Abstract
To ensure proper chromosome segregation during cell division, the centromere in many organisms is transcribed to produce a low level of long non-coding RNA to regulate the activity of the kinetochore. In the budding yeast point centromere, our recent work has shown that the level of centromeric RNAs (cenRNAs) is tightly regulated and repressed by the kinetochore protein Cbf1 and histone H2A variant H2A.ZHtz1, and de-repressed during S phase of the cell cycle. Too little or too much cenRNAs will disrupt centromere activity. Here, we discuss the current advance in the understanding of the action and regulation of cenRNAs at the point centromere of Saccharomyces cerevisiae. We further show that budding yeast cenRNAs are cryptic unstable transcripts (CUTs) that can be degraded by the nuclear RNA decay pathway. CenRNA provides an example that even CUTs, when present at the right time with the right level, can serve important cellular functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
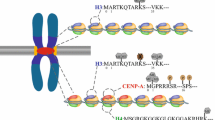
The centromere is a specialized domain on the chromosome responsible for kinetochore assembly and equal chromosome segregation (Dhatchinamoorthy et al. 2018). Almost all active centromeric nucleosomes contain centromeric-specific histone H3 variant, CENP-A, which acts as a base for kinetochore formation. Chromosomes of humans, mice, flies, fission yeast and other higher eukaryotes have regional centromeres, which are made up of tandemly repeated DNA, and can be up to megabase long (Pluta et al. 1995). The regional centromeric domain contains CENP-A nucleosomes interspersing with histone H3-containing nucleosomes. In contrast, chromosomes in budding yeast Saccharomyces cerevisiae contain a short, point centromeres, which are ~ 125 bp, consisting of three well-defined DNA elements, CDEI, II and III, built on a single CENP-ACse4 nucleosome (Furuyama and Biggins 2007). The formation and function of regional centromeres is regulated by epigenetics (Buehl and Kuo 2018; Westhorpe and Straight 2014), whereas point centromeres are thought to be genetically encoded and controlled by the CDE elements, especially the CDEIII element, which recruits CDEIII sequence-dependent CBF3 complex (Lechner and Carbon 1991). In the last decade, there have been tremendous advancements in understanding the non-coding transcription in the regional centromeric chromatin and studies have proposed the roles of centromeric non-coding RNA in regulating the activity of kinetochore proteins, including CENP-A (Quenet and Dalal 2014; Topp et al. 2004), CENP-C (Du et al. 2010; Wong et al. 2007), and components of the chromosomal passenger complex (CPC): Aurora-B, Survivin and INCENP (Ferri et al. 2009; Ideue et al. 2014; Wong et al. 2007). Recently, we showed that budding yeast point centromeres, similar to the regional centromeres, are also transcribed into non-coding RNAs to control point centromeres’ activity epigenetically (Ling and Yuen 2019). Therefore, centromeric transcription is a conserved epigenetic mechanism regulating both regional and point centromeres, and yet, the detailed molecular role of cenRNA is still unclear. Here, we highlight our recent discoveries on the regulation and action of budding yeast cenRNA at point centromeres, and further investigate the post-transcriptional regulation of cenRNA.
Cbf1 and H2A.ZHtz1 maintain centromeric transcription at a low level
Strong transcription activity is not compatible to both regional and point centromere function, and the kinetochore is inactivated in such condition (Bergmann et al. 2012; Hill and Bloom 1987). However, a low level of centromeric transcription, produced by RNA polymerase II (RNAPII), is required for proper centromere activity (Catania et al. 2015; Chan et al. 2012; Ohkuni and Kitagawa 2011). How centromeric transcription is kept at an optimal level is not clear. In budding yeast point centromeres, we found that centromeric transcription is repressed to a low level by kinetochore protein Cbf1 and histone H2A variant H2A.ZHtz1.
