Abstract
Krebs von den Lungen-6 (KL-6) has been described as a promising biomarker in the diagnosis and determining the severity of interstitial lung disease in adults with connective tissue disease. This study was performed to determine whether the serum KL-6 level is useful as a biomarker for detecting the interstitial lung disease (ILD) in pediatric cases of connective tissue disease (CTD). In total, 88 patients [36 patients with systemic juvenile idiopathic arthritis (sJIA), 27 patients with juvenile systemic lupus erythematosus (JSLE), 14 patients with juvenile dermatomyositis (JDM), and 11 patients with juvenile systemic sclerosis (JSSc)] and 68 healthy controls were included in this study. Age, sex, and duration of CTD and ILD (if any) were recorded. Blood samples from all the patients and controls were examined by ELISA. Eleven of the 88 patients with CTD (12.5%) had ILD and all of them were symptomatic. Subgroup analysis indicated that eight patients had JSSc, two had JSLE, and one had systemic JIA. The median serum KL-6 level was 1450.5 U/mL (interquartile range (IQR) 742.9–2603.2) in the CTD with ILD group, 415.9 U/mL (IQR 233.4–748.4) in the CTD without ILD group, and 465.9 U/mL (IQR 273.6–1036) in the control group. KL-6 levels were significantly higher in the CTD with ILD group than the CTD without ILD group and the control group (p = 0.003). At a cut-off of 712.5 U/ml identified by ROC curve, serum KL-6 yielded a sensitivity of 81% and specificity of 72% for CTD with ILD group. There was no significant difference in serum KL-6 level among the disease subgroups (sJIA, JSLE, JSSc, JDM), in either the CTD with ILD group or the CTD without ILD group (p > 0.05). In conclusion, KL-6 is a useful biomarker of CTD with ILD in pediatric patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic juvenile idiopathic arthritis (sJIA), juvenile systemic lupus erythematosus (JSLE), juvenile systemic sclerosis (JSSc), and juvenile dermatomyositis (JDM) are immune-mediated connective tissue diseases (CTD) that can affect many organs [1]. Immune mechanisms involved in the pathogenesis of these diseases may cause structural changes or damage in the various tissues such as the eye, musculoskeletal, cardiovascular, and respiratory systems. Interstitial, alveolar, pleural, airway, vascular, and lymphatic structures may be affected in CTD-related pulmonary disease. Lung involvement often manifests in the form of interstitial lung disease (ILD) [2, 3]. ILD can cause destruction of the pulmonary structure. It is very rare in children and is seen most commonly in JSSc. ILD also may occur in with sJIA, JSLE, and JDM [1, 4]. Although it has been common in JSSc, recent studies have shown that the ILD is increasing in sJIA. It is stated that the increasing ILD in sJRA is associated with early development of disease and macrophage activation syndrome [5, 6]. ILD can cause uncontrolled systemic disease activity in CTD patients. CTD with comorbid ILD can be fatal, and early detection of the disease is, therefore, important [2, 3, 5]. The diagnosis of ILD requires careful evaluation of clinical, radiological, physiological, and pathological changes of disease. There is no definitive test for diagnosis of ILD in CTD. Although pulmonary function tests (PFTs) and radiological imaging are used to detect ILD, they have low sensitivity and specificity and are not able to diagnose ILD at the early stage [1, 7]. Therefore, novel noninvasive methods for early detection are needed. Krebs von den Lungen-6 (KL-6) is a high molecular weight mucin-like protein that shows increased expression in the serum in response to cellular damage and expression of type-2 alveolar pneumocytes and bronchial epithelial cells. The blood level of KL-6 has been reported to be increased in ILD. In many studies, serum KL-6 levels have a positive correlation with ILD severity [2, 3, 7]. Also KL-6 level is suggested as a clinically useful biomarker in screening and evaluating CTD–ILD, especially in adults [8]. There is no study to determine the relationship between KL-6 and CTD–ILD in children.
This study was performed to examine whether KL-6 is useful as a biomarker for ILD in CTD, by comparing serum KL-6 levels in CTD patients with or without ILD to those of healthy controls.
