Abstract
Purpose of Review
This review assesses the long-term remission and predictors of clinical outcome in patients with juvenile idiopathic arthritis (JIA). A comprehensive literature search was performed including articles published between January 1, 2004 and February 28, 2017. Studies, with a minimum follow-up of 24 months, were selected independently by two reviewers based on in- and exclusion criteria. The objective outcome was inactive disease/clinical remission as defined by the Wallace criteria at last follow-up.
Recent Findings
The probability of achieving inactive disease and/or clinical remission is dependent on the JIA subcategories studied in the different articles. Overall, a significant proportion of JIA patients still showed signs of active disease at last follow-up. Some studies include patient populations followed for 15 years or more and these patients were exposed to different treatment protocols at disease presentation than patients diagnosed in the biologic era.
Summary
Although the severity of the morbidity and associated mortality risk has decreased over time, a significant proportion of the current JIA patients still do not reach an inactive disease status within a 2-year follow-up window. Studying the long-term outcome of patients with JIA remains challenging due to the heterogeneity of the study designs and study populations. Although improvement has been shown in the biologic era, we still need to enhance the number of patients with inactive disease within the first 2 years after diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Juvenile idiopathic arthritis (JIA) encompasses a group of chronic childhood arthropathies of unknown etiology defined as a disease persisting for more than 6 weeks, setting in before age 16 years, and with exclusion of other causes of arthritis. JIA is the most common rheumatic disease in childhood. JIA has been divided into seven subcategories based on clinical features in accordance with the International League of Associations for Rheumatology (ILAR, 2001) classification criteria; systemic JIA (sJIA), oligoarticular JIA, polyarticular rheumatoid factor negative (RF−) JIA, polyarticular RF-positive (RF+) JIA, enthesitis-related arthritis (ERA), juvenile psoriatic arthritis (JPsA), and undifferentiated JIA [1].
The clinical course of JIA is unpredictable, with periods of low levels of disease activity followed by resurgence of signs and symptoms on or off medication. Some of the patients may even achieve complete remission over time. The long-term outcome of JIA has improved significantly since the emergence of the first outcome papers around 40 years ago [2].
Since 1999, after the introduction of potent biologic agents for JIA treatment, dramatic improvements in functional outcome measures have been noticed. The advent of these agents heralds a new era in JIA treatment, wherefore comparison with past studies of the long-term, often poor, outcome of JIA makes little sense. Comparison of these studies is hampered by multiple factors. Many early outcome studies were prone to selection bias towards more severe disease subtypes or they combined groups with different outcome, e.g., rheumatoid factor-negative and factor-positive polyarticular JIA patients. Most studies were mainly cross-sectional or retrospective cohort studies, both with the limitation of retrospectively collected data, and very few were prospective, population-based studies. Comparison is further hampered by the pre-biologic era’s lack of standardized outcome measures, which made it difficult to study the outcome of JIA in the long term and, indeed, to draw appropriate conclusions.
Fortunately, these shortcomings have been rectified with the development of validated tools like the Juvenile Arthritis Damage Index (JADI) [3] as a clinical measure of articular and extra-articular damage and the American College of Rheumatology (ACR) Pediatric 30, 50, 70, 90, 100 responses as an efficacy measurement [4]. The well-accepted criteria for inactive disease and clinical remission by Wallace et al. [5] have also facilitated the study of long-term outcome and are an attempt to reduce the variability in activity measurements in recent studies. However, no disease activity score or outcome measure has so far been validated for use in adults with JIA although the development and validation of such measures would clearly further advance the field.
Nowadays, in the management of JIA, clinical remission is the accepted goal. However, remission is much associated with the JIA subtype and inversely with suggested predictors for poor outcome. Acknowledging that the subgroups of JIA do not run a homogeneous course, remission off medication is seen as a proxy for true clinical remission in JIA patients across all JIA subcategories [5].
JIA is not a disease limited to childhood as around half of all children continue to have episodes of active inflammation into adulthood. Therefore, the aim of this review is to describe remission on or off medication over time. The present review will highlight the long-term outcome of JIA with a particular focus on rates of inactive disease, remission, disease flare, and predictors of outcome. Long-term outcome in regard to uveitis is beyond the scope of this paper.
Materials and Methods
A literature search was performed, including studies on clinical outcome in JIA published between January 1, 2004 and February 28, 2017. The time period included was used to minimize the differences between studies in terms of their disease classification, definition of remission, and drug treatment strategy studies, although in some studies with long duration of follow-up, patients treated before the biologic era might still be included.
The literature search included peer-reviewed publications in PubMed using the keywords juvenile idiopathic arthritis or juvenile chronic arthritis or juvenile rheumatoid arthritis. The search identified 5061 publications. One paper was added by screening the references of the selected papers. Study selection was performed systematically and independently by two experienced researchers (MT, MG). The articles were first screened based on their titles, secondly based on their abstract, and finally based on the full-text version. Disagreements were discussed and a decision was taken by consensus.
The following inclusion and exclusion criteria were taken into account. The following are the inclusion criteria: all randomized clinical trials, cohort studies, and observational studies of functional arthritis outcome in JIA with a follow-up of a minimum of 2 years were eligible; studies reported on juvenile idiopathic arthritis (JIA; ILAR classification) [1], juvenile chronic arthritis (JCA; European League Against Rheumatism (EULAR) classification) [6], and juvenile rheumatoid arthritis (JRA; ACR classification) [7] were also included; papers on clinical remission were only included if using the Wallace criteria [5] or modifications hereof [8,9,10,11,12,13,14,15,16,17]. Exclusion criteria were case reports/series with fewer than five patients; studies with less than 2 years of follow-up; reviews, unless they were systematic reviews or meta-analyses; studies that did not report at least one primary or secondary outcome of interest described below; studies only assessing outcome for uveitis; and papers in other languages than English.
