteins (Okuley et al. 1994), and an ER retrieval motif at its C-terminal that comprises a hydrophobic amino acid (aa)-enriched signal peptide (McCartney et al. 2004). FAD2 is FA desaturase that regulates the degrees of phospholipid unsaturation in the ER membrane. Hence, mutant Arabidopsis disrupted the function of FAD2 was inhibited in growth and caused death at 12 °C and at 6 °C, respectively, reflecting lowered fluidity of membranes with reduced PUFAs contents of phospholipid (Miquel et al. 1993).
FAD3, FAD7, and FAD8 genes from Arabidopsis have over 65 % homology and contain seven introns each. These genes encode enzymes that produce trienoic acids of membrane lipids and are associated with tolerance against low temperature (Routaboul et al. 2000) and drought (Torres-Franklin et al. 2009) in plants. Similar to FAD2, FAD3 is an ER membrane-bound FA desaturase (Arondel et al. 1992), whereas FAD7 and FAD8 are involved in the desaturation of galactolipids in plastids (Iba et al. 1993; Yadav et al. 1993; Gibson et al. 1994). FAD8 gene is induced by low temperature; thus, it can compensate for the reduced expression of FAD7 gene under these conditions (McConn et al. 1994; Gibson et al. 1994).
All of the FA desaturases described above are membrane-bound proteins and have three conserved histidine clusters (Los and Murata 1998), which contain histidine boxes (His boxes) comprising eight histidine residues. Histidine residues are moderately conserved in the His boxes of FAD2, whereas those of ω-3 FA desaturases are highly conserved. Conserved His boxes of FAD2 include HXCGH, HXXHH, and HXXHH, and those of ω-3 FA desaturases include HDCGH, HRTHH, and HVIHH (Shanklin et al. 1994; Los and Murata 1998). ER- and chloroplast-localized ω-3 FA desaturase genes have been identified in perilla (Chung et al. 1999; Lee et al. 2001a) and recently we reported expression profiles of FAD2 and ω-3 FA desaturases (FAD3 and FAD7/8) in developing seeds of perilla by transcriptome analysis (Kim et al. 2016).
In this study, we aim to identify how many FA desaturase genes contribute to the high content of the PUFAs in seeds and leaves in perilla. We identified two FAD2 genes, additional FAD3 gene and a chloroplast-localized ω-3 FA desaturase gene from transcriptome data of perilla to contribute to the understanding of fatty acid desaturation in perilla, one of the highest 18:3 accumulating oilseeds. Subsequently, their expression levels and patterns were determined, and subcellular localization and functional analyses in budding yeast indicated precise organelle-specific roles.
Materials and methods
Plant materials and preparation of total RNA
Perilla frutescens var. frutescens cv. Dayudeulkkae was raised in a greenhouse. Four-week-old leaves, stems, roots, and flowers as well as 5-day-old seedlings and 1-, 2-, 3-, and 4-week-old developing seeds were harvested for RNA isolation. Tissue samples were then ground under liquid nitrogen and total RNA was extracted using Plant RNA Purification Reagent (Invitrogen, USA), according to the manufacturer’s instructions.
RNAseq
Coding sequences of perilla genes were determined using sequence data that was generated from developing seed and leaf tissues of perilla (cv. Dayudeulkkae) using a Hiseq2000 sequencing system (Illumina, USA). SolexaQA package software was used for the quality control and for trimming of raw sequence data (Cox et al. 2010) with a Phred quality score as a quality threshold was 20 and the length cutoff set as 25. De novo transcriptome assembly was then performed from the trimmed data using Velvet (Zerbino and Birney 2008) and Oases (Schulz et al. 2012) assembler software (k-mer = 73).
Degenerate PCR
Two primers for degenerate PCR were designed in 5′ to 3′ and 3′ to 5′ directions and were designated as F1, F2, R1, and R2, respectively (Table 1). Because the only one intron of FAD2 gene is out of range of coding sequence, genomic DNA was used as the template. PCRs were performed at 94 °C for 5 min, followed by 30 cycles of 94 °C for 20 s, 54 °C for 30 s, and 72 °C for 1 min as well as an additional extension at 72 °C for 5 min. PCR amplification was performed with four combinations of degenerate primers using Ex Taq polymerase (Takara, Japan).
Random amplification of complementary DNA ends (RACE)
To identify the 5′ and 3′ ends of the perilla FAD2 gene, RACE was performed using SMARTer® RACE 5′/3′ kits (Clontech, USA). Initially, the first strand cDNAs were synthesized for 5′- and 3′-RACE, and RACE PCR was subsequently performed using gene-specific primers (GSPs) and KOD + polymerase (Toyobo, Japan). Perilla FAD2 GSP1 for 5′-RACE and GSP2 for 3′-RACE were designed according to sequences obtained using degenerate PCR using the primers presented in Table 1. RACE experiments were performed according to the user’s manual provided by Clontech.
Gene cloning and vector construction
PCR products were amplified with high fidelity using KOD+ polymerase and were cloned into pENTR/D-TOPO vectors (Invitrogen, USA), according to the manufacturer’s instructions. Gene cloning primer sequences are listed in Table 1. To determine functions, the four perilla genes in pENTR/D-TOPO vectors were subcloned into the yeast expression vector pYES-DEST52 (Clontech, USA) using Gateway® LR clonase® II enzyme (Invitrogen, USA).
Coding sequences of perilla genes without stop codons were subcloned in frame into BamHI sites in front of coding sequence of sGFP (synthetic green fluorescence protein) under the control of the cauliflower mosaic virus 35S promoter in p326-sGFP vectors (Lee et al. 2001b) using In-Fusion HD Cloning Kits (Clontech, USA). In-Fusion and LR reactions were performed according to the manufacturer’s protocol with the p326-sGFP subcloning primer sequences presented in Table 1.
Sequence and phylogenetic analyses
Multiple alignments were performed using the GeneDoc software (Ver. 2.6.002) and DNASTAR® MegAlign (Ver. 8.1.4). Phylogenetic relationships were established using DNASTAR® MegAlign (Ver. 8.1.4) with the ClustalW method. The phylogenetic tree was constructed with TreeView softwares (Ver. 1.6.6). Chloroplast-localized proteins were predicted using the ChloroP prediction server (http://www.cbs.dtu.dk/services/ChloroP). Finally, TMDs were predicted using the TOPCONS website (http://topcons.cbr.su.se/).
