Abstract
Biologic disease-modifying anti-rheumatic drugs (bDMARD) have transformed the treatment paradigm of chronic autoimmune rheumatic diseases (ARDs), but they are often associated with adverse drug reactions. The present study evaluated the frequency, characteristics and type of infections, other than tuberculosis (TB), in ARD patients receiving bDMARDs. The multicentre, cross-sectional, retrospective, observational study was conducted across 12 centers in Karnataka, India, between January to August 2016. The study included patients receiving bDMARD therapy for various ARDs. Outcome variables considered were any infection, minor infections and major infections, other than TB. Clinical variables were compared between infection and no infection group, and the increase in the likelihood of infection with respect to various clinical variables was assessed. The study involved 209 subjects with a median (range) age of 41 (16–84) years and male to female ratio of 0.97:1. A total of 29 (13.88%) subjects developed infection following bDMARD therapy, out of whom a majority had minor infection (n = 26). The likelihood of developing any infection was noted to be more in subjects receiving anti-TNF (golimumab, P = 0.03) and those on three or more conventional synthetic (cs) DMARDs (P < 0.01). Infection risk was higher in patients with systemic lupus erythematosus (P = 0.04), other connective tissue disease (P < 0.01) and in patients with comorbidities (P = 0.13). The risk of infection was associated with the use of anti-TNF therapy and more than three csDMARDs, co morbidities and Adds such as systemic lupus erythematosus and connective tissue disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The introduction of biologic disease-modifying anti-rheumatic drugs (bDMARDs) has revolutionized the treatment paradigm of chronic autoimmune rheumatic diseases (ARDs) like rheumatoid arthritis (RA), ankylosing spondylitis (AS), undifferentiated spondyloarthropathy (unSpA), psoriatic arthritis (PsA), systemic lupus erythematosus (SLE) and other connective tissue diseases (CTD). The commonly used biological agents include: TNF antagonists [infliximab, etanercept, adalimumab, golimumab and certolizumab (not licensed in India)]; IL-6 receptor antagonist (tocilizumab); T-cell costimulatory modulator (abatacept); B-cell depletion therapy type 1 humanized chimeric anti-CD20 monoclonal antibody (rituximab and ocrelizumab); and B cell targeting by BAFF and April inhibitors (belimumab, tabalumab and atacicept) [1, 2]. Despite the therapeutic potential, the drug use is associated with immunological and allergic adverse drug reactions. The adverse drug reactions, which can be delayed or immediate, are caused due to the impact of drug-induced imbalance on the immune system, cofactors and intermediary factors [3]. Recognizing the heterogeneity associated with these adverse drug reactions, Pichler has differentiated their clinical features into five distinct types, namely clinical reactions because of high cytokine levels (type α), hypersensitivity because of an immune reaction against the biological agent (β), immune or cytokine imbalance syndromes (γ), symptoms because of cross-reactivity (δ) and symptoms not directly affecting the immune system (ɛ). This classification is intended to effectively deal with clinical features and to identify the probable individual and general risk factors [4].
Infection has been reported as one of the most common adverse drug reactions noted in subjects receiving bDMARDs [5]. The serious bacterial infections in RA patients receiving bDMARDs include surgical site infection of total joint arthroplasty and respiratory (tuberculosis), skin, urinary tract, bone and joint infections. A systemic review and meta-analysis conducted by Singh et al., involving 42,330 patients with RA, has found an increase in the incidence of serious infections in patients on standard-dose and high-dose biological drugs compared to those who received traditional conventional synthetic (cs) DMARDs [6]. The literature evidence on the safety of biological agents is mainly based on the randomized controlled trials (RCTs), small observational studies and case reports [7]. Based on these findings, presence of co-morbidities, age > 60 years, and concomitant glucocorticoid use have been identified as some of the key factors linked to increased risk of serious infections [5]. However, the prevalence of severe infections noted in RCTs is only < 10% and the researchers are speculative about the extrapolation of safety data obtained from trials in clinical practice. The present study evaluated the frequency, characteristics and type of infections, other than tuberculosis, in ARD patients receiving bDMARDs. It also studied the influence of csDMARDs, corticosteroids and other factors on frequency and type of infections.
