Abstract
DNA polymerases sometimes stall during DNA replication at sites where DNA is damaged, or upon encounter with proteins or secondary structures of DNA. When that happens, the polymerase clamp PCNA can become modified with a single ubiquitin moiety at lysine 164, opening DNA Damage Tolerance (DDT) mechanisms that either repair or bypass the lesions. An alternative repair mechanism is the salvage recombination (SR) pathway, which copies information from the sister chromatid. SUMOylation of PCNA at the same lysine, or at lysine 127, can recruit the Srs2 helicase, which negatively controls SR. Recently, we have dissected the relationship between SR and the DDT pathways, and showed that overexpression of either the PCNA unloader Elg1, or the Rad52 homologous recombination protein, can bypass the repression by Srs2. Our results shed light on the interactions between different DNA damage repair/bypass proteins, and underscore the importance of PCNA modifications in organizing the complex task of dealing with DNA damage during replication of the genetic material.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During cell growth, the genetic material can be damaged by internal or external sources and cells must deal with this threat to faithfully transmit the genetic material to the next generation. In addition to cellular checkpoint pathways that prevent entry into mitosis with damaged chromosomes, sophisticated DNA repair and DNA damage tolerance mechanisms are in place. These are conserved in all eukaryotes, and much of our knowledge about them comes from the study of simple organisms, such as the yeast Saccharomyces cerevisiae (Adames et al. 2019; Ballew and Lacefield 2019; Gkouskou et al. 2019; Tutaj et al. 2019).
The DNA is particularly prone to attack during DNA replication, a time at which the DNA is particularly exposed (Corcoles-Saez et al. 2019; Moriel-Carretero et al. 2019). DNA replication is a complex process, and fork progression may be halted by lesions in the DNA, by secondary structures or by the presence of bound proteins (Owiti et al. 2019). Cells deal with this situation by either repairing the damage or bypassing it completely, thus preventing the situation from escalating into fatal genomic rearrangements (Singh and Wu 2019). The DNA damage tolerance (DDT) pathway (sometimes also known as the post-replication repair pathway) becomes activated when single-stranded DNA gaps are formed during DNA replication, following DNA polymerase stalling and re-start events (Karras and Jentsch 2010). Central to the DDT is PCNA, the processivity factor for replicative DNA polymerases, composed of three identical subunits that form a ring encircling the DNA (Garbacz et al. 2020). Upon polymerase stalling, PCNA becomes mono-ubiquitinated at lysine 164 by the Rad6/Rad18 E2/E3 complex. This modification allows the exchange of the replicative polymerases by specialized trans-lesion synthesis (TLS) polymerases that are able to synthesize DNA past the lesion, albeit in a less accurate fashion, thus creating mutations (Acharya et al. 2019; Bebenek and Ziuzia-Graczyk 2018; Stelter and Ulrich 2003; Szwajczak et al. 2018). The mono-ubiquitinated PCNA molecules can otherwise be further ubiquitinated, creating poly-ubiquitin chains, by the combined activity of the E2 enzymes Ubc13-Mms2 and the E3 Rad5, creating K63-linked ubiquitin chains (Fan et al. 2018). This modification somehow orchestrates a template switch (TS) event, in which information is copied from the newly synthesized sister chromatid. The molecular details of this process, as well as the nature of potential “readers” of the poly-ubiquitin signal, are still mysterious (Branzei et al. 2008). PCNA is also SUMOylated, mainly at lysine 164, and to a lesser extent, at lysine 127 (Hoege et al. 2002). PCNA is loaded onto DNA by RFC, a 5 subunit complex; it is unloaded by a similar complex in which the Elg1 protein replaces the Rfc1 subunit (Kubota et al. 2013; Parnas et al. 2010; Sau and Kupiec 2020; Shemesh et al. 2017). In the absence of Elg1, a higher level of PCNA, and in particular, of SUMOylated PCNA, can be detected in chromatin fractions (Kubota et al. 2013; Parnas et al. 2010). A protein with high affinity to SUMOylated PCNA is Srs2, a UvrD-like helicase that is able to disrupt Rad51 presynaptic filaments, thus preventing homologous recombination (HR) (Bronstein et al. 2018a, b; Krejci et al. 2003; Piazza and Heyer 2019; Veaute et al. 2003).
