Abstract
Optical tomographic imaging (OTI) was reported to be a novel technique for the early diagnosis and disease activity assessment of rheumatoid arthritis (RA). This study aimed to evaluate the clinical utility of OTI for the detection of hand synovitis of RA patients. Manu-scan was used to perform imaging targeting the proximal interphalangeal (PIP) and metacarpophalangeal (MCP) joints in 12 RA patients and three controls. The enrolled RA patients also underwent magnetic resonance imaging (MRI) and bone scintigraphy (BS) to provide reference images. Of the 181 joints feasible for OTI analysis, 140 joints (111 in RA patients and 29 in controls, 77.3%) in which the difference of the OTI indices in the two measurements was within 20% were evaluated. The OTI indices in RA joints were significantly lower than those in control joints (p < 0.001). Overall, the OTI indices in RA joints decreased as the synovitis grades on MRI or BS increased. Moreover, OTI was able to discriminate between RA and control joints (AUC = 0.815, 95% CI 0.739–0.891), even if RA joints were normal on physical examination (AUC = 0.714, 95% CI 0.594–0.834). OTI was in good agreement (kappa = 0.60) with MRI for evaluating synovitis in RA patients and showed positive results in 11.4% of clinically asymptomatic joints. OTI in this study showed the potential to be a supplementary imaging modality for the quantification of synovial inflammation in PIP and MCP joints of RA patients. Further large-scale trials are needed to confirm these findings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by synovitis, joint damage, and progressive disability. It can be successfully managed by early diagnosis and treatment using disease-modifying anti-rheumatic drugs (DMARDs) [1]. This concept requires sensitive indicators that detect joint inflammation. However, current clinical measures, such as the 28-joint Disease Activity Score (DAS28) based on physical examination and serum inflammatory markers, have low sensitivity for subclinical inflammation and can be affected by subjective variables, such as pain levels and mood [2]. Plain radiography has been widely used to detect structural joint damages, but radiographic changes are no longer included in the requirements for RA diagnosis since they are often absent in early disease [3]. On the other hand, ultrasonography (US) and magnetic resonance imaging (MRI) showed a value as a sensitive imaging tools for detecting subclinical synovitis in clinically asymptomatic RA joints [4].
Optical tomographic imaging (OTI), analyzing the degree to which light is transmitted or scattered after irradiation, is a non-invasive and non-ionizing modality used mostly in medical imaging research [5]. Previously developed OTI methods showed better diagnostic performance in proximal interphalangeal (PIP) and metacarpophalangeal (MCP) joints than in the wrist due to the relatively high tissue penetration of light [6,7,8]. OTI was reported to correlate with swollen and tender joint counts, as well as with the US and MRI scores in RA patients [6]. However, the clinical application of laser-based OTI without fluorescence in hand RA has been limited. The aim of this pilot study was to compare the OTI system with established imaging techniques, such as MRI, and to evaluate the clinical utility of OTI to assess synovitis of PIP and MCP joints in RA patients.
Subjects and methods
Subjects
Twelve patients with RA diagnosed according to the 2010 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) classification criteria [3] and three healthy volunteers were recruited at the rheumatology clinic of the Seoul National University Hospital (SNUH). The RA patients had to have at least one swollen and tender joint among the PIP and MCP joints to be eligible for inclusion in the study. No changes to DMARDs and corticosteroids (CS), except for analgesics, were allowed during the study period (for 7 days). The RA patients seeking an increase in DMARDs and CS doses for severe RA status were excluded at screening. There was no limitation on disease duration of RA. The control group participants did not have joint pain or clinical evidence of hand arthritis. All of the participants were over 19 years old. The exclusion criteria included obvious hand deformations, history of trauma, surgery or intra-articular injection of the hand joint within 3 months, females suspected to be pregnant, contraindications for MRI (such as the presence of implantable cardiac devices or claustrophobia), moderate-to-severe renal insufficiency (defined as serum creatinine > 2.0 mg/dL or creatinine clearance < 50 mL/min), and hypersensitivity to contrast agents used in MRI and bone scintigraphy (BS). Patients with Raynaud’s phenomenon were not included. This study was approved by the Institutional Review Board (IRB) of SNUH (IRB No. 1411-011-622) and written informed consent was obtained from all participants.