Patients and methods
The multicenter cross-sectional, retrospective observational study was conducted across 12 centers in Karnataka, India, between January 2016 to August 2016. The study recruited patients receiving bDMARD therapy for any type of ARDs on their routine visit to the center. Clinical diagnosis was established based on 2010 American College of Rheumatology (ACR) criteria for the diagnosis of RA, systemic lupus international collaborating clinics/American College of Rheumatology (SLICC/ACR) criteria (2010) for SLE, Ankylosing Spondylitis International Society criteria (2010) for Ankylosing spondylitis, Classification Criteria for Psoriatic Arthritis (CASPAR) criteria for psoriatic arthritis, the European Spondyloarthropathy Study Group (ESSG) criteria (but these patients failed to fulfil ASAS and CASPAR criteria) for undifferentiated spondyloarthropathy and respective criteria for diagnosis of various ARDs [8,9,10,11,12]. Baseline demographic, clinical and therapeutic data including the history of bDMARDs and concomitant therapy given to the patients were collected retrospectively from patient interviews and clinical records. Patients without details of initiation and follow-up of bDMARD therapy for up to 2 months were excluded. The study was approved by the institutional ethics committee (IEC-CRICR/021/16 dated 19/02/2016) and written consent was obtained from all the recruited subjects to collect data from clinical records.
All the subjects underwent screening for active infections and latent tuberculosis (tuberculin test or QuantiFERON-TB or both) prior to the administration of bDMARDs, as specified by local guidelines. The patient details considered were: age, gender, diagnosis, co-morbidities, and the use of bDMARDs, csDMARDs and steroids. Based on the diagnoses made, the recruited subjects were grouped into six diagnostic groups: RA, SLE, ankylosing spondylitis, psoriatic arthritis, undifferentiated spondyloarthropathy (unSpA) and patients with ARD conditions other than the above diagnoses. Clinical data of co-morbidities were considered as present or absent. Patients receiving single, two and three or above csDMARDs/immunosuppressants therapy were designated as single, double and three or above csDMARDs, respectively. Patients not on any csDMARDs were considered as no csDMARDs. The biosimilars and originator molecules were grouped into the same category; for example, original rituximab and their biosimilars belonged to the same class. Steroids in any form, either oral or intermittent parenteral, were grouped into steroids given or not given. Data pertaining to bacterial, fungal and viral infections including unspecified infections (where the cause of infection could not be ascertained by the treating physician) were collected and classified as minor and major infections. Any bacterial infections, excluding M. tuberculosis, viral or unspecified infections requiring low-profile antibiotics/antivirals without hospitalization, were considered as ‘minor infections’. The category ‘major infections’ included opportunistic fungal infections and bacterial, viral or unspecified infections requiring hospitalization. Occurrence of either minor or major or both infections was considered under the category ‘any infections’.
Statistics
The demographic and clinical variables were given as mean ± SD for normal continuous data, median (min–max) for data without normal distribution, and counts for frequency data. Kolmogorov Smirnov or Shapiro–Wilk normality tests were used to evaluate distribution of the variables. Outcome variables considered were: any infections, minor infections and major infections. Comparison of clinical variables for infection and no infection groups was performed by unpaired t test for normal data, Mann–Whitney U test for non-normal data and Chi square or Fisher’s exact test for frequency data. Adjusted residuals method was used to interpret statistically significant chi square results. Association analysis was performed by univariate and multivariate logistic regression. Variables within the cut-off P value of 0.1 in univariate regression were considered for multivariate regression. Separate analysis was performed for the three outcome variables. The reference groups considered for logistic regression analysis were as follows: gender: male; diagnosis: RA; co-morbidities: absent; bDMARDs: rituximab; csDMARDs: single; and steroids: absent. For all other analyses, P < 0.05 was considered as statistically significant.