The primary outcome evaluated were disease flare, rates of inactive disease, and remission as defined by Wallace et al. [5]. The Wallace criteria for inactive disease include (1) no joints with active arthritis; (2) no fever, rash, serositis, splenomegaly, or generalized lymphadenopathy attributable to JIA; (3) no active uveitis; (4) normal ESR and/or CRP; and (5) physician’s global assessment of disease activity indicating no disease activity. The two types of remission according to Wallace criteria are (1) clinical remission on medication (CRM), the criteria of inactive disease must be met for a minimum of 6 continuous months while the patient is on medication; and (2) clinical remission off medication (CR), the criteria of inactive disease must be met for a minimum of 12 continuous months while off all anti-inflammatory medication [5]. Secondary outcomes evaluated were predictors of inactive disease and/or remission, active and cumulative joint count, sacroiliitis, risk of disease flare, and damage.
For subgroups of the articles, due to heterogeneity of the study populations and study designs, we subdivided the results into the seven JIA subtypes and accordingly into the following two categories:
-
1.
Rates of inactive disease and remission as defined by Wallace et al. [5]
-
2.
Predictors of remission or poor outcome, risk of flare, damage, and sacroiliitis
Results
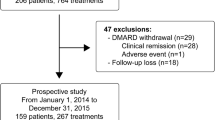
After the selection process, 38 papers were thought to be eligible for inclusion in this review. In total, 29 articles on clinical remission were included [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28, 29•, 30, 31, 32•, 33,34,35, 36••]. Thirteen papers also described predictors for remission or poor outcome and an additional 10 papers specifically discussed predictors of risk of flare, damage, and sacroiliitis [9, 10, 12, 13, 15, 19, 20, 22, 23, 27, 28, 32•, 34, 37,38,39,40,41,42,43,44,45].
Inactive Disease and Remission
Studies describing the achievement of inactive disease and remission on or off medication in the total JIA cohort are summarized in Tables 1, 2, and 3 and are divided according to time of follow-up. Rates of inactive disease and remission varied considerably among the studies. In summary, in six studies, with a follow-up period of 2 to not more than 5 years and reporting on a total of 895 patients, CR was observed in 21.5 ± 15.1% (Table 1). In another five studies, with a follow-up period of 5 to not more than 10 years including 3058 patients, CR was achieved in 36.2 ± 13.8% (Table 2). Finally, in two studies, with a follow-up of more than 17 years covering 262 JIA patients, remission off medication was achieved in 40 and 59%, respectively (Table 3).
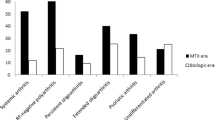
As mentioned in the introduction, the rate of inactive disease and remission of JIA seems to be closely related to the underlying subtype. Therefore, we have extrapolated the data of the manuscripts and reported the seven subtypes, when subtype information was provided, and their clinical outcome separately (Tables 4 and 5) and are described in detail below. Some studies contain a limited amount of patients of certain subtypes, which might lead to an over- or underestimation of clinical remission in those subtypes. Studies were only included in Tables 4 and 5 if ILAR classification for JIA and Wallace criteria for inactive disease and remission on and off medication were applied [1, 5]. The overall remission rate was the lowest in the polyarticular RF+ group; none of these patients were in remission on medication and only 6% obtained CR. On the other hand, the remission rates on or off medication were the most favorable for the oligoarticular-persistent group.
Systemic JIA
In a 2-year cohort study including 27 sJIA patients by Huang et al. [11], complete remission using the EULAR disease activity criteria [46] at the last follow-up was achieved in 46%. The multicenter, retrospective study (sJIA n = 31) of Albers et al. [15] reported clinical remission in 58%. Selvaag et al. [9] performed a 3-year prospective follow-up study and found 29% of sJIA patients in CR.
Vastert et al. [29•] performed a 3-year follow-up study on 20 new-onset sJIA patients treated with anakinra as the first-line treatment; 73% achieved CR and 18%, CRM. In a retrospective study of Russo et al. [20], including 132 sJIA patients, 37% achieved CRM and 9% CR at 3 years follow-up.
In an Italian prospective cohort study with a follow-up of 4.4 years by Romano et al. [28], the causes of discontinuation of biological treatment (n = 301) were studied. They found 8% of the sJIA patients stopped the treatment due to achievement of inactive disease or remission in comparison with 9.2% in the remaining JIA subtypes.