Clinical assessment
Data on demographic and clinical characteristics, including vital signs and medical history, were obtained at the first visit (day 0). DAS28 using erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) and the visual analog scale for pain (pain-VAS) were also measured to assess overall disease activity in patients with RA. The presence or absence of swelling and tenderness in 28 joints, including PIPs and MCPs, was scored (0–1) by a rheumatologist (SJL).
Reference imaging methods
For comparison with the OTI results, gadolinium-enhanced MRI and technetium-99m BS were performed in the hands of RA patients. Due to time constraints, MRI and BS were performed on days 0 and 7, respectively. The MRI and BS images were obtained using a Magnetom Trio 3.0T (Siemens, Erlangen, Germany) scanner and a Discovery NM/CT 670 (GE Healthcare, Milwaukee, WI, USA), respectively. The severity of joint inflammation present on images obtained with each imaging instrument was independently read by experienced physicians (SHH for MRI and GJC for BS) without knowledge of the clinical assessment and was scored on a semi-quantitative scale of 0–3 for each joint (grade 0, normal; 1, mild; 2, moderate; and 3, severe increase of enhancing tissue in the synovial compartment in MRI; 0, normal; 1, moderate; 2, definite; and 3, marked uptake in BS) [9, 10]. Grades of 1 or greater indicated the presence of synovitis. For each imaging process, the participants completed a questionnaire (semi-quantitative 0–10 scales) in which their dissatisfactions (patient boredom, discomfort during the exam, and post-exam fatigue) were examined.
Optical tomographic imaging (OTI)
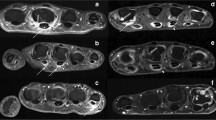
OTI measurements targeting the PIP and MCP joints were performed with the Manu-scan (LG Electronics, Seoul, Korea) administered by a trained research nurse. The first IP joints were not evaluated in this study due to accessibility issues with the device. Manu-scan is a newly developed optical molecular imaging equipment that detects near-infrared light irradiated to the living body and displays the results as a graph or an image. All study participants, including healthy controls, underwent a Manu-scan twice at intervals of a week (day 0 and 7). After executing the gateway program (ViewRex OMI 1.0, Techheim, Seoul, Korea), one hand was inserted through a cylindrical hole in a fixed posture and the PIP or MCP joint to be examined was placed on the seat part. The laser was positioned above (dorsal side) and a photodetector was placed below the joint (palmar side). Each joint was individually transilluminated by the laser at 660-nm and 780-nm wavelengths. Biometric information obtained from the photobiological signal acquired at the seat part was imaged in a two-dimensional light pattern, displayed on a monitor, and stored in a recording medium. The system controlled optical signal measurements at the terminal through serial communication and transmitted the measured signals to the main memory to generate pixelated images through the imaging algorithm. Next, mesh points in the sagittal plane of the finger were taken and the absorption and scattering coefficient values (μa and μs) in the points were mapped (Supplementary Figure S1). The scattering images in the Manu-scans showed a common typical image, regardless of the individual characteristics of the joints, with low optical properties at the center of the joints (synovium and joint fluid) and high optical properties at the edges of the joints (surrounding tissues). The index for the OTI measurement was defined as the contrast ratio of the maximum and minimum scattering coefficients (max μs/min μs), except for the somewhat irregular artifactual parts appearing on the edges above and below the joint. Inflammatory joints were expected to have lower OTI indices due to the relatively increased optical properties of exudative joint fluid and synovium (Fig. 1). When the difference of the OTI indices in the two measurements was within 20%, the mean value was selected.