Results
Clinical records of 272 ARD patients on bDMARD therapy were extracted and assessed for inclusion and exclusion criteria. The details of inclusion and exclusion of patients in the study are provided in the flow chart (Fig. 1). After exclusion of patients with TB and those with inflammatory diseases and incomplete drug history, 209 patients with ARDs were selected for the study. The median (min–max) age of patients was 41 (16–84) years. Among them, 103 (49.28%) were males and 106 (50.72%) were females. The bDMARD therapies administered have been described in Table 1. Six patients received bDMARD of two different categories: etanercept and tocilizumab, infliximab and etanercept, tocilizumab and etanercept, tocilizumab and rituximab, etanercept and golimumab, and infliximab and adalimumab. In these patients, the final bDMARD administered was considered for the data analysis.
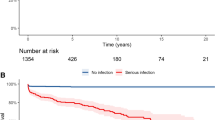
A total of 29 patients developed infections after treatment with bDMARDs. Most patients had minor infections (n = 26). The minor infections reported by the study subjects included eye boils, diarrhea, furunculosis, urinary tract infection, unspecified infections of upper respiratory tract, skin and angioneurotic edema and viral infections of upper respiratory tract, chicken pox and herpes zoster. Major infections (n = 5) included severe cellulitis, candida, and unspecified infections requiring hospitalization. Two patients had both major and minor infections. One patient had minor infection and an infection requiring hospitalization. The second patient had one spell each of viral and fungal infections. In patients with infections, two patients discontinued bDMARDs due to the occurrence of infection as adverse reaction. One patient died due to pulmonary embolism; however it was not related to bDMARD therapy.
Diagnoses of the study subjects have been listed in Table 1. Co-occurring co-morbidities were noted in 33.49% (n = 70) of the study population. Hypertension, diabetes mellitus and ischemic heart disease were observed in 19.14% (n = 40) of the study subjects and 14.35% (n = 30) had other co-morbidities. The corresponding incidence rates noted for hypertension, diabetes mellitus and ischemic heart disease were 7.18% (n = 15), 2.87% (n = 6) and 1.9% (n = 4). Co-occurring co-morbidities reported were: hypertension and diabetes mellitus (2.87%; n = 6), hypertension and ischemic heart disease (2.87%; n = 6), diabetes mellitus and ischemic heart disease (0.48%; n = 1), and all the three conditions, hypertension, diabetes and ischemic heart disease (0.96%; n = 2). The incidence of infection was higher in patients with diabetes mellitus (Suppl. table 1). Other co-morbidities reported were: hypothyroidism, depression, anemia, bronchial asthma, uveitis, osteoarthritic knee, pulmonary thromboembolism, laryngotracheobronchitis, chronic kidney disease, thyroiditis, obesity, anxiety, alcohol cirrhosis, oral lichen planus, bullous lesions and osteonecrosis of the femoral condyles.
The corresponding proportions of subjects who received different types of single csDMARDs/immune suppressants therapy were as follows: 65.29% (n = 79) methotrexate, 16.53% (n = 20) sulfasalazine, 5.79% (n = 7) hydroxychloroquine, 4.13% (n = 5) azathioprine, 4.13% (n = 5) leflunomide, 3.31% (n = 4) mycophenolate mofetil and 0.83% (n = 1) cyclophosphamide. Methotrexate and hydroxychloroquine were the commonly received double csDMARDs (38.1%; n = 24), followed by methotrexate and sulfasalazine (30.16%; n = 19), methotrexate and leflunomide (7.94%; n = 5), hydroxychloroquine and mycophenolate mofetil (7.94%; n = 5), hydroxychloroquine and azathioprine (4.76%; n = 3), methotrexate and tacrolimus (3.17%; n = 2), methotrexate and mycophenolate mofetil (1.59%; n = 1), hydroxychloroquine and leflunomide (1.59%; n = 1), hydroxychloroquine and sulfasalazine (1.59%; n = 1), hydroxychloroquine and tacrolimus (1.59%; n = 1), and leflunomide and tacrolimus (1.59%; n = 1). Patients on three or more csDMARDs received: methotrexate, hydroxychloroquine and leflunomide (40%; n = 6); methotrexate, hydroxychloroquine and sulfasalazine (13.33%; n = 2); methotrexate, hydroxychloroquine and tacrolimus (13.33%; n = 2); hydroxychloroquine, leflunomide and sulfasalazine (13.33%; n = 2); methotrexate, leflunomide and sulfasalazine (6.67%; n = 1); methotrexate, hydroxychloroquine, leflunomide and sulfasalazine (6.67%; n = 1); and methotrexate, hydroxychloroquine, leflunomide, sulfasalazine and tacrolimus (6.67%; n = 1). Around 8% (n = 16) of the study subjects received intermittent parenteral administration of 160 mg methylprednisolone acetate or a maximum dose of 10 mg daily oral therapy.