Quantitative RT-PCR (qRT-PCR)
First strand cDNAs were synthesized using RNA to cDNA EcoDry premix (Clontech, USA) following the manufacturer’s instructions. Subsequently, quantitative RT-PCR was performed using SYBR premix Ex Taq II (Tli RNaseH plus; Takara, Japan) and 20-fold diluted first strand cDNA template using StepOnePlus Real-Time PCR Systems (Applied Biosystems, USA). PCR reactions were performed at 94 °C for 30 s followed by 40 cycles of 94 °C for 5 s, 60 °C for 20 s, and 72 °C for 20 s as well as additional cycles of 94 °C for 15 s and 60 °C for 1 min; this is followed by an increase in temperature at 0.5 °C/min to 94 °C for 15 s. Quantitation was performed using the StepOne software Ver. 2.3 (Applied Biosystems, USA) with perilla β-actin as a reference gene (GenBank accession No. AB002819). Primers for amplicons of approximately 200 base pair (bp) with melting temperatures of approximately 60 °C were designed using the website of Genscript Real-time PCR Primer Design (https://www.genscript.com/ssl-bin/app/primer) and the primer sequences are listed in Table 1.
Transient expression and subcellular localization
p326-sGFP vectors harboring perilla genes were transfected into Arabidopsis protoplasts using the polyethyleneglycol (PEG) transformation method developed by Jin et al. (2001), and the subcellular localization sites of gene products were determined. Images were taken using a fluorescence microscope (Axioplan 2; Carl Zeiss) equipped with a 340/0.75 objective (Plan–NEOFLUAR) and a cooled charge-coupled device camera (Senicam; PCO Imaging) at 20 °C. Filter sets included XF116 (exciter, 474AF20; dichroic, 500DRLP; emitter, 510AF23), XF33/E (exciter, 535DF35; dichroic, 570DRLP; emitter, 605DF50), and XF137 (exciter, 540AF30; dichroic, 570DRLP; emitter, 585ALP; Omega, Inc., Brattleboro, VT) for GFP, red fluorescent protein (RFP), and autofluorescence of chlorophyll, respectively.
Yeast transformation and FA analysis
Saccharomyces cerevisiae INVSc1 (Invitrogen, USA) was used to confirm perilla gene functions. Yeast transformation was performed in accordance with the manufacturer’s protocol, and well-grown yeast colonies were inoculated in synthetic defined (SD)/-Ura drop out (DO) supplement medium (Clontech, USA) with 2 % (w/v) glucose at 30 °C for 24 h. Cells were harvested by centrifugation at 4000 rpm for 5 min and were subsequently diluted and induced using SD/-Ura DO supplement medium with 2 % (w/v) galactose; perilla genes were expressed at 20 °C for 5 days. Culture supernatants were supplemented with 0.1 % (v/v) Tergitol-type nonidet P-40 (Sigma, USA) to facilitate the uptake of 0.5 mM 18:2 substrate into yeast cells harboring the perilla ω-3 FA desaturase gene, and cells were centrifuged and resuspended in sterile distilled water to remove excess 18:2 substrate. Subsequently, cells were harvested and lyophilized for 24 h. FAs of yeasts were extracted and transmethylated in 1 ml of 2.5 % H2SO4 (v/v) in methanol at 85 °C for 2 h. Fatty acid methyl esters (FAMEs) were extracted with 1 ml of 0.9 % NaCl solution and 0.5 ml of n-hexane, which were added and mixed vigorously. Mixtures were then centrifuged at 4000 rpm for 2 min, and n-hexane-dissolved FAMEs were transferred to a glass vial for gas chromatography (GC) analysis. FAMEs were analyzed using a GC-2010 plus instrument (Shimadzu, Japan) equipped flame ionization detector (FID) with a 30 m × 0.25 mm (inner diameter) HP-FFAP column (Agilent, USA), and oven temperature increases from 170 to 180 °C at 1 °C/min for 10 min. Nitrogen gas was used as a carrier gas at a flow rate of 1.4 ml/min.
Results and discussion
Gene cloning of FA desaturase genes from perilla
Perilla seed oil comprises 60 % of ω-3 FAs and approximately 80 % of PUFAs (Table 2), one of the highest PUFA proportions in plant seed oil. Therefore, several FAD2 and FAD3 genes with high activity and/or abundant expression are likely present in developing seeds. To confirm this hypothesis, perilla FAD2 (PfrFAD2) and ω-3 FA desaturase genes, including FAD3 gene (PfrFAD3), were cloned. To clone FAD2 gene that has not been identified in perilla, degenerate PCR and RACE were performed. Degenerate primers for cloning of the PfrFAD2 gene were designed based on the consensus sequences of deduced FAD2 aa sequences from dicotyledonous plants. As a result, one PfrFAD2 gene was identified. However, nucleotide (nt) sequence of PfrFAD2 gene from transcriptome analysis was slightly different from that of PfrFAD2 gene from degenerate PCR and RACE. Sequencing data of PfrFAD2 gene from leaves and developing seeds demonstrated that two different PfrFAD2 genes are expressed in both tissues (Fig. S1). The nt length of two PfrFAD2 genes is 1149 bp encoding 382 aa. The nt identity between two PfrFAD2 genes is 98.4 % (1131 nt/1149 nt; Fig. S1) and the aa identity between their deduced aa sequences is 99.5 % (380 aa/382 aa; Fig. 1a).
Multiple alignments of deduced aa sequences of a PfrFAD2-1, PfrFAD2-2 and AtFAD2 gene and b ω-3 FA desaturase genes from Perilla and Arabidopsis. Black background represents identical aa residues and gray indicate that all, except one, share identical aa residues. Dotted lines and underlines indicate His boxes and transmembrane domains, respectively. Asterisk means different aa residue between a PfrFAD2-1 and PfrFAD2-2, or b PfrFAD3-1 and PfrFAD3-2. Solid triangles represent the cleavage site for cTP according to the prediction by ChloroP prediction server. YNNKL is ER retrieval motif
To identify additional perilla ω-3 FA desaturase genes, we searched homologous transcripts in transcriptome data from the perilla leaf and developing seed tissues using local BLAST. Only a single transcript that had high homology with PfrFAD3 was identified from transcriptome sequences. PfrFAD3 gene cloning was performed based on the sequence reported by Chung et al. (1999). Two different PfrFAD3 genes were cloned from diverse cultivars (Fig. S2). The nt length of two PfrFAD3 genes is 1176 bp encoding 391 aa. The nt identity between two PfrFAD3 genes is 99.1 % (1165 nt/1176 nt; Fig. S2) and the aa identity between their deduced aa sequences is 99.7 % (390 aa/391 aa; Fig. 1b). Only one different aa residue of deduced aa sequence from PfrFAD3-1 (GenBank accession no. AF047039), reported by Chung et al. (1999), and PfrFAD3-2, newly identified in this study, are Asn and Ser at position 61, respectively. Southern blot result reported by Chung et al. (1999) showed two hybridizing bands in lanes of DNA fragmented by EcoRV, NdeI, BclI, Sau3AI and HinfI, whose restriction sites are not in the coding sequences of PfrFAD3 genes. This result supports our finding that two FAD3 genes exist and are expressed in perilla. Since perilla leaf contains high ω-3 FA (56 %) and PUFA (71 %), we identified additional AtFAD7- or AtFAD8-homologous transcripts from transcriptome data (Table 3). Previously reported and newly cloned chloroplast-type ω-3 FA desaturase genes are referred to as PfrFAD7-1 and PfrFAD7-2, respectively. The nucleotide sequences of PfrFAD2-1, PfrFAD2-2, PfrFAD3-2 and PfrFAD7-2 genes, identified in this study, were registered in the GenBank with accession Nos. KP070823, KX228916, KX228917 and KP070824, respectively.