Materials and methods
Study population
Eighty-eight patients (36 patients with sJIA, 27 patients with JSLE, 14 patients with JDM, and 11 patients with JSSc) and 68 healthy controls were included in this study. Age, sex, duration of CTD and ILD (if any), and the treatment and its duration were recorded. ILD was diagnosed on the basis of the American Thoracic Society guidelines [9]. sJIA, JSLE, JSSc, and JDM were diagnosed according to their accepted criteria, based on the clinical and laboratory findings by pediatric rheumatologists [10,11,12,13]. The patients were questioned regarding the pulmonary symptoms, such as cough, dyspnea, tachypnea, chest pain, and hemoptysis, and considered to have ILD if they had findings on clinical exam, chest X-ray, and pulmonary function tests. High-resolution computed tomography (HRCT) and echocardiography were performed in patients with abnormal radiologic findings and symptoms. Bronchoscopy, bronchoalveolar lavage, and/or open lung biopsies were performed if clinically indicated. The patients aged over 18 years, and those with active or chronic infection, malignancy, or liver or kidney disease were excluded. The control subjects were selected within former patients at the department of pediatrics and all had been approved as healthy before invited to be a volunteer. The control group patients were in similar age and had similar gender distribution with the study group.
Laboratory analysis
Blood samples (10 mL) were collected from all the patients and controls, and stored at − 80 °C after centrifugation at 1000 rpm for 15 min. KL-6 levels were measured using ELISA (MyBioSource) after thawing and appropriate dilution of the samples.
Statistical analysis
Mean, standard deviation, interquartile ranges (IQR), median, range, frequency, and ratio data were computed as descriptive statistics. The distribution of variables was examined by the Kolmogorov–Smirnov test. The Kruskal–Wallis and Mann–Whitney U tests were used for the analysis of quantitative independent data. The χ2 test was used for the analysis of qualitative independent data, and Fisher’s test was used when the assumptions for the χ2 test were not met. Spearman’s correlation analysis was used to examine the associations among the variables. Receiver operating characteristic curve was established to define the best threshold value for KL-6 to discriminate patients. Statistical analyses were performed using SPSS software (ver. 22.0; SPSS Inc., Chicago, IL, USA). In all the analyses, p < 0.05 was taken to indicate the statistical significance. A prior sample size calculation was not considered necessary in view of the exploratory nature of the study.
Ethics
The study was approved by the Medical Ethics Committee of Cerrahpasa Medical Faculty, Istanbul University-Cerrahpasa, Istanbul, Turkey (the date of approval: 02.05.2017, document submission number: 83045809-604.01.02) The methods were carried out in accordance with the principles stated in the Declaration of Helsinki, and informed consent was obtained from the parents/legally authorized representatives of all the study and control group patients under 16 years of age and also an informed consent was obtained from all the study and control group patients who were older than 16 years.
Results
Demographic and clinical characteristics of patient
The study population consisted of 88 patients with CTD, 11 (12.5%) of whom had ILD; their data were compared to those of 68 healthy control subjects. The mean age of the CTD without ILD group was 14.4 ± 3.4 years, versus 16.2 ± 2.4 and 14.3 ± 3.3 years in the CTD with ILD and control groups, respectively. Subgroup analysis indicated that 8 patients in the CTD with ILD group had JSSc, 2 had JSLE, and 1 had sJIA, while 35 patients in the CTD without ILD group had sJIA, 25 had JSLE, 3 had JSSc, and 14 had JDM. The mean disease duration of the patients in the CTD with and without ILD groups was 4.95 ± 1.98 and 5.49 ± 4.02 years, respectively (p > 0.05). The mean duration of ILD was 1.64 ± 0.67 years. While 75% (n = 58) of the patients were female and 25% (n = 19) were male in the CTD without ILD group, 91% (n = 10) were female and 9% (n = 1) were male in the CTD with ILD group. The control group consisted of 51% (n = 35) females and 49% (n = 34) males. Thus, the ratio of females was higher in the CTD with and without ILD groups than the control group (p < 0.05) (Table 1).
All ILD patients were symptomatic. Five patients had cough, three patients had dyspnea, two patients had mild tachypnea with normal oxygen saturatıon, and one patient had both cough and pulmonary hypertension.
Pulmonary function tests
In pulmonary function tests, the CTD without ILD group showed a forced expiratory volume in 1 s (FEV1) (% predicted) of 100 ± 10, forced vital capacity (FVC) (% predicted) of 100 ± 10, and diffusing capacity of the lung for carbon monoxide (DLCO) (% predicted) of 80 ± 10. The CTD with ILD group showed a FEV1% of 80 ± 10, FVC% of 90 ± 10, and DLCO% of 70 ± 10; the differences between the two groups were not significant (all, p > 0.05). Also, there were no significant differences in FEV1%, FVC%, or DLCO% between the JDM, sJIA, JSSc, and JSLE subgroups (all, p > 0.05).