A total of 197 patients were considered for comparison and association studies for ‘any infections’, and 12 cases were excluded, as there were no cases for abatacept and no csDMARD use. Infections were reported in 29 (14.72%) patients. Statistically significant difference was noted between infection and no infection groups for age, diagnosis, co-morbidities, bDMARDs and csDMARDs. Gender and steroid usage did not differ significantly between the patient groups (Table 2). Patients with infection were older than those in the no infection group. The infection cases reported in different diagnostic groups showed greater than expected number of infections in other CTDs (29.16%; n = 7) (adj. res. 2.1) and lower number of infections in ankylosing spondylosis (4.17%; n = 2) (adj. res. − 2.4). Patients with co-morbidities showed increased infection (23.88%; n = 16) (adj. res. 2.6) than those without co-morbidities (10%; n = 13) (adj. res. − 2.6). Patients receiving golimumab had highest number of infection cases (50%; n = 4) (adj. res. 2.9), and infliximab (2.70%; n = 1) (adj. res. − 2.3) had lesser than expected infections. The incidences of infections were higher in three or more csDMARDs (38.46%, n = 5) (adj. res. 2.5) than those on single csDMARDs (9.92%, n = 12) (adj. res. − 2.4). The infection percentage noted in patients on steroids was 30% (3/10) compared to 13.9% (26/187) in those not receiving steroids. However, the difference was not statistically significant (P = 0.17). Univariate logistic regression of variables showed that age, co-morbidities, bDMARDs and csDMARDs were significantly associated with infection. Diagnosis was within the cut-off P value of 0.1 (Table 3). Gender and steroid usage with P value > 0.1 were excluded from multivariate analysis. Multivariate logistic regression with age, diagnosis, co-morbidities, bDMARDs and csDMARDs was performed. The model was statistically significant with model Chi square = 37.22, df = 14, P < 0.01 and Nagelkerke R2 = 0.30 (Table 4). The corresponding increased risk of infections noted in patients with SLE and those with other ARDs were 10 times and 12 times when compared to patients with RA. The risk of infection was 11 times more in patients receiving golimumab. Patients on three or more csDMARDs showed 10.5 times increased likelihood of infection than those on single csDMARDs. Presence of co-morbidities in patients receiving bDMARD therapy was associated with a non-significant increase in the infection risk (2 times). Age was not significantly associated with infection risk.
Based on the minor infection status, the patients (n = 179) were grouped as minor infection group (n = 26) and no minor infection (n = 153) group. Patients with unSpA, abatacept and no csDMARD groups did not develop minor infections and these groups were excluded from comparison and association studies (Fig. 1). Significant differences in diagnosis, co-morbidities, biologic and csDMARDs were noted on comparison of minor infection and no minor infection groups (Suppl. table 2). Age, diagnosis, co-morbidities, bDMARDs and csDMARDs were within P < 0.1 cut-off in univariate logistic analysis for inclusion in multivariate logistic regression (Suppl. table 3). Multivariate logistic regression showed statistically significant association between diagnosis and minor infections (Suppl. table 4). The increased likelihood of minor infections noted in patients with other ARDs, SLE and PsA were 11 times, 8.4 times and 4 times, respectively, when compared to RA patients. Patients with ankylosing spondylosis were 40% less likely to have minor infections than RA patients. The point estimates of SLE, PsA and ankylosing spondylosis disease groups did not achieve statistical significance. Patients receiving golimumab demonstrated 13 times increased likelihood of minor infections than those on rituximab. Tocilizumab and etanercept bDMARDs demonstrated two times more non-significant increase in the likelihood of minor infections. Patients receiving adalimumab and infliximab were 27% and 19% less likely to develop minor infections than rituximab receiving group respectively. Patients receiving three or more csDMARDs were eight times more likely to have minor infection than those on single csDMARDs. Those on double csDMARDs had 2.5 times increased likelihood of minor infection. Patients with co-morbidities showed two times increased risk for minor infection than those without co-morbidities. Age was not associated with minor infections in patients receiving bDMARDs.