Cbf1 (centromere-binding protein 1) forms a homodimer that binds to the E-box element (CACGTG) present at gene promoters and the CDEI element of the centromere (Cai and Davis 1990; Mellor et al. 1990). Cbf1 is known as an activator for methionine gene transcription (Bram and Kornberg 1987; Thomas et al. 1992), and a repressor for LAC1 gene transcription in the ceramide biosynthetic pathway (Kolaczkowski et al. 2004). Yet, the role of Cbf1 at centromere is less clear. Cbf1 is not essential, but deletion of it leads to chromosome missegregation (Cai and Davis 1990). Cbf1 is only found in organisms with point centromeres, as no Cbf1 homolog is found in organisms with regional centromeres. A previous study showed that deletion of CBF1 down-regulated cenRNAs (Ohkuni and Kitagawa 2011). On the other hand, we found that Cbf1 is a repressor of centromeric transcription as deletion of CBF1 caused up-regulation of cenRNAs (Ling and Yuen 2019). To further examine the effect of Cbf1 through its binding to the CDEI element, we monitored cenRNA expression on a minichromosome with centromere 8 sequence (CEN8), containing a CDEI mutation (CAT mutation) which impairs the binding of Cbf1 (Baker et al. 1989). The level of cenRNA8 is comparable between cbf1 deletion and in CDEI CAT mutation, and is significantly higher than that in wild type (Fig. 1) (Ling and Yuen 2019). This result matches with our conclusion that Cbf1 is a repressor of centromeric transcription.
Cbf1 repressed centromeric transcription. aCEN8 minichromosome (minichr.) with wild-type (WT) or CAT-mutated CDEI was transformed into yeast strain CEN8::CEN3. b Quantitative reverse transcription PCR (RT-qPCR) analysis of the expression of cenRNA1 from the endogenous chromosome (chr.), cenRNA8 from the minichromosome, and TRP1 from the minichromosome (as an internal control gene). The relative expression of the RNAs was normalized to UBC6 expression. Statistical significances of the expression level (mean ± SD, n = 3) were analyzed with paired t test (*P ≤ 0.05)
Histone H2A variant H2A.Z can be found in organisms containing regional or point centromeres. H2A.Z is enriched in the nucleosomes of promoters controlling transcriptional activation and repression (Kamakaka and Biggins 2005, Zhang, et al. 2005). In the regional centromeric domain, nucleosomes containing H2A.Z and H3 are interspersed nucleosomes containing H2A and CENP-A, and also localized to pericentric heterochromatin non-uniformly (Greaves et al. 2007, Nakagawa and Okita 2019). In budding yeast point centromeres, although there is no defined pericentric heterochromatin, H2A.ZHtz1 nucleosomal domains are found in the flanking pericentric region, starting from 100 to 200 bp away from the CDE elements and spans ~ 600 bp (Albert, et al. 2007). H2A.ZHtz1 is also well documented as a boundary element to hinder the spread of heterochromatin in budding yeast. In htz1 deletion mutant, heterochromatin proteins Sir2 and Sir3 spread to the neighboring euchromatic domain, creating ectopic heterochromatin and repressing gene transcription nearby (Meneghini, et al. 2003). Here instead, we found that deletion of HTZ1 up-regulated cenRNA expression (Ling and Yuen 2019). For fission yeast regional centromeres, which are flanked by heterochromatin, deletion of H2A.ZPht1 also up-regulated cenRNA expression (Hou et al. 2010). These results suggest that H2A.Z may function other than as a heterochromatic boundary element at both regional and point centromeres.
The exact mechanism for repressing centromeric transcription is not clear. We postulated that Cbf1 and H2A.ZHtz1 create a repressive chromatin to hinder the elongation of RNAPII. In fact, RNAPII is accumulated around the point centromeric region in budding yeast (Candelli et al. 2018; Ling and Yuen 2019) and regional centromeric region in fission yeast (Catania et al. 2015). In addition to regulation by centromeric and pericentric protein repressors, we found that point centromeric transcription is also controlled tightly during the cell cycle. In particular, centromeric transcription is de-repressed in S phase (Ling and Yuen 2019). In S phase, kinetochore and nucleosome is transiently disassembled (Kitamura et al. 2007), which may also lead to a transient loss of Cbf1 and H2A.ZHtz1 from the centromere, causing de-repression and inducing transcription across the centromeric region. In humans, centromeric transcription and CENP-A loading are coupled and occur in late mitosis to early G1 phase (Quenet and Dalal 2014). Importantly, in regional centromeres, CENP-A is loaded independent of replication. How centromeric transcription is regulated to couple with and facilitate CENP-A loading is an important question that remains unclear. Interestingly, we found that centromeric transcription and CENP-ACse4 loading also occur coincidently in budding yeast, but in S phase, in a replication-dependent manner. We postulate that budding yeast cenRNA facilitates de novo CENP-A Cse4 loading to the newly synthesized chromatin, by a yet unknown mechanism.