KL-6 levels and relationship between ILD and KL-6
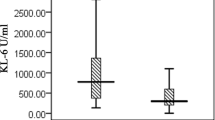
The median serum KL-6 level was 1,450.5 U/mL (IQR 742.9–2603.2, min–max: 271.9–4165) in the CTD with ILD group, 415.9 U/mL (IQR 233.4–748.4, min–max: 75.3–3288) in the CTD without ILD group, and 465.9 U/mL (IQR 273.6–1036, min–max: 48.8–2726) in the control group. KL-6 levels were significantly higher in the CTD with ILD group than the CTD without ILD and control groups (p = 0.003) (Table 2). To assess the diagnostic value of serum KL-6 for CTD with ILD, the receiver of characteristic curve analysis was performed and yielded a cut-off level of 712.5 U/ml. At this cut-off value, the serum, KL-6 level had a sensitivity of 81% and specificity of 72% for the diagnosis of CTD with ILD group (area under the curve (AUC) = 0.80, p < 0,001) (Fig. 1) There were no significant differences in serum KL-6 levels among the disease subgroups (sJIA, JSLE, JSSc, and JDM) in either the CTD with ILD group or the CTD without ILD group (all, p > 0.05). Serum KL-6 was not significantly correlated with the CTD or ILD duration, or with FEV1%, FVC% or DLCO (all, p > 0.05).
Discussion
This study was aimed to evaluate the relationship between ILD and KL-6 in childhood CTD. We confirmed that the serum levels of KL-6 were significantly elevated in CTD patients with versus without ILD.
Childhood CTD is characterized by high morbidity and mortality and can affect many organs. ILD is one of the most serious comorbidities of CTD [2, 3]. A number of studies have been performed to develop methods for early diagnosis of ILD. The serum KL-6 level is increased in regenerated normal lung tissue, and when the air-blood barrier is disrupted in the lungs or expression of alveolar type-2 pneumocytes is increased [14,15,16]. KL-6, which is a sensitive and specific marker for the development of ILD, is also increased in cases of CTD with comorbid ILD [17,18,19,20]. The present study showed that the KL-6 levels were increased in the CTD with ILD patients compared to the CTD without ILD and control groups. At the cut-off value of 712.5 U/ml, calculated by the receiver operating characteristic curve analysis, the serum KL-6 level had a sensitivity of 81% and specificity of 72% for the diagnosis of CTD with ILD patients. Similar results were reported in the literature. In 68 adult patients, Oguz et al. detected increased KL-6 levels in CTD with ILD patients compared to the CTD without ILD and control groups [2]. Similarly, Fathi et al. reported that the KL-6 levels were significantly higher in 12 patients with polymyositis and DM who developed ILD [7]. In addition, Fukaya et al. showed that the KL-6 level was an indicator of ILD in CTD, as well as a biomarker of disease activation and response to treatment [8].
In our study, there was no significant difference in KL-6 level among the CTD subgroups, similar to a previous study of CTD patients with comorbid ILD [2]. In that study, no correlation was found between disease duration and KL-6 level, similar to our study.
The PFT is used to detect the lung involvement in CTD [21,22,23]. Although it is a noninvasive test that can be repeated easily, it requires cooperation from the patient and is not easy to apply, especially in pediatric cases. In a study of polymyositis and DM patients, a negative correlation was found between serum KL-6 level and PFT performance in patients with ILD [7]. In another study, Hu et al. reported polymyositis and DM patients with ILD had a higher KL-6 levels than those without ILD, and they also found that the KL-6 levels were significantly inversely correlated with PFT [14]. In addition, Cao et al. demonstrated that the elevated serum KL-6 correlates with the clinical manifestations of microvascular injury and it may predict deterioration of lung function of SSC–ILD patients [24].
In the present study, there was no relationship between the serum KL-6 level and FEV1%, FVC%, DLCO%. This may have been due to the range of CTD subtypes or the small number of patients in our study.
JIA is the most common CTD of childhood [25, 26]. There were a few studies of children with sJIA and pulmonary complications in the past. But, the recent studies reported that the pulmonary disease is increasingly detected in children with sJIA [6, 27]. Schulert et al. described 18 JIA patients with comorbid lung disease (LD) and found that the developing sJIA before the age of two and history of macrophage activation syndrome are risk factors for sJIA-LD [6]. In another multicenter study, it was found that the macrophage dysfunction and inhibitor exposure may promote life-threatening LD [5]. In this study we have only one patient with sJIA-ILD, so we could not to be able to detect any risk factors for ILD.