Based on the status of major infections, patients (n = 109) were classified as major infection (n = 5) and no major infection (n = 104) groups. However, the analysis of major infections could not be performed, as the number of patients with major infections was only 5 and the sample size was small to obtain reliable estimates.
Discussion
The present study was a conjoint effort by rheumatologists across Karnataka to aggregate Indian data from different centers pertaining to the prescribing patterns and the incidence of infections encountered during bDMARDs use in routine clinical practice. Approximately 14% of the patient developed infections other than TB. SLE and CTD patients on bDMARDs had higher incidence of infections compared to those with other ARDs. The infection was more related to the disease rather than the bDMARDs used. The AS had least infection considering the younger age of the subjects. Use of more than three csDMARDs and presence of co-morbidity like diabetes had increased the incidence of infections.
Infection has been identified as the second most common cause for death in RA subjects (23%) from India [13]. There are studies reporting the increased susceptibility to infections of RA patients compared to non-RA patients. A population-based study by Doran et al. has concluded that the increased frequency of infection in RA patients could be due to immunosuppressive effects of the disease itself or the agents used for the treatment. The researchers have also identified soft tissues, joints, skin and the respiratory tract as the sites with highest risk for infections [14]. A review by Jasvinder Singh has also reported the association between RA disease activity and increased risk of serious infections. The study has suggested that, apart from the bDMARD use, the older age, history of serious infections and glucocorticoid dose may influence the risk of serious infections [15]. In concurrence with these findings, the present study noted increased risk of infection associated with steroid use, but not with the age. The dissimilarities noted on comparison of the present study with that of Jasvinder Singh, which considered only RA, could be due to the consideration of all the ARDs together and subjects with lower mean age.
Contrasting observations have been reported by various studies with reference to infection risk associated with bDMARDs use. The largest prospective study, conducted by the British biological registry, involving 7664 anti-TNF-treated subjects has shown no significant increase in overall rate of serious infections compared to those treated with csDMARDs, after adjustment for baseline risk. However, the study has reported a fourfold increased risk of skin and soft tissue infections in anti-TNF-treated patients [16]. Similarly, a retrospective cohort study conducted by Smitten et al. in 24,530 patients has found only slightly increased risk of hospitalized infection linked to the use of biological DMARDs (RR = 1.21; 95% CI 1.02–1.43) [17]. The study has also noted that oral corticosteroid use increased the risk of infection (RR = 1.92; 95% CI 1.67–2.21). Corroborating these findings, the current study also noted a higher incidence of infection in subjects using steroids; 30% of the subjects receiving steroids had infection, compared to subjects not receiving steroids (13.9%).
Very limited studies have evaluated whether the infection risk associated with the bDMARDs varies among patients with diverse ARDs. AS patients had 40% reduced likelihood of minor infection compared to RA patients. SLE and other CTD had increased incidence of infection. The steroid use was higher in subjects with SLE and other CTD, and this could be the additional risk factor for increased infection, in addition to the disease itself. The AS and unSpA had lesser risk of infection, despite being managed by anti-TNF drugs, whereas, incidence of infections was higher in patients receiving anti-TNF compared to other bDMARDs.
