Abstract
Fibrosis is unregulated tissue repair that may cause impairment of organ function, especially in end-organ damage. Systemic sclerosis (SSc) is the prototype systemic fibrosing disorder. Classical targets for fibrosis in SSc like transforming growth factor Beta (TGF-β), Interleukin-6 (IL-6), and multiple tyrosine kinases, have not yielded therapeutic benefit. There is multitude of evidence from across different tissues like the heart, lung, skin, liver, colon, and, to some extent, the kidney, that interleukin-17 (IL-17) and its downstream pathways are strongly associated with the initiation and propagation of fibrosis. Data from scleroderma patients, as well as from animal models of SSc, mirror these findings. Interestingly, hitherto unknown to be related to IL-17, newer molecules like Programmed Death-protein1 (PD-1), the phosphatase SHP2, along with known signal transducers like signal transducer and activator of transcription (STAT3), have been recently shown to be involved in the pathogenesis of fibrosis. Related molecules include the intracellular signalling molecules Ras/Erk, mammalian target organ of rapamycin (mTOR), and complement components. The biology of these pathways has not yet been fully elucidated to predict regulatory mechanisms, redundancies, and potential off-target effects. All these need to be better understood in the context of each other, in an effort to arrive at the optimal target to modulate fibrosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic sclerosis (SSc) or scleroderma is the prototype systemic fibrosing disorder [1]. The ensuing fibrosis of the skin can be disfiguring, affecting functionality and quality of life, whereas, fibrosis of internal organs and the associated vasculopathy can lead to severe morbidity and increased risk of mortality. Efforts have been ongoing for decades; however, we are yet to develop a drug that can halt the relentless claws of fibrosis in scleroderma. Normally, fibrosis is part of the healing process. At sites of injury, there is deposition of extracellular connective tissue (the most abundant being collagen). In SSc, there is an unregulated repair process in response to endothelial injury. Classically, the Transforming Growth Factor beta (TGF-β) pathways (both canonical and non-canonical) have been implicated in fibrosis, and numerous attempts have been made to target these pathways in SSc [2]. Though myriad preclinical experiments demonstrated benefit of blocking TGF-β in animal models of scleroderma, most human trials targeting this cytokine did not meet their primary end-points [3].
Thus, there is a need to understand the pathogenesis of SSc in greater depth, and identify more feasible targets to halt fibrosis. A drug successful in SSc may have far-reaching consequences, since fibrosis is the final common pathway associated with end-organ damage in almost all tissues, whether renal, hepatic (cirrhosis), neuronal (glial fibrosis), cardiac, or any other organ. Recent insights into the pathogenesis of SSc have demonstrated the role of neutrophil extracellular traps (NETosis), inflammasome activation, endosomal TLR (Toll like receptor) activation, leading to interferon production, and M2 polarized macrophages, and resultant fibrosis [4]. The roles of cytokine mediators such as interleukin-6 (IL-6), TGF-β and downstream pathways, serotonin (via serotonin receptor 2b), are already well known, and have been therapeutically explored with little success [5]. Similarly, evidences that interleukin (IL)-6 might drive TGF-β signalling culminated in the faSScinate trial in SSc [6]. Interestingly, IL-6 leads to the phosphorylation of the transcription factor STAT3 (signal transduction and activator of transcription-3), which, in turn, induces the polarization of naïve T cells towards a T helper 17 (Th17) phenotype. Skin biopsies from SSc patients have been reported to have high levels of expression of the cytokine IL-17 (a major source of which are Th17 cells), and its receptors [7]. Increased serum IL-17 levels have been reported in SSc patients, with higher levels in those having diffuse cutaneous involvement, and in those having interstitial lung disease [8]. Nevertheless, the exact role of IL-17, or the IL-6-STAT3-IL-17 axis has not yet been fully deciphered. Thus, we have reviewed the available literature on Interleukin-17 and fibrosis in different tissues as well as in animal models, and attempted to construct a hypothetical web of interlinked pathways leading to fibrosis.
Search strategy
A search of SCOPUS, MEDLINE, and Pubmed Central was carried out on the 28th of November 2018 with the keywords: interleukin-17, and fibrosis, as per previously published guidelines for writing narrative reviews [9]. The search was limited to articles in English, and preference was given to original articles published in the last 5 years. Reviews, abstracts and conference proceedings were not included. In addition, articles looking at possible mechanisms of action of proposed remedies were not included, unless specific blockers of IL-6, IL-17 or STAT3 were being used to explore these pathways. Of the 1032 search results thus derived, 70 were selected (Fig. 1). Based on the literature reviewed, we attempted to synthesize the different pathways identified into a single schematic to help identify nodes that could be potential future targets to inhibit fibrosis, including in the context of scleroderma.
Evidence for the involvement of IL-17 in human fibrotic disorders
It has been reported that the cytokine IL-27 can activate T lymphocytes from SSc patients to secrete IL-17, and this IL-17 further enhances the expression of the receptor for IL-27 on scleroderma fibroblasts, resulting in their stimulation by IL-27 to secrete greater amounts of extracellular matrix proteins [10]. Increased expression of messenger ribonucleic acid (mRNA), as well as protein levels of IL-17, has been reported in skin biopsies derived from SSc patients [7]. Furthermore, the IL-17A and IL-17F isoforms of IL-17 are elevated in scleroderma skin, when compared with skin biopsies from morphea patients [11]. Interleukin-35 has been reported in SSc patients—it increases the Th17–Treg ratio, and maybe a driver of the IL-17 fibrosis loop [12]. In the setting of keloids, a condition associated with exuberant, uncontrolled fibroblast proliferation, IL-17 has been reported to help maintain a niche of pro-fibrotic stem cells [13]. There is also indirect evidence of the role of IL-17 from patients with stem cell transplantation who develop scleroderma-like graft versus host disease (GVHD). In animal models of hematopoietic stem cell transplant, the use of granulocyte colony-stimulating factor (G-CSF), a potent stimulator of Th17 cell response with resultant IL-17A secretion, resulted in a scleroderma-like phenotype [14].