This study had some limitations. First, it used a cross-sectional design and included a small number of ILD patients, so we could not draw any definitive conclusions regarding whether KL-6 level is a prognostic or predictive marker of childhood CTD with comorbid ILD. Also, it is difficult to detect powerful association between ILD and different CTDs because of the small number of ILD patients. In addition, the study population was heterogeneous and we did not evaluate the active disease phase in the CTD with ILD group. Additionally, we did not perform HRCT to all the patients to minimize radiation exposure and thus asymptomatic ILD patients might be missed. This reduces the power of our work.
Conclusion
KL-6 can be used as a screening biomarker for ILD in children CTDs. Further studies are needed to validate serum KL-6 as a prognostic or predictive biomarker of ILD in childhood CTD.
References
Dell S, Matejka CK, Hagood JS (2012) Diffuse and interstitial lung disease and childhood rheumatologic disorders. Curr Opin Rheumatol 24:530–540
Oguz EO, Kucuksahin O, Turgay M, Yildizgoren MT, Ates A, Demir N, Kumbasar OO, Kinikli G, Duzgun N (2016) Association of serum KL6 levels with interstitial lung disease patients with connective tissue disease: cross sectional study. Clin Rheumatol 35(3):663–666
Satoh H, Kurishima K, Ishikawa H, Ohtsuka M (2006) Increased levels of KL-6 and subsequent mortality in patients with interstitial lung diseases. J Intern Med 260:429–434
Jee AS, Adelstein S, Bleasel J, Keir GJ, Nguyen M, Sahhar J, Youssef P, Corte TJ (2017) Role of autoantibodies in the diagnosis of connective tissue disease ILD (CTD-ILD) and interstitial pneumonia with autoimmune features (IPAF). J Clin Med 6:51
Saper VE, Chen G, Deutsch GH, Guillerman RP, Birgmeler J et al (2019) Emergent high fatality lung disease in systemic juvenile arthritis. Ann Rheum Dis. https://doi.org/10.1136/annrheumdis-2019-216040
Schulert GS, Yasin S, Carey B, Chalk C, Do T, Schapiro AH, Husami A, Watts A, Brunner H, Huggins J, Mellins ED, Morgan EM, Ting T, Trapnell BC, Wikenheiser- Brokamp K, Towe C, Grom AA (2019) Systemic juvenile idiopathic arthritis–associated lung disease: characterization and risk factors. Arthritis Rheumatol 10.1002/art.41073. (Epub ahead of print)
Fathi M, Helmers SB, Lundberg IE (2012) KL6: a serological biomarker for interstitial lung disease in patients with polymyositis and dermatomyositis. J Intern Med 271:589–597
Fukaya S, Oshima H, Kato K, Komatsu Y, Matsumura H, Ishii K, Miyama H, Nagai T, Tanaka I, Mizutani A, Katayama M, Yoshida S, Torikai K (2000) KL-6 as a novel marker for activities of interstitial pneumonia in connective tissue diseases. Rheumatol Int 19:223–225
Kurland G, Deterding RR, Hagood JS, Young RL, Brody AS, Castile RG, Dell S, Fan LL, Hamvas A, Hilman BC, Langston C, Nogee LM, Redding GJ (2013) An official American Thoracic Society clinical practice guideline: classification, evaluation, and management of childhood interstitial lung disease in infancy. Am J Resp Crit Care Med 188:376–394
Petty RE, Southwood TR, Manners P et al (2004) International League of Associations for Rheumatology Classification of Juvenile Idiopathic Arthritis: second revision, Edmonton 2004. J Rheumatol 31(2):390–392
Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheumatol 40(9):1725–1734
Zullan F, Woo P, Athreya BH et al (2007) The Pediatric Rheumatology European Society/American College of Rheumatology/European League against Rheumatism provisional classification criteria for juvenile systemic sclerosis. Arthritis Rheumatol 57:203–212
Bohan A, Peter JB (1975) Polymyositis and dermatomyositis (first of two parts). N Engl J Med 292:344–347
Hu C, Wu C, Yang E, Huang H, Xu D, Hou Y, Zhao J, Li M, Xu Z, Zeng X, Wang Q (2019) Serum KL6 is associated with the severity of interstitial lung disease in Chinese patients with polymyositis and dermatomyositis. Clin Rheumatol 38(8):1–7
Kumanovics G, Gorbe E, Minier T, Simon D, Berki T, Czirjak L (2014) Follow up serum KL6 lung fibrosis biomarker levels in 173 patients with systemic sclerosis. Clin Exp Rheumatol 32:138–144
Salazar GA, Kuwana M, Wu M, Estrada-Y-Martin RM, Ying J et al (2018) KL-6 but not CCL-18 is a predictor of early progression in systemic sclerosis-related interstitial lung disease. J Rheumatol 45(8):1153–1158