Optical tomographic imaging (OTI) with the contrast ratio of the scattering coefficients (μs) according to the degree of synovitis on magnetic resonance imaging (MRI). Representative Manu-scan images of proximal interphalangeal (PIP) and metacarpophalangeal (MCP) joints from control and rheumatoid arthritis (RA) patient are shown. In the effective area, the central portion in which synovium and joint fluid are located has a relatively low scattering coefficient and the peripheral portion around the joint has a relatively high scattering coefficient. The OTI index for evaluating the severity of synovitis was set as the contrast ratio of the maximum and minimum values of the scattering coefficients (max μs/min μs)
Statistical analyses
The Mann–Whitney test (median and interquartile range [IQR]) and Chi-square or Fisher’s exact tests were performed for comparisons between RA joints and control joints. The Kruskal–Wallis nonparametric test for three or more groups was performed to identify differences in the OTI indices according to the presence of joint tenderness or swelling and synovitis grade (by MRI or BS). To reduce the possibility of type I error due to multiple comparisons, a post hoc Bonferroni correction was also used. Correlations between the OTI indices and the synovitis grades were further investigated using Spearman’s rank correlation test. A receiver operating characteristic (ROC) curve analysis was used to identify the diagnostic performance of the OTI index for RA and synovitis lesions on MRI. For all ROC curves, cut-off points with the maximal Youden index were determined. The degree of agreement between two methods was calculated by the kappa statistic. A p value below 0.05 (two-sided tests) was considered to indicate statistical significance. The data analyses were performed using SPSS software version 22.0 (SPSS, Inc., Chicago, IL, USA).
Results
The demographic and clinical findings of the study participants are described in Table 1. Most of the RA patients (83.3%) showed high disease activity (DAS28-ESR > 5.1). During the study, the participants’ body temperatures were within the normal range (< 37.0 °C). OTI indices were derived from a total of 181 hand joints showing typical scattering images in both of the repeated scans, excluding 35 joints with motion artifacts due to inadequate joint positioning or under/over-exposure to the detector. Among them, 140 joints (111 RA and 29 control joints, 77.3%) where the difference of the OTI indices in the two measurements was within 20% were further evaluated. In RA joints, the proportions of those with tenderness, swelling, and synovitis lesions on MRI (MRI grade > 0) were 50.5% (n = 56), 41.4% (n = 46), and 42.3% (n = 47), respectively.
OTI results according to physical examination findings
The OTI indices (median [IQR], 2.06 [1.62–2.43]) in RA joints (n = 111) were lower than those (2.80 [2.56–3.09]) in control joints (n = 29; p < 0.001). When analyzed according to the presence of tenderness or swelling on physical examination, swollen RA joints (n = 46) showed the lowest OTI indices (1.65 [1.51–2.01]). Joint tenderness did not have a significant effect on the OTI index (2.21 [1.88–2.47] in RA joints with only tenderness vs. 2.30 [2.03–2.75] in RA joints without tenderness and swelling; p = 0.423). There was a significant difference in OTI indices between control joints and RA joints that had neither tenderness nor swelling on physical examination (p = 0.002) (Fig. 2).
Optical tomographic imaging (OTI) indices according to rheumatoid arthritis (a) and joint tenderness or swelling on physical examination (b). RA joints, especially swollen joints, showed lower OTI indices. There was a significant difference in OTI indices between RA and controls, even in joints without tenderness and swelling on physical examination. The OTI index was set as the contrast ratio of the maximum and minimum scattering coefficients (max μs/min μs) on the joint scan. The graphs represent the median levels and interquartile ranges (25th and 75th percentiles)
Correlation between OTI and reference imaging methods
In RA joints, the OTI index was significantly different according to the synovitis grade measured by MRI or BS (Kruskal–Wallis test, p < 0.001 in each method). The distribution of the OTI indices according to synovitis grades is shown in Fig. 3. The OTI indices were negatively correlated with synovitis grades on MRI (Spearman’s rho = − 0.523; p < 0.001) and BS (Spearman’s rho = − 0.516; p < 0.001). There was a significant difference in the OTI indices between RA joints without synovitis on MRI (grade = 0) (n = 64) and control joints (2.34 [2.00–2.52] vs. 2.80 [2.56–3.09], respectively; p < 0.001).