It has also been suggested that increased IL-17A in SSc serum and skin maybe due to a negative feedback mechanism, as a consequence of downregulation of the receptor for IL-17A, since the increased IL-17A has also been reported to paradoxically reduce CTGF (connective tissue growth factor) and collagen expression in SSc fibroblasts [15]. When fibroblasts from SSc patients are stimulated with IL-17 and TGF-β together, compared to TGF-β alone, there is a synergistic effect on activation of the mitogen activated protein kinase (MAPK), with increased production of IL-6, which is known to promote fibrosis. However, a paradoxical reduction in type 1 collagen and fibronectin secretion from these same fibroblasts was also seen, when both IL-17 and TGF-β were used, compared to TGF-β alone. This suggests that IL-17 may have a dual role, both driving fibrosis as well as regulating it, in the context of scleroderma [16]. As we shall subsequently see, other fibrotic scenarios also highlight such a dual role of IL-17 in fibrosis.
From humans to preclinical models: role of IL-17
Preclinical models of scleroderma and skin fibrosis
In the bleomycin-induced murine model of scleroderma, it has been reported that administration of bleomycin increases the expression of adhesion molecules, and subsequent recruitment of Th17 cells, and the cytokine IL-17, to the skin and lung tissue of such mice [17]. This was validated in another animal model of scleroderma, the tight skin mouse 1 (TSK-1) model, wherein blockade of IL-17 could reduce the severity of fibrosis, and similar observations were noted in the bleomycin-induced fibrosis model also [18]. Furthermore, IL-17 knockout could also reduce the severity of fibrosis mediated by IL-1 knockout in lungs and skin, in bleomycin as well as GVHD murine models of scleroderma [19]. It has been reported that IL-21 is a key cytokine driving IL-17-mediated fibrosis in bleomycin-induced fibrosis, and the cytokine B-cell activating factor (BAFF) plays a role downstream to IL-17 in this model [20,21,22,23]. Exogenous administration of IL-17 promoted scar formation in an excision wound model of murine fibrosis, in part due to increased recruitment of macrophages to the site of the scar [24].
Preclinical models of cardiac fibrosis
In a rat model of heart failure induced by subcutaneous injection of isoproterenol, IL-17 was identified as a critical mediator of myocardial fibrosis, via further downstream activation of the RANKL [receptor activator of nuclear factor kappa B (NF-κB) ligand], and increased secretion of MMP-1 (matrix metalloproteinase-1) [25]. Furthermore, by blocking IL-17 signalling through a lentivirus carrying IL-17 receptor antagonist in spontaneous hypertension model of rats, a reduction in cardiac fibrosis was noted, along with improvement of cardiac contractile function and compliance [26]. The activation of cardiac myofibroblasts via IL-17 was, in part, mediated through the intracellular mediators p38 MAPK (mitogen associated protein kinase) [27] and ERK1/2 (extracellular signal regulated kinase 1/2) [28], causing downstream activation of the proinflammatory NF-κB. IL-17 induced activation of p38 MAPK enhanced cardiac myocyte apoptosis, and this could eventually result in cardiac fibrosis [29]. In another murine model of hypertension induced by angiotensin II infusion, IL-1β from monocytes further stimulated IL-17 secretion from gamma delta T cells (γδ T-cells), which in turn resulted in cardiac myofibroblast activation, with consequent fibrosis. In another ischemia-induced rabbit model of heart failure, Th17 cells, via secreted IL-17, activated p38 MAPK and enhanced cardiac fibrosis, as well as increased the subsequent risk of ventricular arrhythmia. These adverse scenarios could be ameliorated using a monoclonal antibody against IL-17 [30]. In a cardiac transplantation model, TGF-β, secreted by T lymphocytes, was a key player in cardiac fibrosis and allograft rejection, and this could be abrogated by knocking out IL-17 in these mice [31]. In yet another mouse model of sterile pericarditis induced by intra-pericardial talc injection, blocking IL-17 reduced atrial fibrosis, and the risk of resultant atrial fibrillation [32]. Thus, IL-17 has been demonstrated to play a role in different models of cardiac fibrosis. However, there is literature to suggest that the cytokine IL-23, which plays a role in maintaining the population of differentiated Th17 cells (source of IL-17), has a protective role following myocardial ischemia. In a murine model of ischemic cardiac injury, knocking out IL-23 signalling resulted in decreased Th17 cells in the infarcted area, with disruption of Th1/Th17 ratio, unchecked Th1 lymphocyte activity, and increased cardiac injury and fibrosis [33]. Thus, while abrogating harmful effects of increased IL-17 on the cardiac myocyte may protect against cardiac fibrosis, similar intervention upstream of the source of IL-17 may have counter-productive effects also.
Preclinical models of renal fibrosis
Different types of insults, including obstructive uropathy, ischemic injury and toxic injury, can lead on to renal damage and resultant fibrosis. In an animal model of renal injury due to ureteric obstruction, Il-17, secreted by CD4+ T lymphocytes and γδ T lymphocytes, intensified the inflammatory infiltrate in the kidney, by increasing expression of the chemokine ligand 5 (CCL5 or RANTES), and resulted in greater severity of renal fibrosis [34]. Similar findings were observed in a rat model of ischemia reperfusion injury, wherein IL-17 secreted by CD4+ T lymphocytes and natural killer cells promoted renal fibrosis, which could be ameliorated by blocking this cytokine [35]. In a murine model of obstructive uropathy, the pro-inflammatory cytokine IL-36 was reported to activate the Nod-like receptor (NLR) family pyrin containing domain 3 (NLRP-3) inflammasome complex, an innate immune mechanism, which further resulted in increased activation of dendritic cells, stimulation of Th17 cells, expression of IL-23 and IL-17, and resultant interstitial fibrosis in the kidneys [36]. In the same disease model, the complement component 3a (C3a) could result in activation of T lymphocytes and subsequent IL-17 production, with resultant tubulointerstitial fibrosis [37].