Benyamine A, Heim X, Resseguier N, Bertin D, Gomez C, Ebbo M, Harle JR, Kaplanski G, Rossi P, Bardin N, Granel B (2018) Elevated serum Krebs von den Lungen-6 in systemic sclerosis: a marker of lung fibrosis and severity of the disease. Rheumatol Int 38(5):813–819
Hanaoka M, Katsumata Y, Kawasumi H, Kawaguchi Y, Yamanaka H (2019) KL-6 is a long-term disease-activity biomarker for interstitial lung disease associated with polymyositis/dermatomyositis, but is not a short-term disease-activity biomarker. Mod Rheumatol 29(4):625–632
Hu Y, Wang LS, Jin YP, Du SS, Du YK, He X, Weng D, Zhou Y, Li QH, Shen L, Zhang F, Su YL, Sun XL, Ding JJ, Zhang WH, Cai HR, Dai HP, Dai JH, Li HP (2017) Serum Krebs von den Lungen- 6 level as a diagnostic biomarker for interstitial lung disease in Chinese patients. Clin Respir J 11:337–345
Ishikawa N, Hattori N, Yokoyama A et al (2012) Utility of KL-6/ MUC1 in the clinical management of interstitial lung diseases. Respir Investig 50:3–13
Kinoshita F, Hamano H, Harada H, Kinoshita T, Igıshı T, Hagino H, Ogawa T (2004) Role of KL6 in evaluating the disease severity of rheumatoid lung disease: comparision with HRCT. Respir Med 98:1131–1137
Bonella F, Volpe A, Caramaschi P, Nava C, Ferrari P, Schenk K, Ohshimo S, Costabel U, Ferrari M (2011) Surfactant protein D and KL-6 serum levels in systemic sclerosis: correlation with lung and systemic involvement. Sarcoidosis Vasc Diffuse Lung Dis 28:27–33
Yamakawa H, Hagiwara E, Kitamura H, Yamanaka Y, Ikeda S, Sekine A, Baba T, Okudela K, Iwasawa T, Takemura T, Kuwano K, Ogura T (2017) Serum KL6 and surfactant protein- D as monitoring and predictive markers of interstitial lung disease in patient with systemic sclerosis and mixed connective tissue disease. J Thorac Dis 9:362–371
Cao XY, Hu SS, Xu D, Li M, Wang Q, Hou Y, Zeng X (2019) Serum levels of Krebs von den Lungen-6 as a promising marker for predicting occurrence and deterioration of systemic sclerosis-associated interstitial lung disease from a Chinese cohort. Int J Rheum Dis. 22(1):108–115
Lee JS, Lee YE, Ha YJ, Kang EH, Lee YJ, Song YW (2019) Serum KL-6 levels reflect the severity of interstitial lung disease associated with connective tissue disease. Arthritis Res Ther 21:58
Barut K, Adrovic A, Şahin S, Kasapcopur O (2017) Juvenile Idiopathic Arthritis. Balkan Med J 34(2):90–101
Kimura Y, Weiss JE, Haroldson KL, Lee T, Punaro M, Oliveira S et al (2013) Pulmonary hypertension and other potentially fatal pulmonary complications in systemic juvenile idiopathic arthritis. Arthritis Care Res 65:745–752
Author information
Authors and Affiliations
Contributions
All co-authors contributed substantial contributions to the conception and design of the work; the acquisition, analysis, and interpretation of data for the work. All co-authors made drafting for the work and revising it critically for important intellectual content and made final approval of the version to be published. All co-authors contributed to agreement to be accountable for all aspects of the work in ensuring that the questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All co-authors are familiar and take full responsibility for all aspects of the study and the final manuscript. AAK, HC, AA, and OK contributed to the conception and design of the study, data analysis, and manuscript writing. AAK, AA, AA, and MY had substantially contributed to conception and design of the study and to data analysis. AA, MY, and MK contributed to data collection and data input. HC, KB, OK, and AA contributed to data analysis and manuscript writing. SS, AA, and KB contributed to data analysis and critical revisions. AAK, HC, and OK made final contributions to the conception and design, revision of draft manuscript, and approval of the manuscript to be submitted for publication. All the authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors of this paper has a conflict of interest, including specific financial interests, relationships, and/or affiliations relevant to the subject matter or materials included.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kilinc, A.A., Arslan, A., Yildiz, M. et al. Serum KL-6 level as a biomarker of interstitial lung disease in childhood connective tissue diseases: a pilot study. Rheumatol Int 40, 1701–1706 (2020). https://doi.org/10.1007/s00296-019-04485-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-019-04485-4