Abstract
We investigated serum prolidase activity and oxidative/antioxidative status in patients with knee osteoarthritis (OA) and evaluated its relationships with radiographic severity and clinical parameters. The study population consisted of 137 patients with knee OA and 134 healthy volunteers. The severity of knee OA was classified according to the Kellgren–Lawrence criteria. Each patient was also evaluated clinically according to the Western Ontario and McMaster University Osteoarthritis Index (WOMAC). Serum prolidase activity was measured spectrophotometrically. Oxidative status was assessed by measuring serum lipid hydroperoxide (LOOH) and total oxidative status (TOS). Antioxidative status was assessed by measuring serum-free sulfhydryl groups (–SH = total thiol) and total antioxidant capacity (TAC). Oxidative stress index (OSI) was calculated. Serum prolidase activity was significantly lower in the knee OA group than in the control group (p < 0.001). The serum prolidase activities decreased with the severity of knee OA. Furthermore, serum LOOH, TOS, and OSI levels of the knee OA group were significantly higher than those of the controls (p < 0.001 for all), whereas TAC and –SH levels did not differ between the two groups (p > 0.05). In a multiple regression analysis, WOMAC score was independently associated with serum prolidase activity (β = −0.340, p < 0.001). Decreased serum prolidase activity and elevated LOOH, TOS, and OSI levels may be associated with knee OA, and serum prolidase activity may be a useful adjunctive indicator of the progression of knee OA in follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is a common degenerative joint disease, and knee is the most frequently involved joint. Radiographic appearance and clinical features are still often used for diagnosis of the disease. However, the etiology of OA is not fully understood, although mechanical, biochemical, and genetic factors are accepted to play roles [1, 2]. Intraarticular and periarticular structures are affected in knee OA, and histopathological changes occur in the joint cartilage, subchondral bone, ligaments, periarticular muscle, nerve, and synovial membrane [3]. The general consensus is that these changes help to explain the persistence of knee OA and progression of radiographic severity and clinical parameters. Understanding the histopathological changes that characterize OA and investigating adjunctive tools for evaluation of radiographic severity and clinical parameters of the disease are the most important areas of current research. The major component of the structures involved in knee OA is collagen. Serum prolidase enzyme activity, which is required for collagen biosynthesis, plays a key role in the breakdown of collagen, and prolidase enzyme activity has been shown to be related to the collagen turnover rate [4, 5]. Prolidase enzyme activity and its pathophysiological role have been investigated in the context of different musculoskeletal system diseases [6, 7]. In a recent study, serum prolidase activity was evaluated in patients with knee OA and found to be significantly reduced compared to a control group [8]. These studies showed that serum prolidase enzyme activity reflects the abnormal collagen turnover in affected joints. Nothing on the correlation between serum prolidase enzyme activity and radiographic severity of knee OA and clinical parameters has yet appeared in the literature.
One possible cause of OA is oxidative stress. The levels of pro-inflammatory mediators, such as reactive oxygen species (ROS), are elevated in OA [9, 10]. Thus, the increased levels of these reactive species with oxidative activity mediate the effects of many pro-inflammatory cytokines, such as interleukin (IL)-1 and tumor necrosis factor (TNF)-α [9–11]. IL-1 and TNF-α may play a crucial role in cartilage matrix degradation by stimulating matrix metalloproteinase (MMP) expression in patients with OA [10, 11]. Additionally, recent studies have demonstrated that mitochondrial DNA damage can be induced by these inflammatory cytokines or ROS, and this damage may promote chondrocyte death [12–14]. Thus, ROS contributes to cartilage degradation by reducing cartilage repair capacity and cell death in extracellular cartilage matrix.
Thus, we investigated serum prolidase enzyme activity, oxidative status [lipid hydroperoxide (LOOH), total oxidative status (TOS), and the oxidative stress index (OSI)], antioxidative status (free sulfhydryl groups, –SH = total thiol), and total antioxidative capacity (TAC) in knee OA and evaluated their relationship with radiographic severity of knee OA and clinical parameters.