Sequence analysis of FA desaturase genes from perilla
The coding sequence of the PfrFAD2 gene is up to 1149 bp long, encodes 382 aa residues, and has the highest homology to the FAD2 gene from Olea europaea (76.3 % nt identity and 81.2 % aa identity; Genbank Accession No. AY733077) registered in the NCBI GenBank database (http://www.ncbi.nlm.nih.gov/genbank/). Introns located in 5′- UTRs and 3′-UTRs of the PfrFAD2-1 gene are up to 2491 and 236 bp long, respectively. Sesame FAD2 gene (Genbank Accession No. AF192486) and Arabidopsis FAD2 gene (Genbank Accession No. L26296) have 70.8 and 70.6 % of nt identities, respectively, and 78.0 and 77.5 % of aa identities, respectively. The percentage of identity between PfrFAD3 and PfrFAD7-1 in terms of nt and aa sequence identities is 66.0 and 68.2 %, respectively, and that between PfrFAD3 and PfrFAD7-2 is 67.2 and 67.3 %, respectively. Because of chloroplast transit peptide (cTP) in N-terminal of chloroplast-localized ω-3 FA desaturase, it shares relatively low identity with ER-localized ω-3 FA desaturase. PfrFAD7-1 and PfrFAD7-2 share 80.6 % identity at the nt level and 84.7 % identity at the aa level.
His boxes of deduced aa sequences of PfrFAD2-1 and -2 are HECGHH, HRRHH, and HVAHH (Fig. 1a) and are consistent with the conserved His box of plant FAD2, as described above. Similarly, His boxes of deduced aa sequences encoded by PfrFAD7-1 and PfrFAD7-2 genes are HDCGH, HRTHH, and HVIHH (Fig. 1b) and are identical to the conserved His box of plant ω-3 FA desaturase, as described above. FAD2 carries the ER retrieval motif Φ-X-X-K/R/D/E-Φ (Φ, hydrophobic aa residues) at the C-terminal (McCartney et al. 2004), whereas the ER retrieval motif of PfrFAD2-1, -2 and AtFAD2 is Y-N-N-K-L in accordance with that of the conserved FAD2 of the plant (Fig. 1a). FAD3 also has an ER retrieval motif, but unlike FAD2, the conserved sequence of FAD3 contains a dilysine (McCartney et al. 2004). The ER retrieval motif of PfrFAD3-1 and PfrFAD3-2 is S-K-K-I and contains a dilysine similar to prototype K-K-X-X, whereas that of AtFAD3 is K-S-K-I-N, which is consistent with that of another prototype K-X-K-X-X (Fig. 1b; McCartney et al. 2004). However, PfrFAD7-1 and PfrFAD7-2 have no ER retrieval motifs (Fig. 1b). According to the analysis using the TOPCONS website, similar to other plant FAD2 isozymes, six TMDs are predicted in PfrFAD2-1 and PfrFAD2-2 (Fig. 1a; Okuley et al. 1994; Lee et al. 2012); the first to second and the fifth to sixth TMDs are considered as double-pass membrane domains, whereas the third to fourth TMDs appear to be single-pass membrane domains (Okuley et al. 1994; Hernandez et al. 2005). In contrast, perilla ω-3 FA desaturases have four predicted TMDs that resemble double-pass membrane domains (Fig. 1b).There are two distinct aa residues in TM5 of PfrFAD2s. Ala and Val vary at 222 position and Val and Leu vary at 243 position in PfrFAD2-1 and PfrFAD2-2, respectively (Fig. 1a). Four aa residues are the same chemical property (hydrophobic) in TM5 of PfrFAD2s, hence it is not predicted that the range from 222 to 242 position and function of TM of PfrFAD2 s are changed. One aa residue, Asn and Ser is different at 61 position of PfrFAD3-1 and PfrFAD3-2, respectively (Fig. 1b). The region is not critical to desaturase function. In addition, Ala or Ser is common aa residue in ω-3 FA desaturases from Arabidopsis (Fig. 1b). Therefore, it is expected that both PfrFAD3-1 and -2 have the function of ω-3 FA desaturases.
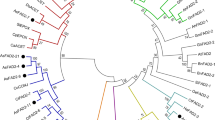
Phylogenetic relationships of FA desaturases
Phylogenetic analyses of six FA desaturase genes from perilla were performed using deduced aa sequences of 32 FAD2 genes and 32 ω-3 FA desaturase genes from 29 plant species (Fig. 2). Multiple FAD2 genes have been identified in various plant species, including soybean (Glycine max; Heppard et al. 1996; Tang et al. 2005; Li et al. 2007), sunflower (Helianthus annuus; Hongtrakul et al. 1998), and Camelina sativa (Kang et al. 2011), and these were separately grouped according to expression patterns (Fig. 2). More than three FAD3 genes have been identified in soybean (Flores et al. 2008), oilseed rape (Brassica napus; Yang et al. 2012) and flax (Linum usitatissimum; Banik et al. 2011, Vrinten et al. 2005).