The relationship between IL-17 and renal fibrosis is not as clear as in other models of end-organ damage. Despite an increase in renal expression of IL-17A in a murine model of ischemia–reperfusion injury, blocking this cytokine did not affect the consequent renal fibrosis [38]. Knocking out IL-17 in a murine obstructive uropathy model paradoxically exacerbated renal fibrosis, and in this particular model, IL-17 inhibited downstream effects of TGF-β on renal fibroblasts [39]. Similarly, in mouse models of diabetic nephropathy, knocking out IL-17 increased the severity of renal injury and fibrosis. Use of low-dose IL-17 had a preventative as well as therapeutic effect on diabetic nephropathy in such mice [40]. A possible reason for the protective effects seen with IL-17 in renal fibrosis could be explained by another set of experiments, wherein the histone deacetylase inhibitor Trichostatin A (TSA) was demonstrated to be therapeutic in a mouse model of obstructive uropathy. The use of TSA resulted in a decrease in renal fibrosis, with a reduction in the number of Th17 cells (which normally secrete IL-17), but an increase in the number of CD4+ FoxP3+ secreting IL-17, with demonstrable plasticity between these subsets further adding to the complexity of the role of IL-17 in renal fibrosis [41].
Preclinical models of peritoneal and gastrointestinal tract fibrosis
In a murine model of peritonitis, IL-17 was demonstrated to play a synergistic role along with TGF-β in driving peritoneal adhesions, and blocking IL-17 (but not TGF-β) helped reduce the severity of such peritoneal fibrosis [42]. It was further demonstrated in another mouse model that CD4+ T lymphocytes were the predominant source of such IL-17 in peritoneal fibrosis [43]. Two agents which reduced peritoneal dialysis induced fibrosis in animal models, paricalcitol (a stimulator of vitamin D receptor) and alanyl-glutamine peptide, did so in part by reducing levels of IL-17 [44, 45]. In a model of chronic colitis in rats induced by 2,4,6-trinitrobenzene sulfonic acid, the reparative phase was associated with increased fibrosis and colonic expression of IL-17 [46], and reduction of colonic fibrosis in this model by blocking TGF-β1 was associated with reduced numbers of Th17 cells, and decreased expression of IL-17 in the colon [47]. Thus, IL-17 plays a role in driving fibrosis in the peritoneal cavity, as well as the gastrointestinal tract also.
Preclinical models of cirrhosis
Cirrhosis is the end-result of fibrosis in the liver occurring due to hepatocyte damage, irrespective of the etiology of such damage. Animal models of liver cirrhosis attempt to replicate this scenario through multiple routes, such as direct exposure to toxin, or biliary obstruction. In a mouse model of liver cirrhosis induced by administration of the toxin carbon tetrachloride (CCl4), the ensuing fibrosis could be reduced by co-administering 1, 25-dihydroxy Vitamin D3, and this acted in part by the reduction of infiltrating Th17 cells and decreased secretion of IL-17 [48]. In another murine model of excessive activation of the NLRP3 inflammasome causing liver fibrosis, genetic knockdown of IL-17A partially ameliorated fibrosis [49]. In murine cirrhosis induced by bile duct ligation, similar increases in IL-17 in the liver, along with the fibrogenic cytokines TGF-β1 and TGF-β2, were noted [50]. The proposed mechanisms by which IL-17 may drive liver cirrhosis in the CCl4 injury and the bile duct ligation models include the induction of inflammatory cytokines IL-1, IL-6 (causing activation of the transcription factor STAT3) and tumor necrosis factor alpha (TNF-α), as well as of TGF-β1, with consequent stellate cell activation, which secrete collagen and result in fibrosis [51]. Blocking the receptor for IL-17A reduces the severity of inflammatory infiltrate and resultant liver fibrosis in the CCl4 injury model [52]. Apart from T lymphocytes, mast cells and neutrophils have also been proposed to be sources of the IL-17 responsible for the induction of fibrosis in models of cirrhosis [53].
Preclinical models of pulmonary fibrosis
Fibrosis plays a role in airway remodeling associated with the chronicity of asthma. In patients with moderate-to-severe asthma, there is increased expression of IL-17 and TGF-β in the bronchial mucosa. While the former cytokine is reduced by oral corticosteroid therapy, this is not the case with TGF-β, resulting in ongoing collagen deposition and fibrosis despite therapy [54]. Eosinophils have been demonstrated to play a role in asthma, and when such eosinophils from asthmatic patients are stimulated ex vivo with IL-17, there is increased production of TGF-β, a process mediated by p38MAPK [55]. In in vitro cultures of human small airway epithelial cells, a synergistic effect of the cytokine IL-17 and growth/differentiation factor 15 (GD15) in driving epithelial-to-mesenchymal transition (EMT) in the presence of cigarette smoke extract has been demonstrated. Such EMT is a precursor to fibrosis, and higher levels of IL-17 and GD15 have also been demonstrated in the lungs of smokers with COPD [56]. NETs are extrusions from activated neutrophils which contain inflammatory mediators, and these have recently been recognised to play a role in fibrosis, including lung fibrosis. It was demonstrated that NETs secreted by neutrophils stimulated with toxins such as cigarette smoke contained IL-17. While IL-17 alone could not differentiate lung fibroblasts into myofibroblasts, it could do so when present along with deoxyribonucleic acid and histones in the NETs generated by such neutrophils [57]. Lung injury and resultant fibrosis can also occur following bone marrow transplant. In a murine model of post-bone marrow transplant pneumonitis and lung fibrosis, blocking IL-17 had beneficial effects on lung fibrosis [58]. A recent study described an association between pulmonary fibrosis and increased expression of the programmed cell death 1 (PD-1) molecule on CD4+ lymphocytes (mainly Th17 cells), leading to STAT3 activation that further leads to increased TGF-β and IL-17A production [59]. Such cells were identified in patients of idiopathic pulmonary fibrosis and sarcoidosis, as well as in the bleomycin-induced murine model of lung fibrosis. Co-culturing human PD-1 + CD4+ cells with human lung fibroblasts resulted in increased deposition of collagen and other extracellular matrix proteins. Furthermore, knocking out PD-1 in these mice ameliorated the severity of lung fibrosis. These experiments identified PD-1 as a potential new target to reduce fibrosis [59].