Bertilsson and colleagues [13] performed a 5-year prospective population-based follow-up study (sJIA n = 6) with a reported CR in 50% of sJIA patients for at least 2 years and 33% achieved inactive disease off treatment for less than 2 years. It should be noted that this study used the EULAR JCA diagnostic criteria for patient inclusion. In a retrospective 6-year follow-up study of 28 sJIA-patients, Tsai et al. [14] reported a modified CR in 28% for more than 2 months. In this study, 46% of the patients had a remitting disease course. One quarter of patients (25%) had a drug-dependent disease course, defined as no clinical remission after onset or achievement of only one episode of remission off medication for less than 2 months’ duration. This is in contrast to the findings of the Nordic Study Group [21]. In their prospective, population-based cohort (sJIA n = 18), 83% achieved CR 8 years after disease onset. None of the remaining sJIA patients were in CRM at the last follow-up in this study. Consistent with the findings of the Nordic Study Group [21], Dewoolkar et al. [33] described remission at 5.5 years follow-up in a prospective cohort of 53 sJIA patients. Overall, 55% of patients achieved clinical remission, 21% on medication and 34% off medication. Patients with a monocyclic course had the highest rate of remission [33]. From the Canadian JIA inception cohort (n = 71), Guzman et al. [36••] reported a probability of 47% for attaining remission within 5 years. Selvaag et al. documented a remission rate off medication in 83% of sJIA patients (n = 12) at 30 years after diagnosis [32•].
Oligoarticular JIA
The disease course of oligoarticular JIA has been divided into two subcategories: persistent oligoarticular JIA including patients with no more than four joints involved during their entire disease course and extended oligoarticular JIA patients with a cumulative number of more than four active joints during the disease course extending 6 months [1].
In the 2-year follow-up study of 117 JIA patients, Huang et al. [11] found that 56% of the patients with an oligoarticular subtype (n = 18) achieved complete remission (EULAR activity criteria). In contrast, in a 3-year prospective follow-up study of 197 patients, Selvaag et al. [9] found a remission rate of 39% in persistent oligoarticular JIA (n = 95) and 6% in extended oligoarticular JIA (n = 16). Sato et al. [18] reported a probability of remission on and off medication of 78% in the group with persistent oligoarticular JIA (n = 44) and 9% in the extended oligoarticular JIA group (n = 20), 4 years after treatment with intra-articular joint injection. In a recent Canadian multicenter inception cohort (ReACCH-OUT) study of 1104 JIA patients, a 57% probability of achieving remission within 5 years of diagnosis was reported for the oligoarticular subtypes (n = 387) [36••]. A multicenter, retrospective study of 311 JIA patients followed for 4.8 years by Albers et al. [15] described a better outcome in persistent oligoarticular JIA compared to the other subgroups. However, Albers et al. used a different definition of disease course than most other studies mentioned here. They found a remitting course (0–35% time with active disease) in 58.1% of the persistent oligoarticular patients (n = 43) and in 34.1% of the patients with the extended subtype (n = 44).
Another retrospective, larger cohort study of 761 JIA patients by Lurati et al. [24] described the remission status 7.3 years after disease onset; 43% of the oligoarticular-persistent patients were in CR (n = 357); in contrast, the number was only 13% in the extended oligoarticular subtype (n = 111).
In a large Nordic multicenter, prospective, population-based 8-year follow-up study of 440 patients by Nordal et al. [21], one out of three oligoarticular-persistent patients progressed to an extended subtype during the disease course. Clinical remission off medication was achieved in 66% of the persistent oligoarticular JIA patients (n = 126), and in 21% of the extended oligoarticular patients (n = 75).
In a prospective 17-year follow-up study, Bertilsson et al. [12] described CR in the first 5 years of disease, for a duration of at least 2 years, in 67% of monoarthritis patients (n = 6) compared to 49% in patients with two to four joints involved, based on the EULAR criteria for JIA (n = 37). After 17 years of follow-up, the proportion of patients in remission remained high: 60% (n = 5) and 40% (n = 30), respectively. Selvaag et al. [32•] reported an improved outcome in a prospective study of 176 patients 30 years after diagnosis: CR in 80% of persistent oligoarticular JIA (n = 50) and 50% in extended oligoarticular JIA (n = 24).
Polyarticular JIA
The diagnosis of polyarticular JIA is based on arthritis affecting five or more joints within the first 6 months of disease and is divided into two subtypes based on the absence or presence of rheumatoid factor (RF) [1]. Historically, the polyarticular subtypes were thought to be associated with a worse outcome than the remaining subtypes.
Wallace et al. [31] performed a 2.3-year follow-up study of the TREAT trial (Trial of Early Aggressive Therapy) of 33 RF− and 15 RF+ polyarticular JIA patients. Fifty-four percent achieved CRM while only 4% were in CR, without any specific information on the presence or absence of RF+ or RF− among the patients in remission.
Ringold et al. [23] described a retrospective cohort study of 104 polyarticular JIA patients with a follow-up of 2.3 years after they were diagnosed. None of the patients achieved CR (modified Wallace criteria); nevertheless, 50% of RF− and 42% of RF+ patients achieved remission on medication. In another 2-year follow-up study, Huang et al. [11] found that 14% of the RF− polyarticular JIA patients were in complete remission off medication (EULAR activity criteria), compared to 33% of the RF+ patients (1 in 3 patients). In contrast, Selvaag et al. [9] reported a remission rate off medication of 16% in RF− polyarticular JIA and 0% in RF+ patients 3 years after diagnosis.
Another seven studies were identified with a disease follow-up from 4.8 to 30 years [12, 15, 21, 24, 26, 32•, 36••]. In a multinational cohort study with a follow-up of 4.8 years, Albers et al. [15] reported a remission rate of 39% among RF− polyarthritis patients and none in the RF+ group, but the latter group was very small (n = 4). Magnani et al. [26] performed a 5-year cohort study with retrospectively collected data of 123 methotrexate-treated polyarticular-course JIA patients (oligoarticular-extended, polyarticular, and systemic JIA). Of the polyarticular-onset JIA patients (RF− and RF+), only 19% achieved inactive disease during the 5-year follow-up. In another 5-year follow-up study by Guzman et al. [36••], the probability of achieving remission was 14% in the RF− group and 0% in the RF+ group. Similarly, in the study of Lurati et al. [24], with a follow-up of 7.6 years, none of the RF+ polyarticular patients achieved remission (n = 26), whereas 22% of the RF− polyarticular patients met the definitions of being in CR.