As an alternative to TLS and TS, a third mechanism, hereafter referred as ‘‘salvage recombination’’ (SR) also exists. This pathway is independent of PCNA ubiquitination, employs some proteins of the homologous recombination pathway (which usually deals with double-stranded DNA breaks) (Bordelet and Dubrana 2019; Marsella et al. 2019; Zimmer and Fabre 2019), and appears to be negatively regulated by the Srs2 helicase (Branzei and Szakal 2016; Urulangodi et al. 2015).
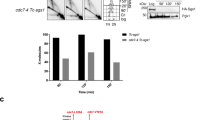
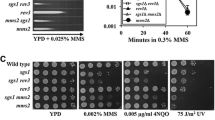
Recently, by performing quantitative serial dilution assays on plates containing the DNA-damaging agent MMS (methylmethane sulfonate) at concentrations differing by small increments, we have dissected the mechanisms that regulate SR (Arbel et al. 2020). A first surprising and paradoxical observation was that yeast strains in which lysine 164 of PCNA was mutated (and thus this residue can neither be ubiquitinated nor SUMOylated) was more sensitive to DNA-damaging agents than isogenic strains that carried an additional mutation at lysine 127 (pol30-KK127,164RR, hereafter referred to as pol30-RR). This result suggested that a modification of PCNA at lysine 127 inhibits repair or tolerance when K164 is mutated. Mutating the SUMO E3 ligase SIZ1 in a pol30-K164R strain led to the same phenotype as that of the pol30-RR strain, indicating that indeed the effect observed is due to SUMOylation of lysine 127. This modification could serve to recruit Srs2. Indeed, strains deleted for SRS2 have the same lower sensitivity as pol30-RR strains, and so does the double mutant pol30-RR srs2Δ strain. These results thus confirm that in addition of binding PCNA SUMOylated at K164 (Hoege et al. 2002), Srs2 can also bind molecules SUMOylated at K127, and exert the same negative regulation of the SR repair mode.
To better understand the nature of this inhibition, we attempted to bypass it by overexpressing, in pol30-K164R strains, proteins from the homologous recombination pathway. Overexpression of Rad52, but not of Rad51, Rad55, rad57, or Rad59, restored resistance to MMS. The lack of effect of Rad51 overexpression was surprising, as Srs2 is known to be able to evict Rad51 from chromatin (Krejci et al. 2003; Veaute et al. 2003) and Rad51 is the main RecA-like strand transfer protein of eukaryotes. Moreover, simultaneous overexpression of Rad51 and Rad52 did not increase resistance beyond that observed in cells overexpressing Rad52. These results indicate that the effect of Srs2 inhibition is through Rad52, and not through Rad51, in accordance with some current models (De Tullio et al. 2017). Overexpression of the Elg1 PCNA unloader also resulted in a suppression of the MMS sensitivity, and no further suppression was obtained by overexpressing it together with Rad52, suggesting that the two proteins work in a single process. Moreover, the effects seen were the same as those seen in srs2Δ or siz1Δ mutants; Rad52 or Elg1 overexpression in these strains had no further increase in MMS sensitivity. We thus concluded that the overexpression allowed the bypass of the negative effect created by the recruitment of Srs2 to SUMOylated lysine 127 in PCNA.
To better understand the overexpression mechanism, we knocked out several genes and asked whether they affect the suppression effect. Our results showed that suppression by overexpression of Rad52 required the activity of Elg1 and vice versa, confirming the common activity. Surprisingly, Rad51 was essential for the suppression. Thus, although not limiting, Rad51 does play a role in the bypass of Srs2 effect. Similarly, Rad59, a protein that works with Rad52 (Bai and Symington 1996; Jablonovich et al. 1999), and the Sgs1 helicase [a RecQ-like protein that can dissolve Holliday Junctions (Chu and Hickson 2009)] were necessary for the suppression. Taken together, our results suggest a model in which the SR pathway involves PCNA unloading by Elg1, followed by invasion of the sister chromatid by a mechanism mediated by the Rad52-Rad59 complex. Intermediates thus created are resolved by the activity of the Sgs1 helicase. The suppression observed by overexpressing Rad52 or Elg1 was independent of PCNA ubiquitination by Rad6/Rad18, and, consistent with lysine 164 being the main target for SUMOylation, recruitment of Srs2 to this lysine had a stronger effect in suppressing the SR pathway.