Exploring new therapeutic targets for fibrosis along the IL-17 pathway
Having looked at the evidence for the role of IL-17 pathway in driving fibrosis in different disease states, it is reasonable to conclude that this cytokine has been implicated as a player of fibrosis in most organs of the body, except for the kidney. In the latter, there are evidences both for and against the role of IL-17 pathway in driving renal fibrosis, and, probably, the IL-17 secreted at a lower concentration, possibly from FoxP3 positive cells, may be anti-fibrotic [40, 41]. This is not at all surprising, since redundancy of biological pathways is a common occurrence. Therefore, there may be a need to explore molecules and transcription factors that lie before and after IL-17, or work in synergism with the same, as therapeutic targets.
STAT3/PD-1 as targets for fibrosis
STAT3, upstream of IL-17, can be potentially a target for ameliorating fibrosis. It is induced by the canonical pathway of TGF-β, and numerous pathways driving fibrosis, including those involving the kinases JAK, JNK, c-ABL and SRC, converge on STAT-3. Furthermore, increased STAT-3 signalling has been demonstrated in activated fibroblasts of patients with scleroderma [60]. But it should be remembered that STAT3 is central in various processes like cellular proliferation, therefore, caution should be exercised while inhibiting this transcription factor due to fear of off-target effects. Strategies to selectively target STAT-3 in activated fibroblasts may need future exploration.
PD-1 has been classically described as a marker of T lymphocyte “exhaustion”, wherein the PD-1-expressing T cell loses most of its proliferative and pro-inflammatory properties. But recent literature has uncovered functions of PD-1 beyond exhaustion [61]. It is increasingly being recognised that PD-1 + T cells have been switched from an pro-inflammatory into a reparative role (as opposed to the previous thought of them roaming around “exhausted”). This makes sense in the light of the data mentioned earlier in the review that PD-1 is linked to fibrosis. The success stories of PD-1 inhibition are exemplified the 2018 Nobel prize in Physiology or Medicine being awarded for negative checkpoint inhibition. However, PD-1 inhibitors only have demonstrable favourable effects in a specific population of patients with refractory malignancies, and can precipitate autoimmune disease in almost one-half of them [62]. This brings to light an ethical concern, i.e., whether, for non-malignant conditions like fibrosis, is it worth risking a new autoimmune or endocrine disease? Thus, PD-1 may not be a favourable molecule for non-selective targeting, and future therapeutic strategies exploring PD-1 inhibition in fibrotic states might require optimisation for specific targeting of this molecule at sites of fibrosis. Furthermore, PD-1 is upstream of SHP2 and it is logical to assume similar or more redundant pathways and off-target effects.
SHP2 as an anti-fibrotic target
It is noteworthy that PD-1 works through Immunoreceptor tyrosine-based inhibitory motifs (ITIMs) that recruit SHP2, a ubiquitous tyrosine phosphatase containing Src Homology 2 (SH2) domains, amongst others. Recently, Zehender et al. demonstrated that SHP2 controls STAT3-induced fibroblast activation and fibrosis [63]. Thus, SHP2 is present both upstream (in CD4+ lymphocytes) and downstream (in fibroblasts) of TGF-β signalling. Logically, this makes it an attractive target for therapy. Inhibition of SHP2 will also inhibit fibroblast activation via fibroblast growth factor receptor 2 (FGFR2) [64]. This simplistic viewpoint needs to be modified in the light of knowledge that, in the presence of TNF-α, SHP2 attenuates the IL-6 induced STAT3 activation via gp130 [65]. Thus, SHP2 inhibition may paradoxically increase STAT3 activation if TNF-α is present in the milieu. Though another recent paper has demonstrated that SHP2 upregulates STAT3 via dephosphorylation of JAK2, in the setting of Noonan syndrome, activated SHP2 downregulates phosphorylation of STAT3 [66]. While downregulation of STAT3 should decrease cellular proliferation, it must also be kept in mind that SHP2 also acts as a protooncogene in breast cancer [67]. SHP2 inhibits both Ras/ERK [68] and Akt/mTOR pathways both of which are implicated in fibrosis. SHP2 has also been observed to be involved in DNA repair pathways including damage checkpoints [69]. Thus at present, understanding regarding SHP2 mediated actions is far from perfect. Though it appears to be a potentially viable target, a more in-depth exploration of all of its regulatory mechanisms, of parallel redundant pathways, and all of its targets is required before targeting of this molecule can be translated clinically.
Complement anaphylatoxins and IL-17
In SSc, complement activation is well known, but is often ascribed to vasculopathy. But the role, if any, of complement activation in fibrosis is still unknown. It has been reported that both TGF-β [70] and IL-17A [71] activate complement during lung fibrosis. IL-17 knockout or blockade reduces bleomycin induced complement activation in the mouse model [71]. C3a and C5a are complement components that can instigate an inflammatory reaction, and hence are often referred to as the anaphylatoxins. C5a has been reported to induce plasticity of CD4+ cells into a Th17 phenotype, in a background of chronic graft versus host disease [72]. Blockade of C3a and C5a receptors have been reported to abrogate endothelial to myofibroblast transition [73] and reduce interstitial fibrosis [74] in diabetic renal fibrosis. Similar blockade of C3a and C5a attenuates lung fibrosis in the murine bleomycin model [75]. The oral C5a receptor antagonist, Avacopan, was successful in a phase 2/3 clinical trial in ANCA associated vasculitis and is being explored in other conditions [76]. Thus, C5a pathway blocking agents safe for human use are already available. Their role may need further exploration in fibrotic states.