Optical tomographic imaging (OTI) indices in rheumatoid arthritis (RA) and control joints according to magnetic resonance imaging (MRI)-grade (a) and bone scintigraphy (BS)-grade (b). Overall, the higher the degree of synovitis on MRI and BS, the lower the OTI index. In RA joints without synovitis on MRI (grade 0), there was a significant difference in OTI indices compared to control joints. The OTI index was set as the contrast ratio of the maximum and minimum scattering coefficients (max μs/min μs) on the joint scan. The graphs represent the median levels and interquartile ranges (25th and 75th percentiles). *There was no significant difference (p > 0.01) after Bonferroni correction for multiple comparisons
Detection of synovitis in hand joints using OTI
The diagnostic performance of OTI for RA at the joint level was evaluated. The ROC analysis showed that the OTI index had good accuracy (area under the curve [AUC] = 0.815, 95% confidence interval [CI] 0.739–0.891; p < 0.001). The cut-off OTI index of 2.49 (RA joint versus control joint) was applied with a high specificity of 83% (Fig. 4a). When separately evaluating RA joints without tenderness and swelling on physical examination, the OTI also demonstrated a fair ability to discriminate (AUC = 0.714, 95% CI 0.594–0.834; p = 0.002) between RA and control joints.
Receiver operating characteristic (ROC) curves of optical tomographic imaging (OTI) to discriminate rheumatoid arthritis (RA) joints from control joints (a) and detect synovitis lesions on MRI (grade > 0) in RA joints (b). The cut-off value (1.86) for the OTI index detecting synovitis on MRI in RA joints was lower than that (2.49) for discriminating RA joints from control joints. The optimal cut-off points were determined at the maximum Youden index
Among the RA joints, further ROC analysis of the OTI for synovitis was performed using MRI as the gold standard (MRI-grade > 0). Instead of 2.49, an OTI index of 1.86 was adopted as a cut-off threshold for synovitis lesions (AUC = 0.805, 95% CI 0.716–0.893; p < 0.001) with a sensitivity of 70% and a specificity of 89% (Fig. 4b). As a result, the kappa agreement between the OTI and MRI for evaluating synovitis was 0.60, better than the agreement between joint swelling and MRI (0.46) and between BS (BS-grade > 0) and MRI (0.34) in this study. OTI showed positive findings for synovitis in 11.4% of the RA joints without tenderness and swelling on physical examination (n = 44).
Examination times and patient satisfactions
OTI was examined individually in each joint, and the average time spent performing the OTI in the entire PIP and MCP joints of both hands was 42 ± 4.4 min (mean ± standard deviation). MRI was performed separately on each hand (35 ± 1.6 min for one hand) and BS was performed simultaneously on both hands (80 ± 8.6 min). The overall dissatisfaction with OTI was lower than that for BS in terms of patient boredom (1.50 [0.00–6.50] vs. 5.00 [5.00–10.00]; p = 0.034), discomfort during the exam (0.00 [0.00–0.00] vs. 3.50 [0.00–5.00]; p = 0.026), and post-exam fatigue (0.00 [0.00–1.75] vs. 4.50 [0.50–9.25]; p = 0.018). OTI tended to be more satisfactory than MRI in regard to patient boredom (1.50 [0.00–6.50] vs. 5.00 [5.00–9.75]; p = 0.052) and discomfort during the exam (0.00 [0.00–0.00] vs. 1.00 [0.00–4.25]; p = 0.084). No adverse reactions were observed during the study.
Discussion
OTI using the Manu-scan correlated with conventional imaging methods for the evaluation of hand RA. This study provided additional evidence for the benefit of OTI for detecting synovitis in PIP and MCP joints with subclinical inflammation. Even in RA joints without tenderness and swelling on physical examination, the OTI indices were significantly lower compared to control joints.
Joint structures have distinct absorption/scattering coefficients when irradiated with light of a particular wavelength. Since inflamed tissue has a higher percentage of proteins and inflammatory cells, synovitis can be demonstrated using OTI without administration of a contrast agent or fluorescent dye [11]. Synovial tissue growth and exudative joint fluid increase the light scattering coefficient inside the joint [5]. In the current study, synovitis was assessed by the contrast ratio of scattering coefficients inside and around the joint, reflecting the composition of the joint. Thus, OTI is distinguished from infra-red thermography, which directly evaluates the infrared emission from the joint surface with high temperature due to increased blood flow [12].