Materials and methods
Study population
This study included 137 patients with knee OA (90 females, 47 males; mean age 58.5 ± 9.8 years, range 40–79) diagnosed according to the American College of Rheumatology criteria [15] and 134 healthy controls (85 females, 49 males; mean age 59.3 ± 8.7 years, range 43–80) who were free of symptoms. Exclusion criteria included the use of supplemental vitamins, secondary posttraumatic OA, previous knee injury, inflammatory rheumatic disease, infectious- or endocrine-related arthropathy, clinically unstable medical illness, or the use of any medication within 4 weeks prior to initiation of the study. The controls were matched in terms of age, body mass index (BMI), and gender with the patients.
Written informed consent was obtained from all subjects. The use of their medical records was approved by the local research ethics committee.
Radiological assessment
All radiographs of the patients (weight-bearing anteroposterior and weight-bearing lateral and Merchant’s X-rays of both knees) were evaluated by a single observer (CE) who was not involved in the clinical care of the patients. The severity of OA was assessed and classified according to the Kellgren–Lawrence (KL) grading scale [16]. Grade 0 was accepted as normal, grade 1 as possible osteophytes only, grade 2 as absolute osteophytes and possible joint space narrowing, grade 3 as moderate osteophytes and/or absolute joint space narrowing, and grade 4 as large osteophytes, severe joint space narrowing, and/or bony sclerosis. The grade used was the highest of the right or left knee. Patients with knee OA were divided into four subgroups according to the KL grading scale.
Clinical assessment
Clinical assessments included the Western Ontario and McMaster University Osteoarthritis Index (WOMAC). The WOMAC questionnaire consists of three items: pain, stiffness, and physical function. The WOMAC scores were recorded on a Likert scale of 0–4 (0 = no pain/limitation, 1 = mild pain/limitation, 2 = moderate pain/limitation, 3 = severe pain/limitation, and 4 = very severe pain/limitation). Maximum scores for pain, stiffness, and physical function were 20, 8, and 68, respectively, for a total score of 96 (0–96 scale with 0 as the best and 96 as the worst) [17]. Additionally, clinical baseline parameters such as weight, height, BMI, age, gender, side of involvement (left or right or both knees) were recorded.
Blood sample collection
Blood samples were collected into Vacutainer serum clot activator tubes (BD®, USA) and immediately stored at 4 °C. Serum samples were then separated from cells by centrifugation (3000 rpm, 10 min). The remaining serum portions were stored at −80 °C until analysis.
Measurement of serum prolidase activity
Prolidase activity was measured by a photometric method, based on the measurement of proline levels produced by prolidase [18]. The samples (100 μL) were mixed with 100 μL of physiological saline (NaCl, 0.9 %). A volume of 25 μL of the mixture was preincubated with 75 μL of the preincubation solution (50 mmol/L Tris–HCl buffer pH 7.0 containing 1 mmol/L GSH, 50 mmol/L MnCl2) at 37 °C for 30 min. The reaction mixture, which contained 144 mmol/L gly-pro, pH 7.8 (100 μL), was incubated with 100 μL of preincubated sample at 37 °C for 5 min. To stop the reaction, 1 mL glacial acetic acid was added. After adding 300 μL Tris–HCl buffer (pH 7.8) and 1 mL ninhydrin solution (3 g/dL ninhydrin was dissolved in 0.5 mol/L orthophosphoric acid), the mixture was incubated at 90 °C for 20 min and then cooled on ice. Absorbance was then measured at 515 nm to determine proline levels, according to the method of Myara et al. [19], as optimized by Ozcan et al. [18]. Intra- and interassay coefficients of variation (CV) were lower than 10 % for the assay.