Phylogenetic analysis of deduced aa sequences of ω-3 fatty acid desaturase and microsomal oleate 12-desaturase genes. Letters and numbers behind scientific names indicate GenBank accession numbers. Blank boxes indicate perilla fatty acid desaturase genes and their GenBank Accession Numbers are as follows. PfrFAD2-1, KP070823; PfrFAD2-2, KX228916; PfrFAD3-1, AF047039; PfrFAD7-1, U59477; PfrFAD7-2, KP070824. Sequences were aligned using DNASTAR® MegAlign (Ver. 8.1.4) with the ClustalW method and 1000 bootstrapping cycles. The phylogenetic tree was generated using TreeView (Ver. 1.6.6) with the aligned data. Bootstrap values greater than 50 are noted at each branch point; Scale bars represent 0.1 aa substitutions per site
PfrFAD2-1 and PfrFAD2-2 genes were grouped in constitutive expressed FAD2 s. Although whole genome sequencing of P. frutescens has not been completed, P. frutescens is known as an allotetraploid plant (2n = 40; Nitta et al. 2005), hybridized from P. citriodora (2n = 20) and unknown wild perilla species (Honda et al. 1994), indicating the potential for multiple FAD2 and FAD3 genes in perilla. On the contrary, the transcriptome data in Table 3 suggest the expression of only single FAD2 and FAD3 genes in perilla. It is likely to result from nucleotide sequences mixing caused by the nearly identical sequences between two FAD2 genes or two FAD3 genes. The near identity (99 %) between PfrFAD2 or PfrFAD3 genes suggests that P. frutescens hybridized from two ancestral wild perilla species recently.
In the present study, ω-3 FA desaturases were separately grouped as chloroplast- and ER-localized (Fig. 2), and the chloroplast-localized perilla ω-3 FA desaturase isoform PfrFAD7-2 was adjacent to PfrFAD7-1. Considering the close relationship between FAD7 and FAD8 enzymes in Arabidopsis, these enzymes are not likely to be present without the other. Moreover, according to the annotation of GenBank, FAD7 isozymes from other plants, such as Solanum lycopersicon, S. tuberosum, O. europaea, and Portulaca oleracea, and FAD8 isozymes from Glycine max, Camellia sinensis, and Brassica rapa were mixed in the same clade. Hence, FAD7 and FAD8 were indistinguishable according to their sequences.
Expression patterns of FA desaturase genes from perilla
Transcriptome and expression analyses of transcripts that correspond with perilla FA desaturase genes during the developmental stages of perilla seeds are described in Table 4. In these analyses, PfrFAD2 and PfrFAD3 genes were expressed in leaves and during all stages of seed development, and their expression levels in 2- to 3-week-old developing seeds were 6.7- and 25-fold higher than their expression in leaves, respectively. The expression level and relative expression to leaves of FAD3 gene were higher than those of FAD2 genes. It suggests that high 18:3 in perilla seed oil is caused by high expression level of FAD3 gene (Table 4). In contrast, although the expression of PfrFAD7-1 and PfrFAD7-2 was similar to that of PfrFAD2 and PfrFAD3 genes in leaves (Table 4), PfrFAD7-1 and PfrFAD7-2 expression was much lower in developing seeds (Table 4). According to Table 4, the expression levels of PfrFAD2 and PfrFAD3 genes were much higher in 2- and 3-week-old developing seeds than those in leaves (Fig. 3). However, PfrFAD7-1 and PfrFAD7-2 genes were expressed at low levels during all stages of developing seeds as compared with expression levels in leaves (Fig. 3).
Differential expression of four perilla FA desaturase genes in four seed developmental stages. The Y axis represents log2 value of expression value of seed developmental stages versus that of leaves from Table 4. S1/L to S4/L in the X axis correspond with 1- to 4-week-old developing seeds (S1 to S4) versus leaves (L)
To confirm the expression levels of perilla FA desaturase genes, total RNAs were prepared from 2-week-old leaves, stems, roots, flowers, 5-day-old seedlings as well as from 1-, 2-, 3-, and 4-week-old developing seeds. Accordingly, PfrFAD2-1, -2 genes were classified as a member of the constitutive FAD2 clade (Fig. 2) and were constitutively expressed, although expression levels differed between tissues. Specifically, the PfrFAD2 genes was expressed in leaves, flowers, and seedlings; it was weakly expressed in stems and roots but it was 2.5- to 5-fold higher expressed in developing seeds than other tissues (Fig. 4a). Expression pattern of two PfrFAD2 genes could not be determined separately because of their too high nucleotide identity. However, two PfrFAD2 genes were cloned from both leaves and developing seeds tissues with similar proportion; therefore, two PfrFAD2 genes are likely to be expressed in both vegetative tissues such as leaves, and developing seeds.
Relative expression levels of a PfrFAD2, b PfrFAD3, c PfrFAD7-1, and d PfrFAD7-2 genes in several perilla tissues. L leaves, St stems, R roots, F flowers, S seedlings, DS1-4 1- to 4-week-old developing seeds, respectively. Perilla β-actin (GenBank Accession No. AB002819) was used as reference gene. Experiments were performed in triplicate and error bars indicate standard deviations
A previous study demonstrated seed-specific expression of the PfrFAD3 gene (Chung et al. 1999). Accordingly, the relative expression of PfrFAD3 was 192-fold greater in 3-week-old developing seeds than in other tissues, and the maximally high 18:3 contents of perilla seed oil likely reflect the high expression of PfrFAD3 in developing seeds. Even though PfrFAD3 was weakly expressed at 0.14- to 1-fold in other tissues, transcripts corresponding to PfrFAD3 were expressed in leaves (Table 4) and AtFAD3 was previously found to be directly responsible for the synthesis of 18:3 in plasma membranes of leaves as well as seeds (Browse et al. 1993; Smith et al. 2003). Like the case of AtFAD3, it is likely that PfrFAD3 contributes to the 18:3 contents in most tissues and is expressed abundantly in seeds. Similar to the case of PfrFAD2 genes, the expression pattern of two PfrFAD3 genes could not be determined because they share 99 % of nucleotide sequence identity. However, PfrFAD3 genes showed seed-specific expression by qRT-PCR (Fig. 4b) and Northern blot analysis by Chung et al. (1999). Hence, it is assumed that two PfrFAD3 genes contribute the expression in developing seed tissues with similar ratio.
Two chloroplast-localized ω-3 FA desaturase genes were expressed at low levels in developing seeds (Fig. 4c, d), whereas PfrFAD7-1 was normally expressed in tissues apart from roots and developing seeds (Fig. 4c). PfrFAD7-2 was highly expressed in leaves and roots (Fig. 4d). Arabidopsis FAD7 and FAD8 gene shares high identity in nt level each other and express abundantly in leaves tissue, however, they show differential expression by the temperature (McConn et al. 1994; Gibson et al. 1994). Given that FAD8 is not expressed at normal growth temperatures and is induced at low temperatures, it is unlikely that PfrFAD7-2 is a transcriptional homolog of FAD8 (Fig. S3). Hence, these two genes are likely responsible for the synthesis of ω-3 FAs in chloroplast membrane in leaves, except developing seeds.