Further analysis demonstrated physical interactions between Srs2 and Rad52. The interactions take place through the same region of Srs2 (amino acids 875–902), which is known to bind Rad51. Thus, our results define Srs2 as a negative regulator of Rad52/59, in addition to its known role in eviction of Rad51 (Krejci et al. 2003; Veaute et al. 2003).
What is the relationship between Srs2 and Elg1? Overexpression of Rad52 overcomes the inhibition by Srs2 because Rad52 is a direct target of Srs2. However, we were unable to detect direct physical interactions between Srs2 and Elg1. Overexpression of Elg1 may remove the inhibition of Srs2 either by a direct competition for binding of SUMOylated PCNA, or because increased PCNA unloading by Elg1 removes Srs2 from the chromatin. The double mutant srs2Δ elg1Δ grows poorly and exhibits higher sensitivity to DNA-damaging agents (Gazy et al. 2013). In the absence of Srs2, the SR pathway should open. However, Elg1 is needed to unload PCNA and allow repair and resumption of DNA replication. Indeed, a triple mutant pol30-K164R srs2Δ elg1Δ has the same sensitivity to DNA-damaging agents as pol30-RR elg1Δ strains. Significantly, mutants still able to recruit Srs2 but for which the SR pathway is blocked (e.g., pol30-K164R elg1Δ) are more sensitive, showing that some DNA repair can be performed in the absence of Elg1 activity, provided Srs2 is not recruited. This is in line with the fact that Elg1 is not an essential protein, and therefore, alternative mechanisms for unloading PCNA must exist.
Our results can be summarized in the following model (Fig. 1): wherever DNA polymerases stall, PCNA becomes SUMOylated, mainly at lysine 164, and, at lower levels, at lysine 127. Modified PCNA may be found at the arrested fork itself, or, if left behind by re-initiation, next to RPA-covered ssDNA gaps. It is possible that these two locations are treated differently, as recent work has shown differences in DNA damage checkpoint signaling between them (Garcia-Rodriguez et al. 2018). Recruitment of Srs2 to SUMOylated PCNA inhibits the activity of Rad52, Rad51 and Elg1, thus precluding unwanted or untimely recombination events (Fig. 1b). The high levels of RPA in proximity to SUMOylated PCNA allow it to become mono-ubiquitinated by Rad6 and Rad18 (Li et al. 2019), or further poly-ubiquitylated by the Rad5/Mms2/Ubc13 proteins to allow TS. How the decision is made regarding whether to stop at a single ubiquitin to allow the error-prone TLS, or to continue into the error-free pathway that requires poly-ubiquitination of PCNA is still an unsolved question.
Potential model for the recombination salvage pathway regulation. a During DNA replication, fork movement can be impaired by lesions or other perturbations (red star) causing stalling. Either at the arrested fork, or at gaps left behind after re-initiation downstream, the ssDNA uncovered gets covered by RPA and Rad51. b During normal DNA replication, binding of Srs2 to the SUMOylated lysines of PCNA inhibits the activity of Rad52 and evicts Rad51, thus preventing potential homologous recombination events. Ubiquitination of PCNA allows damage bypass by trans-lesion synthesis (TLS) or by template switch (TS). Which of these two sub-pathways is chosen may depend on the amount and perhaps the nature of the DNA damage, or by its location (at the fork or behind it, at ssDNA gaps. c Mutation of lysine 164 of PCNA does not impair the recruitment of Srs2 by K127 SUMOylation. Since PCNA cannot undergo ubiquitination, and Srs2 inhibits the activity of both Rad52 and Rad51, the TLS and TS pathways are inactivated, and the cells become extremely sensitive to DNA damage
Cells mutated for lysine 164 (Fig. 1c) are extremely sensitive to DNA damage, because recruitment of Srs2 at lysine 127 precludes the use of the SR pathway. If Srs2 cannot be recruited (either because of mutations in the two lysines of PCNA (pol30-RR), in the SUMOylation machinery (siz1 siz2), or upon deletion of SRS2), however, the SR option opens, and Elg1 is brought to unload PCNA, allowing invasion of the sister chromatid in a process that requires Rad52, Rad51, Rad59 and Sgs1.