A balanced level of cenRNA is required for optimal centromere activity
To manipulate the total pool of cenRNA, we have developed a yeast strain with the same centromeric sequence across all 16 chromosomes, and knocked down all the cenRNAs by introducing the RNA interference (RNAi) pathway and hairpin RNA against the same cenRNA into the budding yeast. It resulted in an increase of minichromosome loss. Not only is down-regulating cenRNA level detrimental, cenRNA over-expression in cbf1 and htz1 deletion also increase minichromosome loss. Importantly, cenRNA knockdown alleviates minichromosome loss in cbf1 and htz1 deletion in a dose-dependent manner, reflecting that a tightly balanced level of cenRNA is required for optimal point centromere function (Ling and Yuen 2019).
Regional and point centromeres are usually transcribed by RNAPII to produce a low level of poly(A)tail-containing non-coding cenRNA (Choi et al. 2011; Ling and Yuen 2019; Pezer and Ugarkovic 2008; Rosic et al. 2014). In budding yeast, we found that cenRNA from centromere 8 has a copy number of only 0.002 transcripts per cell. We hypothesized that such a low level is due to a short centromeric transcription window, in which RNAPII can only elongate across the centromere when the kinetochore is transiently disassembled in S phase, and it only lasts for 1–2 min (Kitamura et al. 2007). Another reason we postulated is that cenRNA may be unstable. Cryptic unstable transcripts (CUTs) are a class of unstable, RNAPII-dependent lncRNAs that originated from intergenic regions, representing more than 10% of intergenic transcripts in the budding yeast (Wyers et al. 2005). CUTs are rapidly degraded by the nuclear exosome, and the degradation is enhanced with the Trf4/Air2/Mtr4 polyadenylation (TRAMP) complex (LaCava et al. 2005; Vanacova et al. 2005; Wyers et al. 2005). Low level CUTs could be hard to be detected, unless there is a defect in the nuclear RNA decay pathway (Neil et al. 2009; Xu et al. 2009). In fission yeast, the cenRNA has a high turnover rate and can only be detected in exosome mutants (Choi et al. 2011). An increase of a ~ 1.2 kb transcript derived from CEN3 is also reported in budding yeast lacking Trf4 (Houseley et al. 2007). We tested if budding yeast cenRNAs are CUTs by deletion of the exosome component RRP6, or TRF4 in the TRAMP complex. Our result showed that there is an accumulation of cenRNAs in rrp6 or trf4 deletion mutants (Fig. 2), suggesting that budding yeast cenRNAs are CUTs that are degraded by the nuclear RNA decay pathway. Cryptic transcription is often described as transcription noise, and CUTs, being quickly degraded, have been proposed to have little function. However, the direct epigenetic role of CUTs, such as gene trans-silencing (Berretta et al. 2008) and histone modification (Camblong et al. 2007), have been reported. Identifying cenRNAs as CUTs suggested that CUTs are not simply junk RNA produced from transcription noise and it would be exciting to see more functions of CUTs being uncovered in the future.
Budding yeast cenRNAs are cryptic unstable transcripts (CUTs). Expression of cenRNA1, 3 and 8 was analyzed by RT-qPCR in the rrp6 and trf4 deletion mutants. The relative expression of the RNAs was normalized to UBC6 expression. Statistical significances of the expression level (mean ± SD, n = 3) were analyzed with paired t test (*P ≤ 0.05 and **P ≤ 0.01)
Although cenRNA is in low abundance, cenRNA is proposed to regulate the formation of kinetochore. In regional centromeres, cenRNA interacts with kinetochore proteins CENP-A (Quenet and Dalal 2014; Topp et al. 2004), CENP-C (Du et al. 2010; Wong et al. 2007), and components of the chromosomal passenger complex (CPC): Aurora-B, Survivin and INCENP (Ferri et al. 2009; Ideue et al. 2014; Wong et al. 2007). Knockdown of cenRNA in regional centromeres resulted in mitotic defects (Ideue et al. 2014; McNulty et al. 2017; Quenet and Dalal 2014; Rosic et al. 2014). It is proposed that cenRNA acts as a scaffold to regulate kinetochore protein assembly, and a stable kinetochore complex may require a precise amount of cenRNA (Fig. 3). In cbf1 htz1 double deletion mutant in which cenRNA is highly expressed, we found a decrease of chromatin localization of CENP-ACse4, CENP-CMif2, and Aurora-BIpl1, suggesting a disruption of the kinetochore complex (Ling and Yuen 2019).