Subcellular localization of FA desaturase genes from perilla
FAD2- and FAD3-mediated desaturation of PC occurs in the ER (Somerville et al. 2000). Accordingly, these desaturases carry ER retrieval motifs at their C-terminal ends (McCartney et al. 2004). Plant ω-3 FA desaturases are highly homologous, with the exception of the chloroplast localizing N-terminal extension of some ω-3 FA desaturases (Fig. 1b). Typical features of chloroplast transit peptides (cTPs) indicate that this N-terminal extension leads to chloroplast localization. Specifically, hydroxylated aa residues (Ser and Thr) are enriched by over 20 %, and few acidic residues are present (Glu and Asp; von Heijne et al. 1989); the Ala following the initial Met is highly conserved (von Heijne et al. 1989). Hence, ω-3 FA desaturases are membrane-bound proteins that are most likely localized in chloroplast inner membranes or thylakoid membranes (Gibson et al. 1994). Proteomic analyses demonstrated that FAD7 and FAD8 are also located in the chloroplast envelope (Ferro et al. 2003; Joyard et al. 2010). In contrast, Andreu et al. (2007) performed immunogold labeling and Western blot analyses of subfractions, and they showed that soybean FAD7 is preferentially localized in thylakoid membranes. Hence, the present data indicate that FAD7 and FAD8 are targeted to chloroplasts, but their precise chloroplast locations remain unknown. According to the ChloroP prediction server (Emanuelsson et al. 1999), two chloroplast-localized ω-3 FA desaturases of Arabidopsis and perilla were predicted to carry the cTP and be targeted to the chloroplast, whereas FAD2 and FAD3 enzymes of perilla were not (Fig. 1).
In this study, it is revealed that perilla contains two FAD2 and FAD3 genes, respectively; however, it is thought that they are almost identical to each other and they are not necessary to be functionally distinct determined. Therefore, subcellular localization and functional analysis were examined for PfrFAD2-1, PfrFAD3-2, PfrFAD7-1 and PfrFAD7-2. To confirm the subcellular localization of these four desaturase genes of perilla, protoplasts of A. thaliana were transformed with p326-sGFP vector containing GFP-fused genes using PEG transformation methods; the patterns of transient expression were observed using confocal microscopy. In these analyses, the GFP signals of PfrFAD2-1:GFP and PfrFAD3-2:GFP seemed to be localized with the ER (Fig. 5a). To confirm this, PfrFAD2-1:GFP and PfrFAD3-2:GFP were cotransfected with the ER marker protein BiP:RFP. GFP signal of PfrFAD2-1:GFP was colocalized with the RFP signal of ER marker protein BiP:RFP (Fig. 5b), in accordance with previous immunocytological ER localization of Arabidopsis FAD2 (Dyer and Mullen 2001) and tung FAD2 (Aleurites fordii; Dyer et al. 2002) as well as the subcellular ER localization of cotton FAD2–4:GFP (Zhang et al. 2009), B. rapa FAD2–1:GFP (Jung et al. 2011), and three yellow fluorescence protein-fused FAD2 enzymes of B. napus (Lee et al. 2013). In agreement with previous immunocytological localization of B. napus FAD3 (Dyer and Mullen 2001) and the subcellular localization of tung FAD3:GFP (O’Quin et al. 2010), rice (Oryza sativa) FAD3:GFP, and three GFP-fused FAD3 of soybean (Liu et al. 2012), PfrFAD3-2 was also colocalized with BiP in the ER (Fig. 5b). Two chloroplast-localized ω-3 FA desaturases of perilla were found to be localized in the chloroplast (Fig. 5c), as shown in a previous study representing the subcellular localization of GFP-fused FAD7 and FAD8 of rice (Liu et al. 2012). Merged image of GFP signal of PfrFAD7-1 and PfrFAD7-2, and red autofluoresence of chlorophyll in chloroplast showed that GFP signal is stronger boundary of chloroplast and the aggregated spot in chloroplast. Boundary green signals and inner orange signals indicate that PfrFAD7-1 and 7-2 are localized in chloroplast envelope. However, aggregated spot might be artifact due to overexpression of chloroplast envelope protein:GFP-fused protein in Arabidopsis protoplasts (Machettira et al. 2012).
Subcellular localization of (a, b) ER-localized FA desaturases (PfrFAD2-1 and PfrFAD3-2) and c chloroplast-localized FA desaturases (PfrFAD7-1 and PfrFAD7-2) fused to GFP after transfection of Arabidopsis protoplasts with p326-sGFP vectors harboring each of these genes for each GFP-fused enzyme using PEG transformation method, respectively. The images were captured using a fluorescence microscope. BiP is an ER marker. Bars indicate 20 μm
Functional analysis of FA desaturase genes from perilla in yeast
To confirm the functions of perilla FA desaturase genes, the budding yeast S. cerevisiae INVSc1 was transformed with pYES-DEST52 yeast expression vectors harboring each of the four perilla genes. FA analyses demonstrated that pYES2-transformed control yeast produced only 16:0, 16:1, 18:0, and 18:1, whereas yeast harboring PfrFAD2 also produced 18:2 and 16:2 (Fig. 6a), which were not detected in wild-type yeast. Conversion ratios of 16:2 and 18:2 by PfrFAD2 were calculated as follows: Conversion ratio = products/(products + substrates), giving (16:2 + 18:2)/(16:1 + 16:2 + 18:1 + 18:2) = (10.38 + 7.54)/(46.90 + 10.38 + 18.40 + 7.54) = 0.215 (21.5 %). Although no additional peaks were detected in pYES2 transformant supplemented with 18:2, pYES2-PfrFAD3, pYES2-PfrFAD7-1, and pYES2-PfrFAD7-2 transformants produced 18:3 when supplemented with 18:2 (Fig. 6b). Although the production of 18:3 was limited (1.3 %), it was similar to that shown in the FA analyses of FAD3 in B. napus and/or PfrFAD3 in yeast (Reed et al. 2000; Dyer et al. 2004; Abdel-Reheem and Hildebrand 2013). Conversion ratios of 18:3 by PfrFAD3 and PfrFAD7-2 were approximately 4 %, and that of 18:3 by PfrFAD7-1 was 1.9 %, which is less than the conversion ratios of other perilla ω-3 FA desaturases. Taken together, these data indicate that PfrFAD2, PfrFAD3, PfrFAD7-1, and PfrFAD7-2 genes encode functional FA desaturase enzymes that produce 18:2 or 18:3, whose peaks have the same retention time as internal standard 18:2 or 18:3, respectively.