It is clear that the role of Srs2 is far from been completely understood and that we are looking through a keyhole, each time gazing at a different part of its activity but still lacking the ability to see the big picture. Arbel et al. used a minimalistic approach in trying to dissect Srs2's regulation of the SR; additional layers or regulation probably exist. Moreover, different types of impediments to the replication fork’s movement may be treated differently. From the many enigmas surrounding Srs2, maybe the most interesting one, is when, where and why (and even if) the SR pathway is activated in WT cells. An appealing answer to this question may be found perhaps in Srs2's role in the DNA damage checkpoint (Berto et al. 2019; Ma et al. 2020). Srs2 is phosphorylated in a Mec1(ATR)-dependent manner, which controls Srs2 turnover (Liberi et al. 2000; Saponaro et al. 2010). Elg1 also undergoes a Mec1(ATR)-dependent phosphorylation (Sau and Kupiec 2020; Shkedy et al. 2015). Thus, one of the functions of the DNA damage response may be to coordinate the activity of the various DNA tolerance pathways. Interestingly, the activity of Srs2 itself is also necessary for the resumption of growth after cells were arrested by the DDR (Vaze et al. 2002). Thus, Srs2 could be involved in a regulatory feedback loop, in which its own activity, regulated by the DDR, in turn inactivates it.
The results obtained by Arbel et al. (2020) shed light on the complex interactions between various players in cells experiencing DNA damage. They also underscore the central role played by protein modifications, such as ubiquitin and SUMO, in orchestrating complex decisions in the cell.
References
Acharya N, Manohar K, Peroumal D, Khandagale P, Patel SK, Sahu SR, Kumari P (2019) Multifaceted activities of DNA polymerase eta: beyond translesion DNA synthesis. Curr Genet 65:649–656. https://doi.org/10.1007/s00294-018-0918-5
Adames NR, Gallegos JE, Peccoud J (2019) Yeast genetic interaction screens in the age of CRISPR/Cas. Curr Genet 65:307–327. https://doi.org/10.1007/s00294-018-0887-8
Arbel M, Bronstein A, Sau S, Liefshitz B, Kupiec M (2020) Access to PCNA by Srs2 and Elg1 controls the choice between alternative repair pathways in yeast. mBio. https://doi.org/10.1128/mBio.00705-20
Bai Y, Symington LS (1996) A Rad52 homolog is required for RAD51-independent mitotic recombination in Saccharomyces cerevisiae. Genes Dev 10:2025–2037
Ballew O, Lacefield S (2019) The DNA damage checkpoint and the spindle position checkpoint: guardians of meiotic commitment. Curr Genet. https://doi.org/10.1007/s00294-019-00981-z
Bebenek A, Ziuzia-Graczyk I (2018) Fidelity of DNA replication-a matter of proofreading. Curr Genet 64:985–996. https://doi.org/10.1007/s00294-018-0820-1
Berto G, Ferreira-Cerca S, De Wulf P (2019) The Rio1 protein kinases/ATPases: conserved regulators of growth, division, and genomic stability. Curr Genet 65:457–466. https://doi.org/10.1007/s00294-018-0912-y
Bordelet H, Dubrana K (2019) Keep moving and stay in a good shape to find your homologous recombination partner. Curr Genet 65:29–39. https://doi.org/10.1007/s00294-018-0873-1
Branzei D, Szakal B (2016) DNA damage tolerance by recombination: Molecular pathways and DNA structures. DNA Repair (Amst) 44:68–75. https://doi.org/10.1016/j.dnarep.2016.05.008
Branzei D, Vanoli F, Foiani M (2008) SUMOylation regulates Rad18-mediated template switch. Nature 456:915–920
Bronstein A, Bramson S, Shemesh K, Liefshitz B, Kupiec M (2018a) Tight regulation of srs2 helicase activity is crucial for proper functioning of DNA repair mechanisms. G3 8:1615–1626. https://doi.org/10.1534/g3.118.200181
Bronstein A, Gershon L, Grinberg G, Alonso-Perez E, Kupiec M (2018b) The main role of Srs2 in DNA repair depends on its helicase activity, rather than on its interactions with PCNA or Rad51. mBio 9:e0119218. https://doi.org/10.1128/mBio.01192-18