Measurement of lipid hydroperoxide levels
Serum LOOH levels were measured by a ferrous ion oxidation-xylenol orange (‘FOX-2’) assay, which involves the oxidation of ferrous ions to ferric ions via the effects of various oxidants. The ferric ion level is then measured using xylenol orange. LOOH levels are reduced by the application of triphenyl phosphine (TPP), a reductant specific for lipids. LOOH levels were then calculated as the difference between the absence and presence of TPP [20]. The CV for the determination of the LOOH level was 5 %.
Measurement of total free sulfhydryl groups
Free sulfhydryl groups (–SH) in serum samples were assayed according to the method of Ellman [21] as modified by Hu et al. [22]. Briefly, 1 mL of buffer containing 0.1 M Tris, 10 mM EDTA (pH 8.2), and 50 µL serum was added to cuvettes, followed by 50 µL of 10 mM DTNB in methanol. Blanks were run for each sample with no DTNB. Following incubation for 15 min at room temperature (RT), sample absorbance was read at 412 nm on a Cecil 3000 spectrophotometer (Cecil Instruments, Cambridge, UK). The concentration of sulfhydryl groups was calculated using reduced glutathione as the free sulfhydryl group standard, and the results are expressed in millimoles. The CV for the measurement of serum –SH levels was 3.6 %.
Measurement of total oxidative status (TOS)
The TOS of serum was determined using an automated measurement method (Rel assay diagnostics kits) developed by Erel [23]. Oxidants present in the sample oxidized the ferrous ion-o-dianisidine complex to ferric ions. The oxidation reaction is enhanced by glycerol molecules, which are abundant in the reaction medium. The ferric ion produces a colored complex with xylenol orange in an acidic medium. The color intensity, which can be measured spectrophotometrically, is related to the total amount of oxidant molecules present in the sample. The assay was calibrated with hydrogen peroxide (H2O2), and the results are expressed as micromoles of H2O2 equivalents per liter (µmol H2O2 equiv/L). The CV for the measurement of the serum TOS level was <3 %.
Measurement of total antioxidative capacity (TAC)
TAC of serum was determined using an automated measurement method (Rel assay diagnostic kits) of Erel [24], in which the hydroxyl radical, the most potent biological radical, is produced. Ferrous ion solution in reagent-1 is mixed with H2O2, which is present in reagent-2. Sequentially produced radicals include the brownish-colored dianisidinyl radical cation, produced by the hydroxyl radical. Using this method, the antioxidative effect of the sample against the potent free radical reactions, which was initiated by the hydroxyl radicals produced, was measured. The data are expressed as mmol Trolox equiv/L. The CV for the measurement of the serum TAC level was <3 %.
Oxidative stress index (OSI)
The ratio of TOS to TAC yielded the OSI, an indicator of the percentage degree of oxidative stress [23, 24]. For calculations, the unit of TAC was changed to µmol/L, and the OSI value was calculated according to the following formula: OSI (arbitrary units): TOS (µmol H2O2 equiv/L)/TAC (µmol Trolox equiv/L) × 10.
Statistical analysis
All analyses were conducted using the SPSS software (version 16; SPSS Inc., Chicago, IL, USA). Continuous variables are expressed as mean ± SD. Comparisons of categorical and continuous variables between the knee OA and control groups were performed using the Chi-square test and independent-samples t test, respectively. The paired-samples t test was used for comparison of continuous variables categorized according to age within both groups. Comparison of laboratory variables between groups categorized according to the grade of knee OA was performed using one-way analysis of variance (ANOVA) with the least significant difference post hoc test. The correlation between serum prolidase activity and clinical and laboratory parameters was evaluated using Pearson’s correlation test. Standardized β-regression coefficients and their significance in a multiple linear regression analysis were reported. A two-tailed p < 0.05 was considered to indicate statistical significance.
Results
Demographic characteristics of patients with knee OA and the control group are shown in Table 1. No significant difference was observed in age, BMI, or female/male ratio between patients and controls.