In addition, patients with co-morbidities were found to have higher number of infections (23.88%) than those without (10%). Co-morbidities, such as hypertension, diabetes mellitus and ischemic heart disease, were observed in 19.14% of the subjects. Among these conditions, the highest infection incidence rate was noted for diabetes mellitus. Similar findings were noted in the study by Quartuccio et al., though majority of the subjects considered were having RA [18].
Studies indicate that the associated risk of hospitalized infections varies across different bDMARD agents used to treat RA. The difference in susceptibility to adverse drug reactions (e.g., serious infection, malignancy and death) noted for these agents could be due to the difference in the immune system components that serve as drug targets. Yun et al. have concluded that the use of etanercept, infliximab or rituximab in RA patients with prior exposure to a biologic agent is linked to 1-year risk of hospitalized infection compared to the risk associated with abatacept [19]. The results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR), involving 11,466 patients with psoriasis, have suggested that the treatment with adalimumab and infliximab has higher risk of serious infections compared to non-methotrexate and non-biologic therapies, whereas the present study has noted that the infection rate was least with infliximab treatment. This could be due to the consideration of only data pertaining to non-tubercular infections for evaluation. Generally, it has been noted that the tubercular infection is more in RA patients treated with infliximab [20]. The respective incidence rates noted in the PSOLAR study for ustekinumab, etanercept, adalimumab and infliximab were 0.83, 1.47, 1.97, and 2.49 per 100 per 100 patient-years [21].
The present study holds greater significance, as there are very limited literature data from India comparing the risks of infections across bDMARDs with different mechanisms of action. The study highlights the need of increasing the awareness among both patients and primary physicians regarding the possibility of serious infection that may develop during the course of the treatment. The present study evaluated various clinically relevant variables to improve the generalizability of results. However, the findings must be interpreted in the light of several limitations. One of the major limitations was not collecting the data on duration of illness and duration of bDMARDs received and hence influence of these on infection risk could not be ascertained. The data used in the current study lacked detailed information on disease severity and some clinical factors (e.g., smoking) that may differ between treatment groups. The current study included real-time data from rheumatology clinical settings. The variability in some of the factors such as other CTD disease group and biosimilars received by study subjects could have contributed to heterogeneity. Adult onset Still’s disease was included in the other CTD group as many studies have considered it as a disease with autoinflammatory-autoimmune continuum [22,23,24,25]. Co-morbidities were not analyzed separately. The possibility of misclassification and residual confounding cannot be excluded. In addition, the number of major infections reported was less to perform analysis. The possibility of selection bias cannot be ruled out, as the patients not reporting infection risk for some of the variables were not considered for comparison and association studies.
Further research involving larger sample size is mandatory to evaluate the magnitude of infection risk associated with diverse bDMARD agents. Such studies should also focus on evaluating the effect of systemic inflammation and diverse drug mechanisms on the risk of serious infections.
Conclusion
The study highlights the need of conducting periodic screening and close monitoring in patients receiving bDMARDs for any suspected infection risk. Though the present study reassures that the incidence of infection other than TB is not higher as perceived earlier, caution is advised in elderly, patients on anti-TNF therapy and those with SLE and CTDs. The putative relationship between susceptibility to infections and bDMARDs and the variation in risk across different biological agents have to be corroborated through further studies.