Joint US has been commonly used in clinical practice in patients with hand RA, as the sensitivity of physical examination for synovitis may be incomplete in some cases [13]. OTI is not superior compared with US in terms of image resolution. However, unlike US, which is semi-quantitatively evaluated and remains highly operator- or reader-dependent [14], OTI results in this study can be automatically quantified without subjectivity of the reader. In addition, the small-size imaging device (Supplementary Figure S1) and ease of test preparation make OTI an easy-to-access arthritis-tracking method that can be used in clinics.
Contrast-enhanced MRI, reflecting both morphological aspects and hypervascularity of arthritis, is an established imaging method and has shown high sensitivity and specificity for synovitis when read by experienced readers [15]. OTI was found to be more advantageous for the detection of subclinical joint inflammation than 1.5T MRI [16, 17] but showed lower sensitivity compared to 3.0T MRI [18, 19]. In our study using 3.0T MRI as a reference, the OTI indices were different between control and RA joints when no synovitis lesions were present on MRI (grade = 0) (Fig. 3a). A possible explanation for this is that the disease activity of RA patients may have affected the OTI results. The diagnostic performance of OTI is known to be better in severe RA [18, 19] and the present study was mainly composed of RA patients with severe disease activity (DAS28-ESR > 5.1). Further confirmation is required in RA patients with mild disease activity or in remission. Another explanation is that joint lesions other than synovitis, such as bone erosion, tendinitis, or osteophytes, may have caused overestimation of the OTI indices in our study [8].
Thus, we performed a second ROC analysis corresponding to synovitis on MRI (grade > 0), in addition to the primary analysis distinguishing RA joints from controls. No definite cut-off values for a clear separation of synovitic from non-synovitic joints could be identified in a previous study using fluorescence optical imaging (FOI) [20] but the current study demonstrated that OTI, with a cut-off value of 1.86, performed well in detecting synovitis lesions on MRI (AUC = 0.805) with high specificity (Fig. 4b). The agreement on synovitis between OTI and MRI was relatively good (kappa = 0.60).
OTI was similar to BS in that both techniques indirectly visualized morphological aspects and functional changes of the joint, such as synovial thickening with joint effusion and hypervascularity. However, OTI was superior to BS in detecting the presence of MRI-based synovitis. RA patients were more satisfied about the exam preparation for OTI that did not need an intravenous injection.
The present study had several limitations. Because OTI was employed only at the individual joint level, we could not determine any association with overall disease activity at the patient level. The time interval of a week (days 0 and 7) for OTI and reference imaging methods may have affected the reproducibility and correlation between them. Due to the small number of subjects, the valid number of joints for OTI analysis was less in comparison to previous studies [8, 21]. The proportion of exclusion by motion artifacts among the assessed joints was higher than an OTI study using Lightscan [21], which is probably due to the visually concealed detector part in our OTI device (Supplementary Figure S1). Unlike indocyanine green (ICG)-based FOI using a commercially available instrument (Xiralite) [16, 18,19,20], OTI in the present study was not advantageous in terms of examination time since the individual joints were measured separately to obtain OTI indices. But, OTI as a real-time imaging had relatively good satisfaction with the examination, and it would take only a short time if needed only in certain hand joints (about 2–3 min for one joint). A standardized examination technique may increase the measurement capability and shorten the procedure time.
This study referred to previous studies of OTI studies using MRI as a reference for synovitis evaluation [18, 19]. However, MRI was not performed in age-matched healthy controls and patients with osteoarthritis, and it was not possible to exclude the possibility of false-positivity of MRI in the OTI evaluation for MRI-based synovitis. Considering the higher specificity of MRI in RA patients with high disease activity than in those with low disease activity [19], we attempted to minimize this risk by performing MRI in hand joints with active joint swelling. Practically speaking, it is difficult to apply “true gold” standard for synovitis such as histology by synovial biopsy. Joint US would have complemented these problems, but unfortunately US was not done in our study.
In this study, OTI in the PIP and MCP joints was able to effectively discriminate RA and was comparable to MRI for detection of synovitis. To establish the role of OTI as a supplementary imaging modality in clinical practice, further large-scale longitudinal studies using OTI and appropriate reference imaging methods are warranted in RA patients with various states of disease activity.