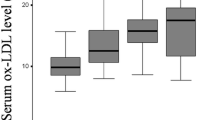
Serum prolidase activity was significantly lower in patients with knee OA compared with controls (p < 0.001). The comparison included 137 patients with OA in four subgroups according to the KL grading scale—grade 1 (n = 34), grade 2 (n = 34), grade 3 (n = 37), and grade 4 (n = 32)—along with 134 controls. Figure 1 demonstrates levels of serum prolidase activity in the patient group divided by X-ray grades of the knee joints. The ANOVA test indicated significant differences between the groups. Serum prolidase activities of the patients with grades 3 and 4 (late knee OA) were significantly lower than those of knee grades 1 and 2 (early knee OA; ANOVA, p < 0.001). Although prolidase activity was significantly lower in the patient group than in the controls (p < 0.001), when all participants were stratified into two groups (40–60 and 61–80 years) according to age, there was no statistically significant difference between the two layers within each group (Table 2). The relationship between serum prolidase activity and clinical characteristics is presented in Table 3. Serum prolidase activity was inversely correlated with WOMAC score in a bivariate analysis (r = −0.516, p < 0.001; Table 3). The correlation between serum prolidase activity and WOMAC score is shown in Fig. 2. In a multiple regression analysis, we observed that WOMAC score (β = −0.340, p < 0.001) was independently associated with serum prolidase activity (Table 3).
Furthermore, oxidant parameters (LOOH, TOS, and OSI) were significantly higher in knee OA patients (p < 0.001 for all). However, antioxidant parameters (TAC and –SH) did not differ between the groups (p > 0.05). In a bivariate analysis, LOOH level was positively correlated with TOS (r = 0.609, p < 0.001) in patients with OA of the knee.
The relationship between WOMAC score and clinical characteristics and laboratory parameters is presented in Table 4. The WOMAC score was positively correlated with severity of knee OA (r = 0.619, p < 0.001), but inversely correlated with serum prolidase activity (r = −0.516, p < 0.001) in a bivariate analysis. In a multiple regression analysis, we found that severity of knee OA (β = 0.429, p < 0.001) and serum prolidase activity (β = −0.298, p < 0.001) were independently associated with the WOMAC score (Table 4).
Discussion
The main results of this study were as follows. First, prolidase activity was lower in the knee OA group than in the control group. Second, after the participants were stratified into two groups according to age, serum prolidase activity was significantly lower in the patients group than in the controls in both age groups. Third, prolidase activity was significantly decreased in late-grade versus early-grade knee OA. Fourth, the oxidative stress parameters (LOOH, TOS, and OSI) of the OA group were significantly greater than those of the control group, whereas the antioxidative stress parameters (–SH groups and TAC) were unchanged. Fifth, the WOMAC score was independently correlated with prolidase activity and knee OA grading.
We observed that serum prolidase activity was significantly lower in patients with knee OA than in the controls. The pathogenic mechanisms underlying this reduction in serum prolidase activity in patients with OA are unclear. However, three major causes have been suggested. First, it may be related to decreased collagen resynthesis in patients with knee OA. Prolidase (E.C. 3.4.13.9) is a cytosolic Mn(II)-activated metalloproteinase that hydrolyzes imidodipeptides and imidotripeptides with a C-terminal proline or hydroxyproline. Then, it releases these two amino acids for collagen resynthesis and cell growth, and its activity determines the rate of collagen turnover [4, 5, 25]. Although prolidase activity is found in a wide variety of diseases, the reports have been inconsistent. Several studies have reported that prolidase activity decreases in diseases such as chronic uremia [26] and cardiomyopathy [27], while increased prolidase activity has been described in Legg–Calve–Perthes disease [6] and idiopathic clubfoot [7]. Under normal circumstances, chondrocytes stimulate synthesis of the new extracellular matrix, such as aggrecan and type II collagen. However, in patients with OA, synovial cells and chondrocytes serve to increase the levels of inflammatory cytokines. Thus, this anabolic phase of matrix remodeling is considered insufficient or defective. Second, the reason for this imbalance in cartilage remodeling in OA may be related to aging [12, 28]. Previous studies have demonstrated that the accumulation of advanced glycation end products (AGEs) is associated with age in OA. The accumulation of AGEs causes damage to matrix synthesis in articular cartilage due to decreased collagen turnover [28]. In this study, to evaluate the effect of age, we stratified all participants into two groups according to age. We found lower prolidase enzyme activity in the patient group in each age group than in the controls. Although a partial decrease was seen in the >60-year-old group, we did not find a statistically significant difference between the two layers within each group. These results may be related to the severity of the knee OA in terms of prolidase activity rather than age. Another possible reason for decreased prolidase activity in knee OA may be related to the low physical activity levels of the elderly population. Physical activity levels decreased in patients with knee OA, and collagen turnover seems to be positively correlated with the degree of exercise [27, 29]. Furthermore, we found significant decreases in the levels of prolidase activity in the late stages of knee OA. The cause of the decreased serum prolidase activity with advancing OA stage is unclear. However, we believe that these results may be due to grades 3 and 4 being the late stages of OA, in which most of the cartilage has already degenerated and morphological changes are manifest.