Chromatograms of FAMEs from a budding yeast harboring PfrFAD2-1 and b 18:2 supplemented budding yeast harboring each of the three perilla ω-3 FA desaturase genes obtained by GC-FID, respectively. Oven temperature increases from 170 to 180 °C at 1 °C/min for 10 min and the flow rate of nitrogen as carrier gas at a of 1.4 ml/min. FAMEs were prepared with the method described in “Materials and methods”. pYES2 is a control. FID flame ionization detector
Conclusion
We newly identified and characterized four FA desaturase genes including two FAD2 and two ω-3 FA desaturase genes from perilla, the highest ω-3 FA content oil crop. Among these, the member of the PfrFAD2 gene family share near identified in protein sequence as do members of the PfrFAD3 gene family. The chloroplast-localized ω-3 FA desaturase genes were identified two distinct genes, PfrFAD7-1 and PfrFAD7-2, by the analysis of transcriptome datasets. Expression analyses demonstrated that PfrFAD2 and PfrFAD3 are expressed in the most tissues and are particularly expressed strongly in developing seeds, and that PfrFAD7-1 and PfrFAD7-2 genes are abundantly expressed in vegetative tissues. Subcellular localization experiments showed that GFP-fused PfrFAD2 and PfrFAD3 are directed to the ER, and that GFP-fused PfrFAD7-1 and PfrFAD7-2 are localized in chloroplasts. Functional analyses in budding yeast confirmed that PfrFAD2 acts as an 18:1 FA desaturase, and that PfrFAD3, PfrFAD7-1, and PfrFAD7-2 have desaturase activity for 18:2, indicating ω-3 FA desaturase. This study demonstrated that the biosynthesis of 18:3 in perilla seeds potentially results from the sequential contributions of two FAD2 isozymes and two FAD3 enzymes and that FAD7-1 and FAD7-2 may each contribute to the biosynthesis of 18:3 expressed in perilla vegetative tissues. This finding will contribute to the research for the regulation of 18:3 content in perilla seed oil. In addition, the presence of two copies of FAD2, FAD3 and FAD7 genes in P. frutescens var. frutescens is consistent with P. frutescens as allotetraploid plant.
Author contribution statement
KRL carried out experiments and manuscript preparation; SBL performed quantitative RT-PCR and vector construction; YL provided the data of subcellular localization; EHK and KHR carried out GC analysis; JBK helped cloning of perilla genes; HCK helped construction of phylogenetic tree and multiple alignment; HUK was designed the experimental plan and provided perilla transcriptome data. All authors were involved in editing and approved the manuscript.
Abbreviations
- PUFA:
-
Polyunsaturated fatty acid
- FA:
-
Fatty acid
- ER:
-
Endoplasmic reticulum
- PC:
-
Phosphatidylcholine
- UTR:
-
Untranslated region
- FAME:
-
Fatty acid methyl ester
- Nt:
-
Nucleotide
- Bp:
-
Base pair
- Aa:
-
Amino acid
- PG:
-
Phosphatidylglycerol
- DAG:
-
Diacylglycerol
- PA:
-
Phosphatidic acid
- TMDs:
-
Transmembrane domains
References
Abdel-Reheem M, Hildebrand D (2013) Activity of Brassica napus and Perilla frutescens microsomal ω-3 desaturases expressed in yeast (Saccharomyces cerevisiae). Turk J Biol 37:591–605
Anders S, Huber W (2010) Differential expression analysis for sequence count data. Genome Biol 11:R106
Andreu V, Collados R, Testillano PS, del C Risueño M, Picorel R, Alfonso M (2007) In situ molecular identification of the plastid ω3 fatty acid desaturase FAD7 from soybean: evidence of thylakoid membrane localization. Plant Physiol 145:1336–1344
Arondel V, Lemleux B, Hwang I, Gibson S, Goodman HM, Somerville CR (1992) Map-based cloning of a gene controlling omega-3 fatty acid desaturation in Arabidopsis. Science 258:1353–1355
Asif M (2011) Health effects of omega-3,6,9 fatty acids: Perilla frutescens is a good example of plant oils. Orient Pharm Exp Med 11:51–59
Banik M, Duguid S, Cloutier S (2011) Transcript profiling and gene characterization of three fatty acid desaturase genes in high, moderate, and low linolenic acid genotypes of flax (Linum usitatissimum L.) and their role in linolenic acid accumulation. Genome 54:471–483
Browse J, McConn M, James D Jr, Miquel M (1993) Mutants of Arabidopsis deficient in the synthesis of α-linolenate. Biochemical and genetic characterization of the endoplasmic reticulum linoleoyl desaturase. J Biol Chem 268(22):16345–16351
Chung CH, Kim JL, Lee YC, Choi YL (1999) Cloning and characterization of a seed-specific ω-3 fatty acid desaturase cDNA from Perilla frutescens. Plant Cell Physiol 40:114–118
Ciftci ON, Przybylski R, Rudzińska M (2012) Lipid components of flax, perilla, and chia seeds. Eur J Lipid Sci Technol 114:794–800
Cox MP, Peterson DA, Biggs PJ (2010) SolexaQA: at-a-glance quality assessment of Illumina second-generation sequencing data. BMC Bioinform 11:485
Dyer JM, Mullen RT (2001) Immunocytological localization of two plant fatty acid desaturases in the endoplasmic reticulum. FEBS Lett 494:44–47
Dyer JM, Chapital DC, Kuan JCW, Mullen RT, Turner C, McKeon TA, Pepperman AB (2002) Molecular analysis of a bifunctional fatty acid conjugase/desaturase from tung. Implications for the evolution of plant fatty acid diversity. Plant Physiol 130:2027–2038
Dyer JM, Chapital DC, Kuan JCW, Shepherd HS, Tang F, Pepperman AB (2004) Production of linolenic acid in yeast cells expressing an omega-3 desaturase from tung (Aleurites fordii). J Am Oil Chem Soc 81:647–651
Emanuelsson O, Nielsen H, von Heijne G (1999) ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Sci 8:978–984
Ferro M, Salvi D, Brugière S, Miras S, Kowalski S, Louwagie M, Garin J, Joyard J, Rolland N (2003) Proteomics of the chloroplast envelope membranes from Arabidopsis thaliana. Mol Cell Proteom 2:325–345