References
Majithia V, Geraci SA (2007) Rheumatoid arthritis: diagnosis and management. Am J Med 120(11):936–939. https://doi.org/10.1016/j.amjmed.2007.04.005
Ton E, Bakker MF, Verstappen SM, Ter Borg EJ, van Albada-Kuipers IA, Schenk Y, van der Veen MJ, Bijlsma JW, Jacobs JW, Utrecht Rheumatoid Arthritis Cohort Study G (2012) Look beyond the disease activity score of 28 joints (DAS28): tender points influence the DAS28 in patients with rheumatoid arthritis. J Rheumatol 39(1):22–27. https://doi.org/10.3899/jrheum.110072
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Menard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovsky J, Wolfe F, Hawker G (2010) 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 69(9):1580–1588. https://doi.org/10.1136/ard.2010.138461
Brown AK, Quinn MA, Karim Z, Conaghan PG, Peterfy CG, Hensor E, Wakefield RJ, O’Connor PJ, Emery P (2006) Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug-induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum 54(12):3761–3773. https://doi.org/10.1002/art.22190
Scheel AK, Backhaus M, Klose AD, Moa-Anderson B, Netz UJ, Hermann KG, Beuthan J, Muller GA, Burmester GR, Hielscher AH (2005) First clinical evaluation of sagittal laser optical tomography for detection of synovitis in arthritic finger joints. Ann Rheum Dis 64(2):239–245. https://doi.org/10.1136/ard.2004.024224
van Onna M, Ten Cate DF, Tsoi KL, Meier AJ, Jacobs JW, Westgeest AA, Meijer PB, van Beek MC, Rensen WH, Bijlsma JW (2016) Assessment of disease activity in patients with rheumatoid arthritis using optical spectral transmission measurements, a non-invasive imaging technique. Ann Rheum Dis 75(3):511–518. https://doi.org/10.1136/annrheumdis-2015-207315
Krabbe S, Ammitzboll-Danielsen M, Ostergaard M, Giard MC, Terslev L (2016) Sensitivity and specificity of optical spectral transmission imaging in detecting joint inflammation in rheumatoid arthritis. Ann Rheum Dis 75(3):632–633. https://doi.org/10.1136/annrheumdis-2015-208399
Besselink NJ, van der Meijde P, Rensen WHJ, Meijer PBL, Marijnissen ACA, van Laar JM, Lafeber F, Jacobs JWG (2018) Optical spectral transmission to assess inflammation in hand and wrist joints of rheumatoid arthritis patients. Rheumatol (Oxf) 57(5):865–872. https://doi.org/10.1093/rheumatology/kex531
Ostergaard M, Peterfy C, Conaghan P, McQueen F, Bird P, Ejbjerg B, Shnier R, O’Connor P, Klarlund M, Emery P, Genant H, Lassere M, Edmonds J (2003) OMERACT rheumatoid arthritis magnetic resonance imaging studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J Rheumatol 30(6):1385–1386
Jamar F, Houssiau FA, Devogelaer JP, Chapman PT, Haskard DO, Beaujean V, Beckers C, Manicourt DH, Peters AM (2002) Scintigraphy using a technetium 99m-labelled anti-E-selectin Fab fragment in rheumatoid arthritis. Rheumatol (Oxf) 41(1):53–61
Scheel AK, Krause A, Rheinbaben IM, Metzger G, Rost H, Tresp V, Mayer P, Reuss-Borst M, Muller GA (2002) Assessment of proximal finger joint inflammation in patients with rheumatoid arthritis, using a novel laser-based imaging technique. Arthritis Rheum 46(5):1177–1184. https://doi.org/10.1002/art.10226
Brenner M, Braun C, Oster M, Gulko PS (2006) Thermal signature analysis as a novel method for evaluating inflammatory arthritis activity. Ann Rheum Dis 65(3):306–311. https://doi.org/10.1136/ard.2004.035246
Garrigues F, Jousse-Joulin S, Bouttier R, Nonent M, Bressollette L, Saraux A (2013) Concordance between clinical and ultrasound findings in rheumatoid arthritis. Jt Bone Spine 80(6):597–603. https://doi.org/10.1016/j.jbspin.2013.03.011