In our study, we observed significantly elevated oxidant parameters in subjects with knee OA compared to the controls. These results confirmed the presence of oxidative stress. Under healthy conditions, when ROS production decreases, LOOH and TOS are inhibited by various antioxidants present in plasma. However, in the case of excessive ROS production, as demonstrated in our study by the rise in LOOH and TOS, this protection may be inadequate as a defense mechanism against continuing oxidative stress. Oxidative stress is associated with OA, but the precise mechanism is unclear. However, oxidative stress causes telomere shortening and aging in chondrocytes. Senescent cells have decreased tolerance to oxidative stress [14, 30]. Furthermore, ROS promote cellular senescence and apoptosis. Thus, ROS may play important roles in the development of OA. We used serum –SH groups and TOS levels as markers of antioxidant status. In a previous study in our laboratory, Altindag et al. [8] showed that serum –SH groups, TAC levels, and prolidase activity were lower, whereas LOOH levels were higher, in patients with knee OA compared with healthy individuals. However, we detected no difference in the serum levels of –SH groups or TAC in patients with knee OA compared with controls. As a result, the cause of the unchanged antioxidative stress parameters (–SH groups and TAC) may be attributable to increased oxidative stress compensatory mechanisms in patients with knee OA.
In the present study, the WOMAC score was positively correlated with the severity of knee OA, but inversely correlated with serum prolidase activity. The WOMAC scale allows detailed analysis of pain and dysfunction [31]. The articular cartilage is aneural and avascular and thus does not directly generate pain. However, the changes in articulation caused by structural and associated degenerative changes in articular cartilage may result in the manifestation of pain in joint tissues [32].
BMI is routinely used as a screening tool for obesity, and higher BMI has been associated consistently with an elevated risk of knee OA [33]. However, in our study, there was no relationship between the BMI and serum prolidase activity. This may be due to our patient’s BMI values not being very high, and as mentioned recently, BMI does not describe the pattern of fat distribution or body composition and does not distinguish adipose from non-adipose body mass due to the changes in fat-free mass and fat mass with height, weight, and age [34].
Several limitations of this study should be noted. First, it had a cross-sectional design and was a single-center study. Second, radiographically visualized degeneration is only part of knee OA. Thus, these findings should be confirmed by magnetic resonance imaging or arthroscopy, which we did not perform. Third, prolidase enzyme activity was evaluated only in serum samples. We were unable to obtain synovial fluid samples due to ethical restrictions. Further prospective longitudinal studies are needed to evaluate whether the results in serum are comparable to those in synovial fluid or cartilage and to determine the molecular mechanisms of the association between prolidase activity and the severity of knee OA.