References
Yamanaka H, Goto K, Suzuki M (2012) Bacterial infection in the limbs of patients with rheumatoid arthritis during biological agent therapy. Open J Rheumatol Autoimmune Dis 2:47–52
Hofmann K, Clauder A-K, Manz RA (2018) Targeting B cells and plasma cells in autoimmune diseases. Front Immunol 9:835
Boyman O, Comte D, Spertini F (2014) Adverse reactions to biologic agents and their medical management. Nat Rev Rheumatol 10:612–627
Pichler WJ (2006) Adverse side-effects to biological agents. Allergy 61:912–920
Her M, Kavanaugh A (2016) Alterations in immune function with biologic therapies for autoimmune disease. J Allergy Clin Immunol 137:19–27
Singh JA, Cameron C, Noorbaloochi S et al (2015) Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Lancet 386:258–265
Díaz-Lagares C, Pérez-Alvarez R, García-Hernández FJ et al (2011) Rates of, and risk factors for, severe infections in patients with systemic autoimmune diseases receiving biological agents off-label. Arthritis Res Ther 13:R112
Aletaha D, Neogi T, Silman AJ et al (2010) 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 69:1580–1588
Petri M, Orbai A-M, Alarcón GS et al (2012) Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 64:2677–2686
Rudwaleit M, van der Heijde D, Landewé R et al (2011) The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 70:25–31
Taylor W, Gladman D, Helliwell P et al (2006) Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 54:2665–2673
Dougados M, van der Linden S, Juhlin R et al (1991) The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum 34:1218–1227
Chopra A (2012) Epidemiology of rheumatoid arthritis. In: Mukherjee S, Ghosh A (ed) Monograph on rheumatoid arthritis. Indian College of Physicians, Academic Wing of API India, Marksman Media Service, Kolkata, pp 1–9
Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE (2002) Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum 46:2287–2293
Singh JA (2016) Infections with biologics in rheumatoid arthritis and related conditions: a scoping review of serious or hospitalized infections in observational studies. Curr Rheumatol Rep 18:61
Dixon WG, Watson K, Lunt M, Hyrich KL, Silman AJ, Symmons DPM (2006) Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum 54:2368–2376
Smitten AL, Choi HK, Hochberg MC et al (2008) The risk of hospitalized infection in patients with rheumatoid arthritis. J Rheumatol 35:387–393
Quartuccio L, Zabotti A, Zotto SD, Zanier L, De Vita S, Valent F (2018) Risk of serious infection among patients receiving biologics for chronic inflammatory diseases: use-fulness of administrative data. J Adv Res. https://doi.org/10.1016/j.jare.2018.09.003
Yun H, Xie F, Delzell E et al (2016) Comparative risk of hospitalized infection associated with biologic agents in rheumatoid arthritis patients enrolled in medicare. Arthritis Rheumatol 68:56–66
Wolfe F, Michaud K, Anderson J, Urbansky K (2004) Tuberculosis infection in patients with rheumatoid arthritis and the effect of infliximab therapy. Arthritis Rheum 50:372–379
Kalb RE, Fiorentino DF, Lebwohl MG et al (2015) Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol 151:961–969
Gerfaud-Valentin M, Jamilloux Y, Iwaz J, Sève P (2014) Adult-onset Still’s disease. Autoimmun Rev 13:708–722
Ruscitti P, Giacomelli R (2018) Pathogenesis of adult onset still’s disease: current understanding and new insights. Expert Rev Clin Immunol 14:965–976
Maria AT, Le Quellec A, Jorgensen C, Touitou I, Rivière S, Guilpain P (2014) Adult onset Still’s disease (AOSD) in the era of biologic therapies: dichotomous view for cytokine and clinical expressions. Autoimmun Rev 13:1149–1159
Peckham D, Scambler T, Savic S, McDermott MF (2017) The burgeoning field of innate immune-mediated disease and autoinflammation. J Pathol 241:123–139
Acknowledgements
The authors acknowledge the help of Research Assist (http://www.research-assist.com) for editing and formatting the manuscript.
Funding
Karnataka Rheumatology Association (Association of rheumatologists).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors S. Chandrashekara, Vineeta Shobha, Vijay Rao, Anu Desai, Ramesh Jois, B. G. Dharmanand, Sharath Kumar, Pradeep Kumar, Chethana Dharmapalaiah, Kurugodu Mathada Mahendranath, Shiva Prasad, Manisha Ashwin Daware, Yogesh Singh, Uma Karjigi, Nagaraj S, and K. R. Anupama declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chandrashekara, S., Shobha, V., Rao, V. et al. Incidence of infection other than tuberculosis in patients with autoimmune rheumatic diseases treated with bDMARDs: a real-time clinical experience from India. Rheumatol Int 39, 497–507 (2019). https://doi.org/10.1007/s00296-019-04245-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-019-04245-4