Flores T, Karpova O, Su X, Zeng P, Bilyeu K, Sleper DA, Nguyen HT, Zhang ZJ (2008) Silencing of GmFAD3 gene by siRNA leads to low α-linolenic acids (18:3) of fad3-mutant phenotype in soybean [Glycine max (Merr.)]. Transgenic Res 17:839–850
Gibson S, Arondel V, Iba K, Somerville C (1994) Cloning of a temperature-regulated gene encoding a chloroplast ω-3 desaturase from Arabidopsis thaliana. Plant Physiol 106:1615–1621
Heppard EP, Kinney AJ, Stecca KL, Miao GH (1996) Developmental and growth temperature regulation of two different microsomal ω-6 desaturases in soybean. Plant Physiol 110:311–319
Hernandez ML, Mancha M, Martinez-Rivas JM (2005) Molecular cloning and characterization of genes encoding two microsomal oleate desaturases (FAD2) from olive. Phytochemistry 66:1417–1426
Honda G, Yuba A, Kojima T, Tabata M (1994) Chemotaxonomic and cytogenetic studies on Perilla frutescens var. citriodora (“Lemon Egoma”). Nat Med 48:185–190
Hongtrakul V, Slabaugh MB, Knapp SJ (1998) A seed specific Δ-12 oleate desaturase is duplicated, rearranged, and weakly expressed in high oleic acid sunflower lines. Crop Sci 38:1245–1249
Iba K, Gibson S, Nishiuch T, Fuse T, Nishimura M, Arondel V, Hugly S, Somerville C (1993) A gene encoding a chloroplast ω-3 fatty acid desaturase complements alterations in fatty acid desaturation and chloroplast copy number of the fad7 mutant of Arabidopsis thaliana. J Biol Chem 268:24099–24105
Jin JB, Kim YA, Kim SJ, Lee SH, Kim DH, Cheong GW, Hwang I (2001) A new dynamin-like protein, ADL6, is involved in trafficking from the trans-Golgi network to the central vacuole in Arabidopsis. Plant Cell 13:1511–1526
Joyard J, Block MA, Malherbe A, Maréchal E, Douce R (1993) Origin and synthesis of galactolipid and sulfolipid head groups. In: Moore TS Jr (ed) Lipid metabolism in plants. CRC Press, Boca Raton, pp 231–258
Joyard J, Ferro M, Masselon C, Seigneurin-Berny D, Salvi D, Garin J, Rolland N (2010) Chloroplast proteomics highlights the subcellular compartmentation of lipid metabolism. Prog Lipid Res 49:128–158
Jung JH, Kim H, Go YS, Lee SB, Hur C-G, Kim HU, Suh MC (2011) Identification of functional BrFAD2-1 gene encoding microsomal delta-12 fatty acid desaturase from Brassica rapa and development of Brassica napus containing high oleic acid contents. Plant Cell Rep 30:1881–1892
Kang J, Snapp AR, Lu C (2011) Identification of three genes encoding microsomal oleate desaturase (FAD2) from the oilseed crop Camelina sativa. Plant Physiol Biochem 49:223–229
Kim HU, Lee K-R, Shim D, Lee JH, Chen GQ, Hwang S (2016) Transcriptome analysis and identification of genes associated with ω-3 fatty acid biosynthesis in Perilla frutescens (L.) var. frutescens. BMC Genom 17:474
Kurdrid P, Subudhi S, Hongsthong A, Ruengjitchatchawalya M, Tanticharoen M (2005) Functional expression of Spirulina-Δ6 desaturase gene in yeast, Saccharomyces cerevisiae. Mol Biol Rep 32:215–226
Lee S-K, Kim K-H, Kwon M-S, Hwang Y-S (2001a) Molecular cloning and characterization of expression patterns of a plastid ω-3 fatty acid desaturase cDNA from Perilla frutescens. Agric Chem Biotechnol 44:6–11
Lee YJ, Kim DH, Kim Y-W, Hwang I (2001b) Identification of a signal that distinguishes between the chloroplast outer envelope membrane and the endomembrane system in vivo. Plant Cell 13:2175–2190
Lee K-R, Kim SH, Go Y-S, Jung SM, Roh KH, Kim J-B, Suh M-C, Lee S, Kim HU (2012) Molecular cloning and functional analysis of two FAD2 genes from American grape (Vitis labrusca L.). Gene 509:189–194
Lee K-R, Sohn SI, Jung JH, Kim SH, Roh KH, Kim JB, Suh MC, Kim HU (2013) Functional analysis and tissue-differential expression of four FAD2 genes in amphidiploid Brassica napus derived from Brassica rapa and Brassica oleracea. Gene 531:253–262
Li L, Wang X, Gai J, Yu D (2007) Molecular cloning and characterization of a novel microsomal oleate desaturase gene from soybean. J Plant Physiol 164:1516–1526
Liu HL, Yin ZJ, Xiao L, Xu YN, le Qu Q (2012) Identification and evaluation of ω-3 fatty acid desaturase genes for hyperfortifying α-linolenic acid in transgenic rice seed. J Exp Bot 63:3279–3287
Los DA, Murata N (1998) Structure and expression of fatty acid desaturases. Biochim Biophys Acta 1394:3–15
Machettira AB, Groß LE, Tillmann B, Weis BL, Englich G, Sommer MS, Königer M, Schleiff E (2012) Protein-induced modulation of chloroplast membrane morphology. Front Plant Sci 2:118
McCartney AW, Dyer JM, Dhanos PK, Kim PK, Andrews DW, McNew JA, Mullen RT (2004) Membrane-bound fatty acid desaturases are inserted co-translationally into the ER and contain different ER retrieval motifs at their carboxy termini. Plant J 37:156–173
McConn M, Hugly S, Browse J, Somerville C (1994) A mutation at the fad8 locus of Arabidopsis identifies a second chloroplast ω-3 desaturase. Plant Physiol 106:1609–1614
Miquel M, James D, Dooner H, Browse J (1993) Arabidopsis requires polyunsaturated lipids for low-temperature survival. Proc Natl Aca Sci USA 90:6208–6212
Nitta M, Lee JK, Kang CW, Katsuta M, Yasumoto S, Liu D, Nagamine T, Ohnishi O (2005) The distribution of Perilla species. Genet Resour Crop Ev 52:797–804
Ohlrogge J, Browse J (1995) Lipid biosynthesis. Plant Cell 7:957–970
Okuley J, Lightner J, Feldmann K, Yadav N, Lark E, Browse J (1994) Arabidopsis FAD2 gene encodes the enzyme that is essential for polyunsaturated lipid synthesis. Plant Cell 6:147–158
O’Quin JB, Bourassa L, Zhang D, Shockey JM, Gidda SK, Fosnot S, Chapman KD, Mullen RT, Dyer JM (2010) Temperature-sensitive post-translational regulation of plant omega-3 fatty-acid desaturases is mediated by the endoplasmic reticulum-associated degradation pathway. J Biol Chem 285:21781–21796