Scheel AK, Schmidt WA, Hermann KG, Bruyn GA, D’Agostino MA, Grassi W, Iagnocco A, Koski JM, Machold KP, Naredo E, Sattler H, Swen N, Szkudlarek M, Wakefield RJ, Ziswiler HR, Pasewaldt D, Werner C, Backhaus M (2005) Interobserver reliability of rheumatologists performing musculoskeletal ultrasonography: results from a EULAR “Train the trainers” course. Ann Rheum Dis 64(7):1043–1049. https://doi.org/10.1136/ard.2004.030387
Ejbjerg BJ, Narvestad E, Jacobsen S, Thomsen HS, Ostergaard M (2005) Optimised, low cost, low field dedicated extremity MRI is highly specific and sensitive for synovitis and bone erosions in rheumatoid arthritis wrist and finger joints: comparison with conventional high field MRI and radiography. Ann Rheum Dis 64(9):1280–1287. https://doi.org/10.1136/ard.2004.029850
Werner SG, Langer HE, Ohrndorf S, Bahner M, Schott P, Schwenke C, Schirner M, Bastian H, Lind-Albrecht G, Kurtz B, Burmester GR, Backhaus M (2012) Inflammation assessment in patients with arthritis using a novel in vivo fluorescence optical imaging technology. Ann Rheum Dis 71(4):504–510. https://doi.org/10.1136/annrheumdis-2010-148288
Werner SG, Langer HE, Schott P, Bahner M, Schwenke C, Lind-Albrecht G, Spiecker F, Kurtz B, Burmester GR, Backhaus M (2013) Indocyanine green-enhanced fluorescence optical imaging in patients with early and very early arthritis: a comparative study with magnetic resonance imaging. Arthritis Rheum 65(12):3036–3044. https://doi.org/10.1002/art.38175
Meier R, Thurmel K, Moog P, Noel PB, Ahari C, Sievert M, Dorn F, Waldt S, Schaeffeler C, Golovko D, Haller B, Ganter C, Weckbach S, Woertler K, Rummeny EJ (2012) Detection of synovitis in the hands of patients with rheumatologic disorders: diagnostic performance of optical imaging in comparison with magnetic resonance imaging. Arthritis Rheum 64(8):2489–2498. https://doi.org/10.1002/art.34467
Thuermel K, Neumann J, Jungmann PM, Schaffeler C, Waldt S, Heinze A, Beckmann A, Hauser C, Hasenau AL, Wildgruber M, Clotten S, Sievert M, Haller B, Woertler K, Harasser N, Rummeny EJ, Meier R (2017) Fluorescence optical imaging and 3T-MRI for detection of synovitis in patients with rheumatoid arthritis in comparison to a composite standard of reference. Eur J Radiol 90:6–13. https://doi.org/10.1016/j.ejrad.2017.02.016
Schafer VS, Hartung W, Hoffstetter P, Berger J, Stroszczynski C, Muller M, Fleck M, Ehrenstein B (2013) Quantitative assessment of synovitis in patients with rheumatoid arthritis using fluorescence optical imaging. Arthritis Res Ther 15(5):R124
Amitai I, Werner S, Schicke B, Burmester GR, Minet O, Zabarylo U, Backhaus M, Ohrndorf S (2015) Comparison of photo optical imaging with musculoskeletal ultrasound and clinical examination in the assessment of inflammatory activity in proximal interphalangeal joints in rheumatoid arthritis and osteoarthritis. J Rheumatol 42(9):1595–1602. https://doi.org/10.3899/jrheum.150098
Funding
The study was supported by LG Electronics and the OTI device (Manu-scan) was also provided by LG Electronics. This research was partly supported by a grant from the Ministry of Science, ICT and Future planning (NRF-2015M3A9B6052011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have declared no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Go, D.J., Lee, S.J., Joo, S.H. et al. Potential clinical utility of a novel optical tomographic imaging for the quantitative assessment of hand rheumatoid arthritis. Rheumatol Int 39, 2103–2110 (2019). https://doi.org/10.1007/s00296-019-04424-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-019-04424-3