Remission off medication was achieved in 28% of the polyarticular RF− patients and in 33% (1 out of 3 patients) of the RF+ in the 8-year follow-up study by Nordal et al. [21]. In a prospective, population-based study, Bertilsson et al. [12] described follow-up data of polyarticular- and oligoarticular-extended patients. A remission frequency of 24% was described for the polyarticular-course JIA patients after 5 years increasing to 39% at the 17-year follow-up. The longest follow-up study of 30 years was performed by Selvaag et al. [32•], with a reported remission rate of 52% in the RF− polyarticular patients, which is in contrast to 17% among RF+ patients.
Juvenile Psoriatic Arthritis
JPsA represents less than 10% of all JIA cases with a substantial clinical heterogeneity. Subsequently, no large-scale long-term follow-up study focusing exclusively on JPsA has been performed.
In a 3-year prospective follow-up study including JPsA patients, Selvaag et al. [9] observed 20% in CR (JPsA, n = 12). In a larger retrospective cohort study of 119 JPsA patients 5 years after disease onset, Butbyl et al. [16] reported inactive disease at the last follow-up, defined as the absence of clinically evident synovitis and enthesitis for a minimum of 3 months, in 58% of JPsA patients, and 30% had achieved inactive disease off medication.
In the Canadian ReACCh-Out study [36••], 47% of JPsA patients attained remission within 5 years (n = 56). An 8-year follow-up study performed by Nordal et al. [21], including JPsA patients (n = 14), reported CR as defined by the Wallace criteria in 23% of JPsA patients, and CRM in 23%. In contrast, 54% of the JPsA patients did not achieve remission. In a 15-year prospective follow-up study of 31 JPsA patients, Flatø et al. [25] found that 55% were in CR. Later, at 30-year follow-up, Selvaag et al. [32•] described a decreasing CR rate to 48% for the same prospective cohort (JPsA n = 21).
In a 17-year follow-up study by Bertilsson et al. [12], at the last follow-up, 25% of JPsA patients were in remission (defined as no evidence of active synovitis and/or active extra-articular features and without drugs for ≥ 2 years) and 25% achieved an inactive disease status, defined as no evidence of active synovitis and/or active extra-articular features and without drugs for < 2 years. However, the cohort only consisted of eight JPsA patients and the EULAR criteria for diagnosis were used.
Enthesitis-Related Arthritis
The long-term outcome of ERA, just as JPsA, has only been studied to a limited extent due to the lower prevalence. In the past decade, there has been a growing interest in the field of enthesopathies, and recently, larger-scaled studies have been performed.
In a retrospective study on 27 ERA patients, Huang et al. [11] found 66% in complete remission off medication using the EULAR criteria for remission [46] 2 years after disease onset. In another follow-up study, Guzman et al. [36••] reported only 1.9% of ERA patients (N = 144) to be in remission 2 years after diagnosis; however, the number increased to 47% at 5 years after diagnosis. In the 4-year follow-up study by Sato et al. [18], none were in remission on or off medication (n = 6). Bertilsson et al. [12] found similar remission rates in their ERA population (n = 6) of 0 and 20% in remission, at respectively 5 and 17 years follow-up. However, it should be noted that the EULAR diagnostic criteria for JCA were used. In a 7-year follow-up study of 59 ERA patients, Pagnini et al. [22] reported 20% in CR, 66% in CRM remission on medication, and 13% still had active disease on medication. In a population-based cohort study, Nordal et al. [21] described 49 ERA patients with a follow-up of 8 years. They found CR in 30% and CRM in 8% of the patients. Flatø et al. [27] and Selvaag et al. [32•] reported a prospective cohort study of 55 ERA patients, with a 44% rate of CR after 15 years (n = 55) decreasing to 37% at 30 years of follow-up (n = 27).
Predictors of Remission or Poor Outcome
For the physician, the patients, and their families, it is crucial to know the individual prognosis of the disease, preferably as early as possible to institute timely and tailored treatment in order to prevent a poor outcome. To predict this individually, it is essential to know the predictors of poor outcome or remission, which has been summarized in Table 6.
Albers et al. [15] studied a cohort with oligoarticular, polyarticular, and systemic JIA patients and found that the amount of time with ongoing active disease during the first 2 years of disease was the most significant factor for predicting active disease in the following years. Using a modified remission definition, they found a positive predictive value of 90.9% of having a remitting course, defined by 0–35% of time with active disease during the first 2 years of disease, for the proceeding 3 years. This is consistent with the previous CARRA studies [31, 47] on early aggressive therapy. Glerup et al. studied the role of ANA on remission and found no significant difference in remission rate between ANA-negative and ANA-positive patients [34].
In a prospective study on 197 JRA patients (EULAR classification), Selvaag et al. [10] studied predictors for the CHAQ disability index at 3 years of follow-up. The CHAQ score and the patient’s global assessment during the first 6 months of the disease course were predictors for the 3-year CHAQ disability index (standardized beta = 0.324 and 0.231, p < 0.001 and p = 0.006, respectively). Further, Selvaag et al. [9] identified numbers of active joints and negative ANA at baseline as predictors of persistent disease 3 years after onset.