Reed DW, Schäfer UA, Covello PS (2000) Characterization of the Brassica napus extraplastidial linoleate desaturase by expression in Saccharomyces cerevisiae. Plant Physiol 122:715–720
Routaboul J-M, Fischer SF, Browse J (2000) Trienoic fatty acids are required to maintain chloroplast function at low temperatures. Plant Physiol 124:1697–1705
Schulz MH, Zerbino DR, Vingron M, Birney E (2012) Oases: robust de novo RNA-seq assembly across the dynamic range of expression levels. Bioinformatics 28:1086–1092
Shanklin J, Whittle E, Fox BG (1994) Eight histidine residues are catalytically essential in a membrane-associated iron enzyme, stearoyl-CoA desaturase, and are conserved in alkane hydroxylase and xylene monooxygenase. Biochemistry 33:12787–12794
Shin HS, Kim SW (1994) Lipid composition of perilla seed. J Am Oil Chem Soc 71:619–622
Smith MA, Moon H, Chowrira G, Kunst L (2003) Heterologous expression of a fatty acid hydroxylase gene in developing seeds of Arabidopsis thaliana. Planta 217:507–516
Somerville C, Browse J, Jaworski JG, Ohlrogge JB (2000) Lipids. In: Buchanan BB, Gruissem W, Jones RL (eds) Biochemistry and molecular biology of plants. American Society of Plant Physiologists, Rockville, pp 456–527
Tang GQ, Novitzky WP, Griffin HC, Huber SC, Dewey RE (2005) Oleate desaturase enzymes of soybean: evidence of regulation through differential stability and phosphorylation. Plant J 44:433–446
Torres-Franklin M-L, Repellin A, Huynh V-B, d’Arcy-Lameta A, Zuily-Fodil Y, Pham-Thi A-T (2009) Omega-3 fatty acid desaturase (FAD3, FAD7, FAD8) gene expression and linolenic acid content in cowpea leaves submitted to drought and after rehydration. Environ Exp Bot 65:162–169
von Heijne G, Steppuhn J, Herrmann RG (1989) Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem 180:535–545
Vrinten P, Hu Z, Munchinsky M-A, Rowland G, Qiu X (2005) Two FAD3 desaturase genes control the level of linolenic acid in flax seed. Plant Physiol 139:79–87
Yadav NS, Wierzbicki A, Aegerter M, Caster CS, Pérez-Grau L, Kinney AJ, Hitz WD, Booth JR Jr, Schweiger B, Stecca KL, Allen SM, Blackwell M, Reiter RS, Carlson TJ, Russell SH, Feldmann KA, Pierce J, Browse J (1993) Cloning of higher plant ω-3 fatty acid desaturases. Plant Physiol 103:467–476
Yang Q, Fan C, Guo Z, Qin J, Wu J, Li Q, Fu T, Zhou Y (2012) Identification of FAD2 and FAD3 genes in Brassica napus genome and development of allele-specific markers for high oleic and low linolenic acid contents. Theor Appl Genet 125:715–729
Zerbino DR, Birney E (2008) Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res 18:821–829
Zhang D, Pirtle IL, Park SJ, Nampaisansuk M, Neogi P, Wanjie SW, Pirtle RM, Chapman KD (2009) Identification and expression of a new delta-12 fatty acid desaturase (FAD2-4) gene in upland cotton and its functional expression in yeast and Arabidopsis thaliana plants. Plant Physiol Biochem 47:462–471
Acknowledgments
The perilla seeds used in this study were kindly provided by Dr. Myung-Hee Lee of the Dept. of the Southern Area Crop Science, National Institute of Crop Science, in Miryang, Republic of Korea. This study was conducted with the support of the Research Program for Agricultural Science & Technology Development (Project No. PJ01007504), the National Institute of Agricultural Science, Rural Development Administration, and the Next-Generation BioGreen 21 Program (SSAC, Grant No. PJ01108101), Republic of Korea as well as the faculty research fund (Project No. 20160163) of Sejong University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by J. Sheop Shin.
Electronic supplementary material
Below is the link to the electronic supplementary material.
299_2016_2053_MOESM1_ESM.pptx
Supplementary Fig. S1 Multiple sequence alignment of two FAD2 genes from perilla leaves and developing seeds tissues. Black box and dotted box indicate PfrFAD2-1 and PfrFAD2-2 specific sequence, respectively. Multiple sequence alignment was performed using DNASTAR® MegAlign (Ver. 8.1.4) (PPTX 887 kb)
299_2016_2053_MOESM2_ESM.pptx
Supplementary Fig. S2 Multiple sequence alignment of two FAD3 genes from diverse perilla cultivar. Black box and dotted box indicate PfrFAD3-1 and PfrFAD3-2 specific sequence, respectively. Exceptionally, there is a single nucleotide polymorphism at 300 bp position in F2 and F4. 13 sequences except PfrFAD3-1 and PfrFAD3-2 were from perilla cultivar as follows. A1,2, Dayudeulkkae; B2, K131012; C1,3, K135903; D4, K131017; E5,6, Anyu; F2,3,4, K135858; G2,3, K126202. These cultivars were obtained from National Agrobiodiversity Center, National Institute of Agricultural Sciences, Republic of Korea. Multiple sequence alignment was performed using DNASTAR® MegAlign (Ver. 8.1.4) (PPTX 588 kb)
299_2016_2053_MOESM3_ESM.pptx
Supplementary Fig. S3 Relative expression levels of PfrFAD2, PfrFAD3, PfrFAD7-1, and PfrFAD7-2 genes from leaves of perilla treated or untreated at low temperature (15˚C) for two weeks. Unattached any letter in front of gene name and LT-gene name indicate untreated and treated low temperature, respectively. Experiments were performed in triplicate and error bars indicate standard deviations (PPTX 48 kb)
Rights and permissions
About this article
Cite this article
Lee, KR., Lee, Y., Kim, EH. et al. Functional identification of oleate 12-desaturase and ω-3 fatty acid desaturase genes from Perilla frutescens var. frutescens . Plant Cell Rep 35, 2523–2537 (2016). https://doi.org/10.1007/s00299-016-2053-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-016-2053-4