Chu WK, Hickson ID (2009) RecQ helicases: multifunctional genome caretakers. Nat Rev Cancer 9:644–654. https://doi.org/10.1038/nrc2682
Corcoles-Saez I, Dong K, Cha RS (2019) Versatility of the Mec1(ATM/ATR) signaling network in mediating resistance to replication, genotoxic, and proteotoxic stresses. Curr Genet 65:657–661. https://doi.org/10.1007/s00294-018-0920-y
De Tullio L, Kaniecki K, Kwon Y, Crickard JB, Sung P, Greene EC (2017) Yeast Srs2 helicase promotes redistribution of single-stranded DNA-bound RPA and Rad52 in homologous recombination regulation. Cell Rep 21:570–577. https://doi.org/10.1016/j.celrep.2017.09.073
Fan Q, Xu X, Zhao X, Wang Q, Xiao W, Guo Y, Fu YV (2018) Rad5 coordinates translesion DNA synthesis pathway by recognizing specific DNA structures in Saccharomyces cerevisiae. Curr Genet 64:889–899. https://doi.org/10.1007/s00294-018-0807-y
Garbacz MA, Lujan SA, Kunkel TA (2020) Opportunities for new studies of nuclear DNA replication enzymology in budding yeast. Curr Genet 66:299–302. https://doi.org/10.1007/s00294-019-01023-4
Garcia-Rodriguez N, Morawska M, Wong RP, Daigaku Y, Ulrich HD (2018) Spatial separation between replisome- and template-induced replication stress signaling. EMBO J. https://doi.org/10.15252/embj.201798369
Gazy I, Liefshitz B, Bronstein A, Parnas O, Atias N, Sharan R, Kupiec M (2013) A genetic screen for high copy number suppressors of the synthetic lethality between elg1Delta and srs2Delta in yeast. G3 3:917–926. https://doi.org/10.1534/g3.113.005561
Gkouskou K, Fragiadakis GS, Voutsina A, Alexandraki D (2019) Distinct associations of the Saccharomyces cerevisiae Rad9 protein link Mac1-regulated transcription to DNA repair. Curr Genet. https://doi.org/10.1007/s00294-019-01047-w
Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S (2002) RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 419:135–141. https://doi.org/10.1038/nature00991
Jablonovich Z, Liefshitz B, Steinlauf R, Kupiec M (1999) Characterization of the role played by the RAD59 gene of Saccharomyces cerevisiae in ectopic recombination. Curr Genet 36:13–20
Karras GI, Jentsch S (2010) The RAD6 DNA damage tolerance pathway operates uncoupled from the replication fork and is functional beyond S phase. Cell 141:255–267. https://doi.org/10.1016/j.cell.2010.02.028
Krejci L, Van Komen S, Li Y, Villemain J, Reddy MS, Klein H, Ellenberger T, Sung P (2003) DNA helicase Srs2 disrupts the Rad51 presynaptic filament. Nature 423:305–309. https://doi.org/10.1038/nature01577
Kubota T, Nishimura K, Kanemaki MT, Donaldson AD (2013) The Elg1 replication factor C-like complex functions in PCNA unloading during DNA replication. Mol Cell 50:273–280. https://doi.org/10.1016/j.molcel.2013.02.012
Li S, Dong Z, Yang S, Feng J, Li Q (2019) Chaperoning RPA during DNA metabolism. Curr Genet. https://doi.org/10.1007/s00294-019-00945-3
Liberi G, Chiolo I, Pellicioli A, Lopes M, Plevani P, Muzi-Falconi M, Foiani M (2000) Srs2 DNA helicase is involved in checkpoint response and its regulation requires a functional Mec1-dependent pathway and Cdk1 activity. Embo J 19:5027–5038. https://doi.org/10.1093/emboj/19.18.5027
Ma M, Rodriguez A, Sugimoto K (2020) Activation of ATR-related protein kinase upon DNA damage recognition. Curr Genet 66:327–333. https://doi.org/10.1007/s00294-019-01039-w
Marsella A, Cassani C, Casari E, Tisi R, Longhese MP (2019) Structure-function relationships of the Mre11 protein in the control of DNA end bridging and processing. Curr Genet 65:11–16. https://doi.org/10.1007/s00294-018-0861-5
Moriel-Carretero M, Pasero P, Pardo B (2019) DDR Inc., one business, two associates. Curr Genet 65:445–451. https://doi.org/10.1007/s00294-018-0908-7