Romano et al. [28] performed a 5-year follow-up study on 301 JIA patients on biological treatment. Male gender and patients with shorter disease duration before starting biological treatment showed higher remission rates (HR 1.54, p = 0.05).
The Nordic Study Group [19] documented the odds of not being in remission after 8 years of disease to be twice as high for HLA-B27-positive patients compared to HLA-B27-negative patients (N = 399). The group also [38] reported that HLA-B27 positivity in boys with older age predicts more active joints within the first 3 years of the disease course. However, this finding was not persistent since at the 8-year follow-up, they found no difference in the cumulative active joints between HLA-B27-positive and HLA-B27-negative patients, even when stratified by gender [19].
Other predictors of not being in remission in this study were the ERA subtype, hip arthritis, and clinical signs of sacroiliitis [20]. In another study of the same cohort, the group found that patients with ankle arthritis within the first year of disease were twice as likely not to achieve remission at 8 years of follow-up (OR 2; CI 1.3–3.0) [40] as in patients without ankle involvement.
In ERA patients, Flatø et al. [27] reported that predictors of failure to achieve remission, 15.3 years after onset, were HLA-DPB1*08, a family history of ankylosing spondylitis in a first-degree relative, and arthritis in ankle joints within the first 6 months of disease.
Bertilsson et al. [13] described that active disease at 5-year follow-up was positively associated with accumulated active joint count during the first year of disease course. At the 17-year follow-up, Bertilsson et al. [12] found that remission was best predicted by characteristics at 5-year follow-up rather than by variables at onset. Probability of achieving remission was inversely associated with CHAQ > 0, number of involved joints, and activity status at the 5-year follow-up.
Selvaag et al. identified predictors of active disease, at 30 years of follow-up, to be HLA-DRB1*01 positivity, a short total time in remission during the disease course, and physician’s global assessment of disease activity (no value given) at 15-year follow-up [32•].
Predictors of Risk of Flare
Nusman et al. [45] studied the risk of flare in 32 patients, who were clinically described with an inactive disease in a study using contrast-enhanced MRI. During a 2-year follow-up period, they reported flares detected by MRI in 12 patients (38%). They were unable to identify any risk factors for developing a flare, which might be due to their relative small study population with all subtypes represented [45].
The ReACCh-Out investigators [37] described features associated with risk of flare after attaining inactive disease and found RF positivity to have the highest probability of flare HR = 1.53 (CI 0.99–2.39) while sJIA had the lowest probability (HR = 0.60). Furthermore, they also reported a severe disease course (an active joint count > 4, the use of biologics and patient global assessment > 30 mm) and ANA positivity before attaining inactive disease to be associated with a higher risk of flare.
Papadopoulou et al. analyzed the risk of flare after ≥ 3 simultaneously injected joints with a follow-up time of 3 years [41]. Predictors of flare were a positive CRP, negative ANA, polyarticular course, and lack of response to methotrexate treatment. The flare rate was the highest within the first year after intra-articular injection and decreased thereafter.
Predictors of Damage
In a study of polyarticular JIA, Ringold et al. [23] found that patients older than 13.5 years at first visit were twofold more likely to have radiological evidence of damage during the first 6 months of disease (RR = 2.67, CI 1.38–5.16).
In a Nordic study, Berntson et al. [39] analyzed antibodies against type II collagen, anti-CCP, IgM-RF, and IgA-RF detected within the first months of disease. At 8 years of disease duration, they found a significant association of these antibodies with joint damage assessed by JADI-A. However, they found no association between these antibodies and the cumulative number of affected joints, remission, or ongoing disease activity.
Elhai et al. [42] reported the use of increased disease activity and use of multiple biologics or DMARD as predictors for the presence of more radiologically confirmed cervical lesions in a cohort of 57 polyarticular JIA patients at 12.8 years after disease onset. However, to note, this cohort was relatively small and the prevalence of cervical lesions may be overestimated due to selection bias towards more severe cases.
In sJIA, Batthish et al. [43] found that a polyarticular course within the first 6 months of disease was the strongest predictor for radiographic hip damage.
Predictors of Sacroiliitis
Three papers have looked at predictors of sacroiliitis. In patients with early ERA, Pagnini et al. reported that the numbers of active joints or entheses at onset were predictors of sacroiliitis at 3.2 years after disease onset [22]. Flatø et al. [27] reported that persistently elevated erythrocyte sedimentation rate (ESR) for more than 6 months and hip arthritis within the first 6 months of disease were risk factors for developing sacroiliitis in patients with ERA. In contrast, they reported the presence of HLA-DPB1*02 to be protective against developing sacroiliitis.
In a 3.5-year follow-up study of a SpA cohort (according to the Amor criteria), Stoll et al. [44] also showed hip arthritis to be the major risk factor for sacroiliitis and, surprisingly, the presence of dactylitis protected against developing sacroiliitis.
Discussion
In the pre-DMARD and biologic era, many patients still experienced considerable morbidity related to their JIA diagnosis. In recent years, less functional disabilities, growth retardation, and delayed puberty are noted in the newly diagnosed and treated patients. However, although many studies and reviews mentioned the increased remission rate in JIA after introduction of biologics, many patients still do not fulfill the remission criteria after 2-, 5-, 8-, 15-, and even 30-year follow-up [11, 12, 15, 21, 32•, 36••]. It remains difficult to compare remission rates presumably due to the diversity of study design and study populations. Only a few studies have dealt with population-based cohorts, the majority being derived from cohorts with either a clear selection bias, or biologic registries which do not include the less severe subtypes. Although this could potentially lead to a less positive view on remission in JIA, it could be argued to be an overestimation. The patients in these cohorts are usually treated with the newest medication, while the “less severe” unincluded patients are either untreated or treated with non-biological DMARDs, which might decrease the probability to achieve remission over time.