Conclusion
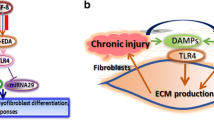
The review has identified the following nodal points related to IL-17 and fibrosis: IL-6, PD-1, SHP2, STAT3, IL-17A, and C5a/C3a. In the case of renal fibrosis, there is conflicting evidence, since low-dose Il-17 has also been reported to reduce renal fibrosis [40]. Most of the literature, however, makes a strong case in favour of the role of IL-17 in fibrosis. The associated pathways have been summarised in Fig. 2 [3, 62, 76,77,78,79,80,81,82], with possible points of interventions with currently available drugs also mentioned. However, the biology, control mechanisms, redundant pathways and potential off-target effects of PD-1, SHP2, STAT3, IL-17A, or C5a/C3a need to be better understood to have a clinically relevant and viable remedy to break the mesh of fibrosis. We may be tantalizingly close! However, one needs to be cautious in drawing comfort from targeting only IL-17 pathways. We know that TGF-β, the most potent pro-fibrotic cytokine, mediates fibrosis through canonical (Smad2/3 dependent) as well as non-canonical pathways (ERK1/2, STAT3, MAPK etc.) [82]. Targeting non-canonical pathways may thus have potential to ameliorate fibrosis to some extent but not fully. Thus, targeting more than one node should also be explored, in the future search of the elusive holy grail of an anti-fibrotic agent.
Abbreviations
- BAFF:
-
B-cell activating factor
- C3a:
-
Complement component 3a
- CCl4:
-
Carbon tetrachloride
- CCL5:
-
Chemokine ligand 5
- CTGF:
-
Connective tissue growth factor
- EMT:
-
Epithelial-to-mesenchymal transition
- ERK 1/2:
-
Extracellular signal-regulated kinase 1/2
- FGFR2:
-
Fibroblast growth factor receptor 2
- γδ T-cells:
-
Gamma delta T cells
- G-CSF:
-
Granulocyte colony-stimulating factor
- GD15:
-
Growth/differentiation factor 15
- GVHD:
-
Graft versus host disease
- IL:
-
Interleukin
- ITIM:
-
Immunoreceptor tyrosine-based inhibitory motifs
- MAPK:
-
Mitogen-activated protein kinase
- MMP-1:
-
Matrix metalloproteinase-1
- mRNA:
-
Messenger ribonucleic acid
- NET:
-
Neutrophil extracellular traps
- NLR:
-
NOD-like receptor
- NLRP-3:
-
NOD-like receptor (NLR) family pyrin containing domain 3
- NF-κB:
-
Nuclear factor kappa B
- PD-1:
-
Programmed cell death 1
- RANKL:
-
Receptor activator of nuclear factor kappa B ligand
- SH2:
-
Src homology 2
- SSc:
-
Systemic sclerosis
- Stat-3:
-
Signal transduction and activator of transcription-3
- TGF-β:
-
Transforming growth factor beta
- Th:
-
T helper cell
- TLR:
-
Toll like receptor
- TNF-α:
-
Tumor necrosis factor alpha
- Treg:
-
T regulatory cell
- TSA:
-
Trichostatin A
- Tsk-1:
-
Tight skin mouse 1
References
Varga J, Abraham D (2007) Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest 117:557–567. https://doi.org/10.1172/JCI31139
Lafyatis R (2014) Transforming growth factor β–at the centre of systemic sclerosis. Nat Rev Rheumatol 10:706–719. https://doi.org/10.1038/nrrheum.2014.137
Baron M (2016) Targeted therapy in systemic sclerosis. Rambam Maimonides Med J 7(4):e0030. https://doi.org/10.5041/RMMJ.10257
Gupta L, Ahmed S, Zanwar A (2017) The pathogenesis of scleroderma. Indian J Rheumatol 12:142–148. https://doi.org/10.4103/0973-3698.219083
Distler O, Cozzio A (2016) Systemic sclerosis and localized scleroderma–current concepts and novel targets for therapy. Semin Immunopathol 38:87–95. https://doi.org/10.1007/s00281-015-0551-z
Khanna D, Denton CP, Jahreis A et al (2016) Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet 387:2630–2640. https://doi.org/10.1016/S0140-6736(16)00232-4
Zhou Y, Hou W, Xu K et al (2015) The elevated expression of Th17-related cytokines and receptors is associated with skin lesion severity in early systemic sclerosis. Hum Immunol 76:22–29. https://doi.org/10.1016/j.humimm.2014.12.008
Wakhlu A, Sahoo RR, Parida JR et al (2018) Serum Interleukin-6, Interleukin-17A, and transforming growth factor beta are raised in systemic sclerosis with interstitial lung disease. Indian J Rheumatol 13:107–112. https://doi.org/10.4103/injr.injr_106_17
Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD (2011) Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int 31:1409–1417. https://doi.org/10.1007/s00296-011-1999-3
Yoshizaki A, Yanaba K, Iwata Y et al (2011) Elevated serum interleukin-27 levels in patients with systemic sclerosis: association with T cell, B cell and fibroblast activation. Ann Rheum Dis 70:194–200. https://doi.org/10.1136/ard.2009.121053
Lonati PA, Brembilla NC, Montanari E et al (2014) High IL-17E and low IL-17C dermal expression identifies a fibrosis-specific motif common to morphea and systemic sclerosis. PLoS One 9:e105008. https://doi.org/10.1371/journal.pone.0105008
Tang J, Lei L, Pan J et al (2018) Higher levels of serum interleukin-35 are associated with the severity of pulmonary fibrosis and Th2 responses in patients with systemic sclerosis. Rheumatol Int 38:1511–1519. https://doi.org/10.1007/s00296-018-4071-8
Zhang Q, Yamaza T, Kelly AP et al (2009) Tumor-like stem cells derived from human keloid are governed by the inflammatory niche driven by IL-17/IL-6 axis. PLoS One 4:e7798. https://doi.org/10.1371/journal.pone.0007798