Conclusions
In conclusion, decreased serum prolidase activity, correlated inversely with WOMAC scores, and elevated LOOH, TOS, and OSI levels may be associated with knee OA. Additionally, according to the significant decreases in the levels of prolidase activity in the late stages of knee OA, prolidase enzyme activity may be a useful adjunctive indicator to assess the progression of knee OA.
References
Ertürk C, Altay MA, Selek S, Koçyiğit A (2012) Paraoxonase-1 activity and oxidative status in patients with knee osteoarthritis and their relationship with radiological and clinical parameters. Scand J Clin Lab Invest 72(5):433–439
Brandt KD, Dieppe P, Radin EL (2009) Commentary: Is it useful to subset “primary” osteoarthritis? A critique based on evidence regarding the etiopathogenesis of osteoarthritis. Semin Arthritis Rheum 39(2):81–95
Ertürk C, Altay MA, Altay N, Kalender AM, Oztürk IA (2014) Will a single periarticular lidocaine-corticosteroid injection improve the clinical efficacy of intraarticular hyaluronic acid treatment of symptomatic knee osteoarthritis? Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-014-3398-2
Miltyk W, Surazynski A, Kasprzak KS, Fivash MJ Jr, Buzard GS, Phang JM (2005) Inhibition of prolidase activity by nickel causes decreased growth of proline auxotrophic CHO cells. J Cell Biochem 94(15):1210–1217
Surazynski A, Miltyk W, Palka J, Phang JM (2008) Prolidase-dependent regulation of collagen biosynthesis. Amino Acids 35(4):731–738
Altay MA, Erturk C, Aksoy N, Taskin A, Bilge A, Celik H, Isikan UE (2011) Serum prolidase activity and oxidative-antioxidative status in Legg–Calve–Perthes disease. J Pediatr Orthop B 20(4):222–226
Altay MA, Erturk C, Aksoy N, Taskın A, Isıkan UE (2011) A preliminary study pointing out the role of serum prolidase activity and oxidative-antioxidative status parameters during the treatment process of patients with idiopathic clubfoot. Scand J Clin Lab Invest 71(7):576–582
Altindag O, Erel O, Aksoy N, Selek S, Celik H, Karaoglanoglu M (2007) Increased oxidative stress and its relation with collagen metabolism in knee osteoarthritis. Rheumatol Int 27(4):339–344
Ziskoven C, Jäger M, Zilkens C, Bloch W, Brixius K, Krauspe R (2010) Oxidative stress in secondary osteoarthritis: From cartilage destruction to clinical presentation? Orthop Rev (Pavia) 2(2):e23
Davies CM, Guilak F, Weinberg JB, Fermor B (2008) Reactive nitrogen and oxygen species in interleukin-1-mediated DNA damage associated with osteoarthritis. Osteoarthr Cartil 16(5):624–630
Martel-Pelletier J, Pelletier JP (2010) Is osteoarthritis a disease involving only cartilage or other articular tissues? Eklem Hastalik Cerrahisi 21(1):2–14
Loeser RF (2011) Aging and osteoarthritis. Curr Opin Rheumatol 23(5):492–496
Kim J, Xu M, Xo R, Mates A, Wilson GL, Pearsall AW 4th, Grishko V (2010) Mitochondrial DNA damage is involved in apoptosis caused by pro-inflammatory cytokines in human OA chondrocytes. Osteoarthr Cartil 3:424–432
Brandl A, Hartmann A, Bechmann V, Graf B, Nerlich M, Angele P (2011) Oxidative stress induces senescence in chondrocytes. J Orthop Res 29(7):1114–1120
Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M et al (1986) Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and therapeutic criteria committee of the American Rheumatism Association. Arthritis Rheum 29(8):1039–1049
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteoarthrosis. Ann Rheum Dis 16:494–502
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15(12):1833–1840
Ozcan O, Gultepe M, Ipcioglu O, Bolat B, Kayadibi H (2007) Optimization of the photometric enzyme activity assay for evaluating real activity of prolidase. Turk J Biochem 32:12–16