Systemic JIA portrays a wide range of disease trajectories, spanning from a monocyclic course to a more severe and chronic course with continuous systemic symptoms, with high inflammatory markers and risk for associated macrophage activation syndrome (MAS), and a destructive polyarticular course [48]. Consequently, the long-term outcome is dependent on the clinical course. The majority of the recent studies are on sJIA populations treated with anti-IL1 therapy leaving out the mild courses, which could also lead to an overestimation of worse outcome.
Several studies showed quite favorable rates of inactive disease for certain subtypes of patients. Surprisingly, in some studies, oligoarticular-persistent patients, known to have the mildest arthritis and disease course, long-term remission was not achieved in about 50% at the 5-year follow-up and still in 20% even after 30 years of disease duration [32]. This might be due to different treatment strategies in patients with persistent oligoarticular disease compared to patients with a polyarticular course.
The oligoarticular-extended subtype has lower remission rates than the oligoarticular-persistent subtype; however, they receive more DMARDS and biologics than the oligoarticular-persistent patients. Remarkably, remission rates in patients with polyarticular RF− JIA is more favorable than the oligoarticular-extended ones even though they have comparable clinical characteristics and trajectories. However, the pooled data of numerous papers also showed a less favorable clinical outcome in polyarticular RF+ patients. ERA patients also tend to remain active and flare during the disease course [9, 11, 12, 15, 21, 23, 24, 26•, 31, 32•, 36••, 44].
Predictors for poor outcome are possibly associated with a high number of active joints at diagnosis or cumulative active joints during the first year of disease, but the study results are not uniform and more studies are necessary to verify these results and to look for other possible predictors for poor outcome [9, 38].
Recently, studies have looked into predictors for damage, and antibody positivity (ANA, anti-CCP, RF) seems to be related to an increased risk of joint damage [39]. A recent paper by Glerup et al. [34] did not show a difference in remission rates in 625 JIA patients based on ANA positivity. This is in contrast to that of Ravelli et al. [49], who showed an increased arthritis activity in patients with a negative ANA and a more favorable oligoarthritis-persistent subtype in the ANA-positive patients. Results will have to be reproduced in other studies to evaluate which antibodies are real predictors of flare and not confounders of other risk factors.
Overall, compared to data from the last century, the disease outcome has not improved significantly [50, 51]. Unfortunately, a significant number of patients still require medical treatment and are not fulfilling criteria of remission while treated in the biologic era. Despite hereof, there seems no doubt that the severity of the functional impact has decreased significantly, and patients have less severe disabilities than in the pre-biologic era.
More population-based studies, such as the Nordic study and the ReaCCH-Out cohort are necessary to really study the remission rate in JIA in the biologic era. This review emphasizes the need for better predictive models to enhance precise, personalized treatment strategies for children with juvenile idiopathic arthritis.
References
Recently published papers of particular interest have been highlighted as: • Of importance •• Of major importance
Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–2.
Hill RHHA, Walters K. Juvenile rheumatoid arthritis: follow-up into adulthood—medical, sexual and social status. Can Med Assoc J. 1976;114(9):790–6.
Viola S, Felici E, Magni-Manzoni S, Pistorio A, Buoncompagni A, Ruperto N, et al. Development and validation of a clinical index for assessment of long-term damage in juvenile idiopathic arthritis. Arthritis Rheum. 2005;52(7):2092–102.
Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum. 1997;40(7):1202–9.
Wallace CA, Ruperto N, Giannini E. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol. 2004;31(11):2290–4.
Rheumatism ELA. EULAR Bulletin No. 4: nomenclature and classification of arthritis in children. Basel: National Zeitung AG; 1977. p. 3.
Brewer EJ Jr, Bass J, Baum J, Cassidy JT, Fink C, Jacobs J, et al. Current proposed revision of JRA Criteria. JRA Criteria Subcommittee of the Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Section of The Arthritis Foundation. Arthritis Rheum. 1977;20(2 Suppl):195–9.
Anink J, Dolman KM, van den Merlijn Berg J, van Veenendaal M, Kuijpers TW, van Rossum MA. Two-year outcome of juvenile idiopathic arthritis in current daily practice: what can we tell our patients? Clin Exp Rheumatol. 2012;30(6):972–8.
Selvaag AM, Flato B, Dale K, Lien G, Vinje O, Smerdel-Ramoya A, et al. Radiographic and clinical outcome in early juvenile rheumatoid arthritis and juvenile spondyloarthropathy: a 3-year prospective study. J Rheumatol. 2006;33(7):1382–91.
Selvaag AM, Lien G, Sorskaar D, Vinje O, Forre O, Flato B. Early disease course and predictors of disability in juvenile rheumatoid arthritis and juvenile spondyloarthropathy: a 3 year prospective study. J Rheumatol. 2005;32(6):1122–30.
Huang HQX, Yu H, Li J, Zhang Y. Clinical analysis in 202 children with juvenile idiopathic arthritis. Clin Rheumatol. 2013;32(7):1021–7.