Hill GR, Olver SD, Kuns RD et al (2010) Stem cell mobilization with G-CSF induces type 17 differentiation and promotes scleroderma. Blood 116:819–828. https://doi.org/10.1182/blood-2009-11-256495
Nakashima T, Jinnin M, Yamane K et al (2012) Impaired IL-17 signaling pathway contributes to the increased collagen expression in scleroderma fibroblasts. J Immunol 188:3573–3583. https://doi.org/10.4049/jimmunol.1100591
Dufour AM, Alvarez M, Russo B, Chizzolini C (2018) Interleukin-6 and Type-I collagen production by systemic sclerosis fibroblasts are differentially regulated by Interleukin-17A in the presence of transforming growth factor-beta 1. Front Immunol 9:1865. https://doi.org/10.3389/fimmu.2018.01865
Yoshizaki A, Yanaba K, Iwata Y et al (2010) Cell adhesion molecules regulate fibrotic process via Th1/Th2/Th17 cell balance in a bleomycin-induced scleroderma model. J Immunol 185:2502–2515. https://doi.org/10.4049/jimmunol.0901778
Okamoto Y, Hasegawa M, Matsushita T et al (2012) Potential roles of interleukin-17A in the development of skin fibrosis in mice. Arthritis Rheum 64:3726–3735. https://doi.org/10.1002/art.34643
Park M-J, Moon S-J, Lee E-J et al (2018) IL-1-IL-17 signaling axis contributes to fibrosis and inflammation in two different murine models of systemic sclerosis. Front Immunol 9:1611. https://doi.org/10.3389/fimmu.2018.01611
Lei L, Zhong X-N, He Z-Y et al (2015) IL-21 induction of CD4+ T cell differentiation into Th17 cells contributes to bleomycin-induced fibrosis in mice. Cell Biol Int 39:388–399. https://doi.org/10.1002/cbin.10410
François A, Gombault A, Villeret B et al (2015) B cell activating factor is central to bleomycin- and IL-17-mediated experimental pulmonary fibrosis. J Autoimmun 56:1–11. https://doi.org/10.1016/j.jaut.2014.08.003
Lei L, He Z-Y, Zhao C et al (2016) Elevated frequencies of CD4(+) IL-21(+) T, CD4(+) IL-21R(+) T and IL-21(+) Th17 cells, and increased levels of IL-21 in bleomycin-induced mice may be associated with dermal and pulmonary inflammation and fibrosis. Int J Rheum Dis 19:392–404. https://doi.org/10.1111/1756-185X.12522
Lei L, Zhao C, Qin F et al (2016) Th17 cells and IL-17 promote the skin and lung inflammation and fibrosis process in a bleomycin-induced murine model of systemic sclerosis. Clin Exp Rheumatol 34(Suppl 100):14–22
Zhang J, Qiao Q, Liu M et al (2018) IL-17 promotes scar formation by inducing macrophage infiltration. Am J Pathol 188:1693–1702. https://doi.org/10.1016/j.ajpath.2018.04.005
Feng W, Li W, Liu W et al (2009) IL-17 induces myocardial fibrosis and enhances RANKL/OPG and MMP/TIMP signaling in isoproterenol-induced heart failure. Exp Mol Pathol 87:212–218. https://doi.org/10.1016/j.yexmp.2009.06.001
Liu W, Wang X, Feng W et al (2011) Lentivirus mediated IL-17R blockade improves diastolic cardiac function in spontaneously hypertensive rats. Exp Mol Pathol 91:362–367. https://doi.org/10.1016/j.yexmp.2011.04.003
Valente AJ, Yoshida T, Gardner JD et al (2012) Interleukin-17A stimulates cardiac fibroblast proliferation and migration via negative regulation of the dual-specificity phosphatase MKP-1/DUSP-1. Cell Signal 24:560–568. https://doi.org/10.1016/j.cellsig.2011.10.010
Liu Y, Zhu H, Su Z et al (2012) IL-17 contributes to cardiac fibrosis following experimental autoimmune myocarditis by a PKCβ/Erk1/2/NF-κB-dependent signaling pathway. Int Immunol 24:605–612. https://doi.org/10.1093/intimm/dxs056
Zhou S-F, Yuan J, Liao M-Y et al (2014) IL-17A promotes ventricular remodeling after myocardial infarction. J Mol Med (Berl) 92:1105–1116. https://doi.org/10.1007/s00109-014-1176-8
Chang S-L, Hsiao Y-W, Tsai Y-N et al (2018) Interleukin-17 enhances cardiac ventricular remodeling via activating MAPK pathway in ischemic heart failure. J Mol Cell Cardiol 122:69–79. https://doi.org/10.1016/j.yjmcc.2018.08.005
Faust SM, Lu G, Marini BL et al (2009) Role of T cell TGFbeta signaling and IL-17 in allograft acceptance and fibrosis associated with chronic rejection. J Immunol 183:7297–7306. https://doi.org/10.4049/jimmunol.0902446
Fu X-X, Zhao N, Dong Q et al (2015) Interleukin-17A contributes to the development of post-operative atrial fibrillation by regulating inflammation and fibrosis in rats with sterile pericarditis. Int J Mol Med 36:83–92. https://doi.org/10.3892/ijmm.2015.2204
Savvatis K, Pappritz K, Becher PM et al (2014) Interleukin-23 deficiency leads to impaired wound healing and adverse prognosis after myocardial infarction. Circ Heart Fail 7:161–171. https://doi.org/10.1161/CIRCHEARTFAILURE.113.000604
Peng X, Xiao Z, Zhang J et al (2015) IL-17A produced by both γδ T and Th17 cells promotes renal fibrosis via RANTES-mediated leukocyte infiltration after renal obstruction. J Pathol 235:79–89. https://doi.org/10.1002/path.4430
Mehrotra P, Collett JA, McKinney SD et al (2017) IL-17 mediates neutrophil infiltration and renal fibrosis following recovery from ischemia reperfusion: compensatory role of natural killer cells in athymic rats. Am J Physiol Renal Physiol 312:F385–F397. https://doi.org/10.1152/ajprenal.00462.2016