Owiti N, Stokdyk K, Kim N (2019) The etiology of uracil residues in the Saccharomyces cerevisiae genomic DNA. Curr Genet 65:393–399. https://doi.org/10.1007/s00294-018-0895-8
Parnas O, Zipin-Roitman A, Pfander B, Liefshitz B, Mazor Y, Ben-Aroya S, Jentsch S, Kupiec M (2010) Elg1, an alternative subunit of the RFC clamp loader, preferentially interacts with SUMOylated PCNA. EMBO J 29:2611–2622. https://doi.org/10.1038/emboj.2010.128
Piazza A, Heyer WD (2019) Moving forward one step back at a time: reversibility during homologous recombination. Curr Genet 65:1333–1340. https://doi.org/10.1007/s00294-019-00995-7
Saponaro M, Callahan D, Zheng X, Krejci L, Haber JE, Klein HL, Liberi G (2010) Cdk1 targets Srs2 to complete synthesis-dependent strand annealing and to promote recombinational repair. PLoS Genet 6:e1000858. https://doi.org/10.1371/journal.pgen.1000858
Sau S, Kupiec M (2020) A role for the yeast PCNA unloader Elg1 in eliciting the DNA damage checkpoint. Curr Genet 66:79–84. https://doi.org/10.1007/s00294-019-01020-7
Shemesh K, Sebesta M, Pacesa M, Sau S, Bronstein A, Parnas O, Liefshitz B, Venclovas C, Krejci L, Kupiec M (2017) A structure-function analysis of the yeast Elg1 protein reveals the importance of PCNA unloading in genome stability maintenance. Nucleic Acids Res 45:3189–3203. https://doi.org/10.1093/nar/gkw1348
Shkedy D, Singh N, Shemesh K, Amir A, Geiger T, Liefshitz B, Harari Y, Kupiec M (2015) Regulation of Elg1 activity by phosphorylation. Cell Cycle 14:3689–3697. https://doi.org/10.1080/15384101.2015.1068475
Singh B, Wu PJ (2019) Linking the organization of DNA replication with genome maintenance. Curr Genet 65:677–683. https://doi.org/10.1007/s00294-018-0923-8
Stelter P, Ulrich HD (2003) Control of spontaneous and damage-induced mutagenesis by SUMO and ubiquitin conjugation. Nature 425:188–191. https://doi.org/10.1038/nature01965
Szwajczak E, Fijalkowska IJ, Suski C (2018) The importance of an interaction network for proper DNA polymerase zeta heterotetramer activity. Curr Genet 64:575–580. https://doi.org/10.1007/s00294-017-0789-1
Tutaj H, Pogoda E, Tomala K, Korona R (2019) Gene overexpression screen for chromosome instability in yeast primarily identifies cell cycle progression genes. Curr Genet 65:483–492. https://doi.org/10.1007/s00294-018-0885-x
Urulangodi M, Sebesta M, Menolfi D, Szakal B, Sollier J, Sisakova A, Krejci L, Branzei D (2015) Local regulation of the Srs2 helicase by the SUMO-like domain protein Esc2 promotes recombination at sites of stalled replication. Genes Dev 29:2067–2080. https://doi.org/10.1101/gad.265629.115
Vaze MB, Pellicioli A, Lee SE, Ira G, Liberi G, Arbel-Eden A, Foiani M, Haber JE (2002) Recovery from checkpoint-mediated arrest after repair of a double-strand break requires Srs2 helicase. Mol Cell 10:373–385. https://doi.org/10.1016/s1097-2765(02)00593-2
Veaute X, Jeusset J, Soustelle C, Kowalczykowski SC, Le Cam E, Fabre F (2003) The Srs2 helicase prevents recombination by disrupting Rad51 nucleoprotein filaments. Nature 423:309–312. https://doi.org/10.1038/nature01585
Zimmer C, Fabre E (2019) Chromatin mobility upon DNA damage: state of the art and remaining questions. Curr Genet 65:1–9. https://doi.org/10.1007/s00294-018-0852-6
Acknowledgements
We thank past and present members of the Kupiec lab for support, ideas and support. This work was supported by grants from the Israel Science Foundation, the Minerva Center and the Israel Cancer Research Fund to MK.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Judith Berman.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arbel, M., Liefshitz, B. & Kupiec, M. How yeast cells deal with stalled replication forks. Curr Genet 66, 911–915 (2020). https://doi.org/10.1007/s00294-020-01082-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-020-01082-y