Bertilsson L, Andersson-Gare B, Fasth A, Petersson IF, Forsblad-D’elia H. Disease course, outcome, and predictors of outcome in a population-based juvenile chronic arthritis cohort followed for 17 years. J Rheumatol. 2013;40(5):715–24.
Bertilsson L, Andersson-Gare B, Fasth A, Forsblad-d'Elia H. A 5-year prospective population-based study of juvenile chronic arthritis: onset, disease process, and outcome. Scand J Rheumatol. 2012;41(5):379–82.
Tsai HY, Lee JH, Yu HH, Wang LC, Yang YH, Chiang BL. Initial manifestations and clinical course of systemic onset juvenile idiopathic arthritis: a ten-year retrospective study. J Formos Med Assoc. 2012;111(10):542–9.
Albers HM, Brinkman DM, Kamphuis SS, van Suijlekom-Smit LW, van Rossum MA, Hoppenreijs EP, et al. Clinical course and prognostic value of disease activity in the first two years in different subtypes of juvenile idiopathic arthritis. Arthritis Care Res. 2010;62(2):204–12.
Butbul Aviel Y, Tyrrell P, Schneider R, Dhillon S, Feldman BM, Laxer R, et al. Juvenile psoriatic arthritis (JPsA): juvenile arthritis with psoriasis? Pediatr Rheumatol Online. 2013;11(1):11.
Alberdi-Saugstrup M, Enevold C, Zak M, Nielsen S, Nordal E, Berntson L, et al. Nordic Study Group of Pediatric Rheumatology (NoSPeR). Scand J Rheumatol 2017;46(5):1–8.
Sato JFT, Nascimento C, Corrente J, Saad-Magalhaes C. Probability of remission of juvenile idiopathic arthritis following treatment with steroid injection. Clin Exp Rheumatol. 2014;32(2):291–6.
Berntson L, Nordal E, Aalto K, Peltoniemi S, Herlin T, Zak M, et al. HLA-B27 predicts a more chronic disease course in an 8-year followup cohort of patients with juvenile idiopathic arthritis. J Rheumatol. 2013;40(5):725–31.
Russo RA, Katsicas MM. Patients with very early-onset systemic juvenile idiopathic arthritis exhibit more inflammatory features and a worse outcome. J Rheumatol. 2013;40(3):329–34.
Nordal E, Zak M, Aalto K, Berntson L, Fasth A, Herlin T, et al. Ongoing disease activity and changing categories in a long-term nordic cohort study of juvenile idiopathic arthritis. Arthritis Rheum. 2011;63(9):2809–18.
Pagnini I, Savelli S, Matucci-Cerinic M, Fonda C, Cimaz R, Simonini G. Early predictors of juvenile sacroiliitis in enthesitis-related arthritis. J Rheumatol. 2010;37(11):2395–401.
Ringold S, Seidel KD, Koepsell TD, Wallace CA. Inactive disease in polyarticular juvenile idiopathic arthritis: current patterns and associations. Rheumatology (Oxford). 2009;48(8):972–7.
Lurati A, Salmaso A, Gerloni V, Gattinara M, Fantini F. Accuracy of Wallace criteria for clinical remission in juvenile idiopathic arthritis: a cohort study of 761 consecutive cases. J Rheumatol. 2009;36(7):1532–5.
Flato B, Lien G, Smerdel-Ramoya A, Vinje O. Juvenile psoriatic arthritis: longterm outcome and differentiation from other subtypes of juvenile idiopathic arthritis. J Rheumatol. 2009;36(3):642–50.
Magnani A, Pistorio A, Magni-Manzoni S, Falcone A, Lombardini G, Bandeira M, et al. Achievement of a state of inactive disease at least once in the first 5 years predicts better outcome of patients with polyarticular juvenile idiopathic arthritis. J Rheumatol. 2009;36(3):628–34.
Flato B, Hoffmann-Vold AM, Reiff A, Forre O, Lien G, Vinje O. Long-term outcome and prognostic factors in enthesitis-related arthritis: a case-control study. Arthritis Rheum. 2006;54(11):3573–82.
Romano M, Pontikaki I, Gattinara M, Ardoino I, Donati C, Boracchi P, et al. Drug survival and reasons for discontinuation of the first course of biological therapy in 301 juvenile idiopathic arthritis patients. Reumatismo. 2013;65(6):278–85.
• Vastert SJ, de Jager W, Noordman BJ, Holzinger D, Kuis W, Prakken BJ, et al. Effectiveness of first-line treatment with recombinant interleukin-1 receptor antagonist in steroid-naive patients with new-onset systemic juvenile idiopathic arthritis: results of a prospective cohort study. Arthritis Rheumatol. 2014;66(4):1034–43. First study to show the efficacy of a short course of Il-1 blocker on the disease course of systemic JIA
Baszis K, Garbutt J, Toib D, Mao J, King A, White A, et al. Clinical outcomes after withdrawal of anti-tumor necrosis factor alpha therapy in patients with juvenile idiopathic arthritis: a twelve-year experience. Arthritis Rheum. 2011;63(10):3163–8.
Wallace CA, Ringold S, Bohnsack J, Spalding SJ, Brunner HI, Milojevic D, et al. Extension study of participants from the trial of early aggressive therapy in juvenile idiopathic arthritis. J Rheumatol. 2014;41(12):2459–65.