Chi H-H, Hua K-F, Lin Y-C et al (2017) IL-36 signaling facilitates activation of the NLRP3 INFLAMMASOME and IL-23/IL-17 axis in renal inflammation and fibrosis. J Am Soc Nephrol 28:2022–2037. https://doi.org/10.1681/ASN.2016080840
Liu Y, Wang K, Liang X et al (2018) Complement C3 produced by macrophages promotes renal fibrosis via IL-17A secretion. Front Immunol 9:2385. https://doi.org/10.3389/fimmu.2018.02385
Thorenz A, Völker N, Bräsen JH et al (2017) IL-17A blockade or deficiency does not affect progressive renal fibrosis following renal ischaemia reperfusion injury in mice. J Pharm Pharmacol 69:1125–1135. https://doi.org/10.1111/jphp.12747
Sun B, Wang H, Zhang L et al (2018) Role of interleukin 17 in TGF-β signaling-mediated renal interstitial fibrosis. Cytokine 106:80–88. https://doi.org/10.1016/j.cyto.2017.10.015
Mohamed R, Jayakumar C, Chen F et al (2016) Low-dose IL-17 therapy prevents and reverses diabetic nephropathy, metabolic syndrome, and associated organ fibrosis. J Am Soc Nephrol 27:745–765. https://doi.org/10.1681/ASN.2014111136
Wu W-P, Tsai Y-G, Lin T-Y et al (2017) The attenuation of renal fibrosis by histone deacetylase inhibitors is associated with the plasticity of FOXP3+ IL-17+ T cells. BMC Nephrol 18:225. https://doi.org/10.1186/s12882-017-0630-6
Wang G, Wu K, Li W et al (2014) Role of IL-17 and TGF-β in peritoneal adhesion formation after surgical trauma. Wound Repair Regen 22:631–639. https://doi.org/10.1111/wrr.12203
Chung DR, Chitnis T, Panzo RJ et al (2002) CD4+ T cells regulate surgical and postinfectious adhesion formation. J Exp Med 195:1471–1478
González-Mateo GT, Fernández-Míllara V, Bellón T et al (2014) Paricalcitol reduces peritoneal fibrosis in mice through the activation of regulatory T cells and reduction in IL-17 production. PLoS One 9:e108477. https://doi.org/10.1371/journal.pone.0108477
Ferrantelli E, Liappas G, Vila Cuenca M et al (2016) The dipeptide alanyl-glutamine ameliorates peritoneal fibrosis and attenuates IL-17 dependent pathways during peritoneal dialysis. Kidney Int 89:625–635. https://doi.org/10.1016/j.kint.2015.12.005
Zhu MY, Lu YM, Ou YX et al (2012) Dynamic progress of 2,4,6-trinitrobenzene sulfonic acid induced chronic colitis and fibrosis in rat model. J Dig Dis 13:421–429. https://doi.org/10.1111/j.1751-2980.2012.00607.x
Ma Y, Guan Q, Bai A et al (2010) Targeting TGF-beta1 by employing a vaccine ameliorates fibrosis in a mouse model of chronic colitis. Inflamm Bowel Dis 16:1040–1050. https://doi.org/10.1002/ibd.21167
Gu L, Xu Q, Cao H (2017) 1,25(OH)2D3 protects liver fibrosis through decreasing the generation of TH17 cells. Med Sci Monit 23:2049–2058
Wree A, McGeough MD, Inzaugarat ME et al (2017) NLRP3 inflammasome driven liver injury and fibrosis: roles of IL-17 and TNF in mice. Hepatol. https://doi.org/10.1002/hep.29523. (Epub ahead of print)
Zepeda-Morales ASM, Del Toro-Arreola S, García-Benavides L et al (2016) Liver fibrosis in bile duct-ligated rats correlates with increased hepatic IL-17 and TGF-β2 expression. Ann Hepatol 15:418–426. https://doi.org/10.5604/16652681.1198820
Meng F, Wang K, Aoyama T et al (2012) Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology 143:765-776.e3. https://doi.org/10.1053/j.gastro.2012.05.049
Tan Z, Qian X, Jiang R et al (2013) IL-17A plays a critical role in the pathogenesis of liver fibrosis through hepatic stellate cell activation. J Immunol 191:1835–1844. https://doi.org/10.4049/jimmunol.1203013 (1950)
Fabre T, Molina MF, Soucy G et al (2018) Type 3 cytokines IL-17A and IL-22 drive TGF-β-dependent liver fibrosis. Sci Immunol. https://doi.org/10.1126/sciimmunol.aar7754
Chakir J, Shannon J, Molet S et al (2003) Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol 111:1293–1298
Al-Muhsen S, Letuve S, Vazquez-Tello A et al (2013) Th17 cytokines induce pro-fibrotic cytokines release from human eosinophils. Respir Res 14:34. https://doi.org/10.1186/1465-9921-14-34
Jiang G, Liu C-T, Zhang W-D (2018) IL-17A and GDF15 are able to induce epithelial-mesenchymal transition of lung epithelial cells in response to cigarette smoke. Exp Ther Med 16:12–20. https://doi.org/10.3892/etm.2018.6145
Chrysanthopoulou A, Mitroulis I, Apostolidou E et al (2014) Neutrophil extracellular traps promote differentiation and function of fibroblasts. J Pathol 233:294–307. https://doi.org/10.1002/path.4359
Zhou X, Loomis-King H, Gurczynski SJ et al (2016) Bone marrow transplantation alters lung antigen-presenting cells to promote TH17 response and the development of pneumonitis and fibrosis following gammaherpesvirus infection. Mucosal Immunol 9:610–620. https://doi.org/10.1038/mi.2015.85
Celada LJ, Kropski JA, Herazo-Maya JD et al (2018) PD-1 up-regulation on CD4+ T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-β1 production. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aar8356
Chakraborty D, Šumová B, Mallano T et al (2017) Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis. Nat Commun 8:1130. https://doi.org/10.1038/s41467-017-01236-6