Myara I, Charpentier C, Lemonnier A (1982) Optimal conditions for prolidase assay by proline colorimetric determination: application to iminodipeptiduria. Clin Chim Acta 125:193–205
Nourooz-Zadeh J (1999) Ferrous ion oxidation in presence of xylenol orange for detection of lipid hydroperoxides in plasma. Methods Enzymol 300:58–62
Ellman GL (1959) Tissue sulphydryl groups. Arch Biochem Biophys 82:70–77
Hu ML, Louie S, Cross CE, Motchnik P, Halliwell B (1993) Antioxidant protection against hypochlorous acid in human plasma. J Lab Clin Med 121:257–262
Erel O (2005) A new automated colorimetric method for measuring total oxidant status. Clin Biochem 38:1103–1111
Erel O (2004) A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem 37:112–119
Gencer M, Aksoy N, Dagli EC, Uzer E, Aksoy S, Selek S, Celik H, Cakir H (2011) Prolidase activity dysregulation and its correlation with oxidative-antioxidative status in chronic obstructive pulmonary disease. J Clin Lab Anal 25(1):8–13
Evrenkaya TR, Atasoyu EM, Kara M, Unver S, Gultepe M (2006) The role of prolidase activity in the diagnosis of uremic bone disease. Ren Fail 28(4):271–274
Sezen Y, Bas M, Altiparmak H, Yildiz A, Buyukhatipoglu H, Faruk Dag O, Kaya Z, Aksoy N (2010) Serum prolidase activity in idiopathic and ischemic cardiomyopathy patients. J Clin Lab Anal 24(4):213–218
DeGroot J, Verzijl N, Jacobs KM, Budde M, Bank RA, Bijlsma JW, TeKoppele JM, Lafeber FP (2001) Accumulation of advanced glycation endproducts reduces chondrocyte-mediated extracellular matrix turnover in human articular cartilage. Osteoarthr Cartil 9(8):720–726
Langberg H, Skovgaard D, Asp S, Kjaer M (2000) Time pattern of exercise-induced changes in type I collagen turnover after prolonged endurance exercise in humans. Calcif Tissue Int 67(1):41–44
Yudoh K, Nguyen T, Nakamura H, Hongo-Masuko K, Kato T, Nishioka K (2005) Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res Ther 7(2):380–391
Bedson J, Croft PR (2008) The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord 9:116
Ishijima M, Watari T, Naito K, Kaneko H, Futami I, Yoshimura-Ishida K, Tomonaga A, Yamaguchi H, Yamamoto T, Nagaoka I, Kurosawa H, Poole RA, Kaneko K (2011) Relationships between biomarkers of cartilage, bone, synovial metabolism and knee pain provide insights into the origins of pain in early knee osteoarthritis. Arthritis Res Ther 13(1):22
Martin KR, Kuh D, Harris TB, Guralnik JM, Coggon D, Wills AK (2013) Body mass index, occupational activity, and leisure-time physical activity: an exploration of risk factors and modifiers for knee osteoarthritis in the 1946 British birth cohort. BMC Musculoskelet Disord 14:219. doi:10.1186/1471-2474-14-219
Ertürk C, Altay MA, Sert C, Levent A, Yaptı M, Yüce K (2015) The body composition of patients with knee osteoarthritis: relationship with clinical parameters and radiographic severity. Aging Clin Exp Res. doi:10.1007/s40520-015-0325-4
Acknowledgments
Approval of local ethics committee reference number: B.30.2.HRÜ0.0.20.05.00.050.01.04-90. The authors declare that they have no relevant financial involvement with any commercial organization with direct financial interest in the subject or materials discussed in this manuscript.
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Altay, M.A., Ertürk, C., Bilge, A. et al. Evaluation of prolidase activity and oxidative status in patients with knee osteoarthritis: relationships with radiographic severity and clinical parameters. Rheumatol Int 35, 1725–1731 (2015). https://doi.org/10.1007/s00296-015-3290-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-015-3290-5