• Selvaag AM, Aulie HA, Lilleby V, Flato B. Disease progression into adulthood and predictors of long-term active disease in juvenile idiopathic arthritis. Ann Rheum Dis. 2016;75(1):190–5. This study is the longest prospective follow-up study on remission in JIA. They use standardized outcome such as the Wallace remission criteria
Dewoolkar M, Cimaz R, Chickermane PR, Khubchandani RP. Course, outcome and complications in children with systemic onset juvenile idiopathic arthritis. Indian J Pediatr. 2017;84(4):294–8.
Glerup MHT, Twilt M. Remission rate is not dependent on the presence of antinuclear antibodies in juvenile idiopathic arthritis. Clin Rheumatol. 2017;36(3):671–6.
Ekelund M, Aalto K, Fasth A, Herlin T, Nielsen S, Nordal E, et al. Psoriasis and associated variables in classification and outcome of juvenile idiopathic arthritis—an eight-year follow-up study. Pediatr Rheumatol Online. 2017;15(1):13.
•• Guzman J, Oen K, Tucker LB, Huber AM, Shiff N, Boire G, et al. The outcomes of juvenile idiopathic arthritis in children managed with contemporary treatments: results from the ReACCh-Out cohort. Ann Rheum Dis. 2015;74(10):1854–60. This is the largest, prospective inception cohort published. They provide information on the probability of achieving remission 5 years after diagnosis
Guzman J, Oen K, Huber AM, Watanabe Duffy K, Boire G, Shiff N, et al. The risk and nature of flares in juvenile idiopathic arthritis: results from the ReACCh-Out cohort. Ann Rheum Dis. 2015;75(6):1092–8.
Berntson L, Damgard M, Andersson-Gare B, Herlin T, Nielsen S, Nordal E, et al. HLA-B27 predicts a more extended disease with increasing age at onset in boys with juvenile idiopathic arthritis. J Rheumatol. 2008;35(10):2055–61.
Berntson L, Nordal E, Fasth A, Aalto K, Herlin T, Nielsen S, et al. Anti-type II collagen antibodies, anti-CCP, IgA RF and IgM RF are associated with joint damage, assessed eight years after onset of juvenile idiopathic arthritis (JIA). Pediatr Rheumatol Online. 2014;12:22.
Esbjornsson AC, Aalto K, Brostrom EW, Fasth A, Herlin T, Nielsen S, et al. Ankle arthritis predicts polyarticular disease course and unfavourable outcome in children with juvenile idiopathic arthritis. Clin Exp Rheumatol. 2015;33(5):751–7.
Papadopoulou C, Kostik M, Gonzalez-Fernandez MI, Bohm M, Nieto-Gonzalez JC, Pistorio A, et al. Delineating the role of multiple intraarticular corticosteroid injections in the management of juvenile idiopathic arthritis in the biologic era. Arthritis Care Res. 2013;65(7):1112–20.
Elhai M, Wipff J, Bazeli R, Freire V, Feydy A, Drape JL, et al. Radiological cervical spine involvement in young adults with polyarticular juvenile idiopathic arthritis. Rheumatology (Oxford). 2013;52(2):267–75.
Batthish M, Feldman BM, Babyn PS, Tyrrell PN, Schneider R. Predictors of hip disease in the systemic arthritis subtype of juvenile idiopathic arthritis. J Rheumatol. 2011;38(5):954–8.
Stoll ML, Bhore R, Dempsey-Robertson M, Punaro M. Spondyloarthritis in a pediatric population: risk factors for sacroiliitis. J Rheumatol. 2010;37(11):2402–8.
Nusman CM, Hemke R, Lavini C, Schonenberg-Meinema D, van Rossum MA, Dolman KM, et al. Dynamic contrast-enhanced magnetic resonance imaging can play a role in predicting flare in juvenile idiopathic arthritis. Eur J Radiol. 2017;88:77–81.
Smolen JS, Landewe R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73(3):492–509.
Wallace CA, Giannini EH, Spalding SJ, Hashkes PJ, O’Neil KM, Zeft AS, et al. Clinically inactive disease in a cohort of children with new-onset polyarticular juvenile idiopathic arthritis treated with early aggressive therapy: time to achievement, total duration, and predictors. J Rheumatol. 2014;41(6):1163–70.
Cimaz R. Systemic-onset juvenile idiopathic arthritis. Autoimm Rev. 2016;15(9):931–4.
Ravelli A, Felici E, Magni-Manzoni S, Pistorio A, Novarini C, Bozzola E, et al. Patients with antinuclear antibody-positive juvenile idiopathic arthritis constitute a homogeneous subgroup irrespective of the course of joint disease. Arthritis Rheum. 2005;52(3):826–32.
Oen K, Malleson PN, Cabral DA, Rosenberg AM, Petty RE, Cheang M. Disease course and outcome of juvenile rheumatoid arthritis in a multicenter cohort. J Rheumatol. 2002;29(9):1989–99.
Fantini F, Gerloni V, Gattinara M, Cimaz R, Arnoldi C, Lupi E. Remission in juvenile chronic arthritis: a cohort study of 683 consecutive cases with a mean 10 year followup. J Rheumatol. 2003;30(3):579–84.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pediatric Rheumatology
Rights and permissions
About this article
Cite this article
Glerup, M., Herlin, T. & Twilt, M. Clinical Outcome and Long-term Remission in JIA. Curr Rheumatol Rep 19, 75 (2017). https://doi.org/10.1007/s11926-017-0702-4
Published:
DOI: https://doi.org/10.1007/s11926-017-0702-4