Sharpe AH, Pauken KE (2018) The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol 18:153–167. https://doi.org/10.1038/nri.2017.108
Kottschade LA (2018) Incidence and management of immune-related adverse events in patients undergoing treatment with immune checkpoint inhibitors. Curr Oncol Rep 20:24. https://doi.org/10.1007/s11912-018-0671-4
Zehender A, Huang J, Györfi A-H et al (2018) The tyrosine phosphatase SHP2 controls TGFβ-induced STAT3 signaling to regulate fibroblast activation and fibrosis. Nat Commun 9:3259. https://doi.org/10.1038/s41467-018-05768-3
Ahmed Z, Lin C-C, Suen KM et al (2013) Grb2 controls phosphorylation of FGFR2 by inhibiting receptor kinase and Shp2 phosphatase activity. J Cell Biol 200:493–504. https://doi.org/10.1083/jcb.201204106
Bode JG, Schweigart J, Kehrmann J et al (2003) TNF-alpha induces tyrosine phosphorylation and recruitment of the Src homology protein-tyrosine phosphatase 2 to the gp130 signal-transducing subunit of the IL-6 receptor complex. J Immunol 171:257–266
Zhang W, Chan RJ, Chen H et al (2009) Negative regulation of Stat3 by activating PTPN11 mutants contributes to the pathogenesis of Noonan syndrome and juvenile myelomonocytic leukemia. J Biol Chem 284:22353–22363. https://doi.org/10.1074/jbc.M109.020495
Zhou X-D, Agazie YM (2008) Inhibition of SHP2 leads to mesenchymal to epithelial transition in breast cancer cells. Cell Death Differ 15:988–996. https://doi.org/10.1038/cdd.2008.54
Maroun CR, Naujokas MA, Holgado-Madruga M et al (2000) The tyrosine phosphatase SHP-2 is required for sustained activation of extracellular signal-regulated kinase and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol Cell Biol 20:8513–8525
Tsang YH, Han X, Man WY et al (2012) Novel functions of the phosphatase SHP2 in the DNA replication and damage checkpoints. PLoS One 7:e49943. https://doi.org/10.1371/journal.pone.0049943
Fisher AJ, Cipolla E, Varre A et al (2017) Potential mechanisms underlying TGF-β-mediated complement activation in lung fibrosis. Cell Mol Med Open Access 3:14
Cipolla E, Fisher AJ, Gu H et al (2017) IL-17A deficiency mitigates bleomycin-induced complement activation during lung fibrosis. FASEB J 31:5543–5556. https://doi.org/10.1096/fj.201700289R
Chen X, Lai P, Wang Y et al (2018) Emerging role of C5a/C5aR IL-17A axis in cGVHD. Am J Transl Res 10:2148–2157
Li L, Chen L, Zang J et al (2015) C3a and C5a receptor antagonists ameliorate endothelial-myofibroblast transition via the Wnt/β-catenin signaling pathway in diabetic kidney disease. Metabolism 64:597–610. https://doi.org/10.1016/j.metabol.2015.01.014
Yiu WH, Li RX, Wong DWL et al (2017) Complement C5a inhibition moderates lipid metabolism and reduces tubulointerstitial fibrosis in diabetic nephropathy. Nephrol Dial Transplant 33:1323–1332. https://doi.org/10.1093/ndt/gfx336
Gu H, Fisher AJ, Mickler EA et al (2016) Contribution of the anaphylatoxin receptors, C3aR and C5aR, to the pathogenesis of pulmonary fibrosis. FASEB J 30:2336–2350. https://doi.org/10.1096/fj.201500044
Jayne DRW, Bruchfeld AN, Harper L et al (2017) Randomized Trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J Am Soc Nephrol 28:2756–2767. https://doi.org/10.1681/ASN.2016111179
Hellmuth K, Grosskopf S, Lum CT et al (2008) Specific inhibitors of the protein tyrosine phosphatase Shp2 identified by high-throughput docking. Proc Natl Acad Sci USA 105:7275–7280
Brown JR, Byrd JC, Coutre SE et al (2014) Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood 123:3390–3397
Calabrese LH, Rose-John S (2014) IL-6 biology: implications for clinical targeting in rheumatic disease. Nat Rev Rheumatol 10:720–727
Ballou LM, Lin RZ (2008) Rapamycin and mTOR kinase inhibitors. J Chem Biol 1:27–36
Kurschus FC, Moos S (2017) IL-17 for therapy. J Dermatol Sci 87:221–227
Weiss A, Attisano L (2013) The TGFbeta superfamily signaling pathway. Wiley Interdiscip Rev Dev Biol 2:47–63. https://doi.org/10.1002/wdev.86
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
The conception and design of the study, acquisition of data, analysis and interpretation of data—SA, DPM, VA. Drafting the article—SA; Revising it critically for important intellectual content—DPM, VA. Final approval of the version to be submitted—SA, DPM, VA. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved—SA, DPM, VA.
Corresponding author
Ethics declarations
Conflict of interest
Sakir Ahmed declares that he has no conflict of interest, including no relationship with pharmaceutical companies. Durga Prasanna Misra declares that he has no conflict of interest, including no relationship with pharmaceutical companies. Vikas Agarwal declares that he has no conflict of interest, including no relationship with pharmaceutical companies.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmed, S., Misra, D.P. & Agarwal, V. Interleukin-17 pathways in systemic sclerosis-associated fibrosis. Rheumatol Int 39, 1135–1143 (2019). https://doi.org/10.1007/s00296-019-04317-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-019-04317-5