CenRNA in point centromere acts in trans
Fluorescence in situ hybridization (FISH) experiments showed that cenRNAs in human (McNulty et al. 2017) and Drosophila (Bobkov et al. 2018) regional centromere are localized in cis. On the other hand, cenRNAs from Xenopus regional centromeres are trans-acting (Blower 2016). In point centromeres, we do not know yet if cenRNAs are localized to the cis centromeres, but functionally, cenRNAs work in trans, as knockdown all cenRNAs from the endogenous chromosomes, but not the cenRNA from the minichromosome, still increased minichromosome loss (Ling and Yuen 2019). In budding yeast, centromeres are tethered by microtubules to a confined region near the spindle pole body during most of the cell cycle, resulting in centromere clustering (Jin et al. 2000; Kitamura et al. 2007). Furthermore, pericentromeric regions of multiple chromosomes are physically linked by condensin and cohesin (Stephens et al. 2013). When point centromeres are transcribed, there may be a local “cenRNA cloud” at the centromere cluster, allowing cenRNAs to interact with all centromeres and kinetochores. Across the 16 centromeres of the budding yeast, we found that some of the centromeres seem to produce more cenRNA than the others (Ling and Yuen 2019). It is tempting to hypothesize that the one with higher expression is the “dominant” cenRNA which supports the majority of the action.
Role of centromeric transcription per se
RNAPII transcription causes transient displacement of histones from the nucleosome (Kulaeva et al. 2007). The coupling of centromeric transcription to CENP-A loading may indicate a direct role of centromeric transcription on CENP-A dynamics in the chromatin. In regional centromeres, it is shown that centromeric transcription could facilitate histone exchange at the centromere to promote CENP-A incorporation (Bobkov et al. 2018; Chen et al. 2015). The low level of centromeric transcription may indicate that a single passage of RNAPII is sufficient to disrupt the chromatin for new CENP-A loading and a low level of transcription may favor the loading of CENP-A over histone H3 (Talbert and Henikoff 2018). To address the importance of centromeric transcription in point centromeres, we engineered a minichromosome with a centromere flanked by lac operon (lacO), and blocked centromeric transcription by expressing lac repressor (lacI). The result indicated that the stability of the minichromosome required centromeric transcription (Ling and Yuen 2019). One should note that blocking centromeric transcription in this experiment concomitantly inhibited cenRNA production. On the other hand, our RNAi knockdown experiment showed that even when the cis cenRNA is knocked down from a centromere, the trans cenRNAs from other centromeres could still support the function of that centromere. Therefore, the centromeric transcription activity per se, independent of the cenRNA, appeared to be required for proper centromere function. Further investigation is needed to determine if the centromeric transcription machinery or transcriptionally induced chromatin changes co-operate with cenRNA, CENP-A chaperone or other kinetochore protein to facilitate CENP-A loading.
Conclusion
Centromeric transcription is a conserved epigenetic mechanism crucial for proper function of regional centromeres in higher eukaryotes and point centromere in budding yeast S. cerevisiae. In point centromeres, centromeric transcription is repressed to a low level by the kinetochore protein Cbf1 and histone H2A variant H2A.ZHtz1, and is de-repressed during S phase, coupled with CENP-ACse4 loading. Centromeric transcription may promote histone exchange to facilitate CENP-ACse4 incorporation. At the same time, cenRNA could interact with kinetochore proteins to facilitate kinetochore complex assembly. CenRNAs can be degraded by nuclear exosome, resulting in a balanced low level of cenRNA that is required for maintaining optimal function of the point centromere.
References
Albert I, Mavrich TN, Tomsho LP, Qi J, Zanton SJ, Schuster SC, Pugh BF (2007) Translational and rotational settings of H2A.Z nucleosomes across the Saccharomyces cerevisiae genome. Nature 446:572–576. https://doi.org/10.1038/nature05632
Baker RE, Fitzgerald-Hayes M, O’Brien TC (1989) Purification of the yeast centromere binding protein CP1 and a mutational analysis of its binding site. J Biol Chem 264:10843–10850
Bergmann JH, Jakubsche JN, Martins NM, Kagansky A, Nakano M, Kimura H, Kelly DA, Turner BM, Masumoto H, Larionov V, Earnshaw WC (2012) Epigenetic engineering: histone H3K9 acetylation is compatible with kinetochore structure and function. J Cell Sci 125:411–421. https://doi.org/10.1242/jcs.090639
Berretta J, Pinskaya M, Morillon A (2008) A cryptic unstable transcript mediates transcriptional trans-silencing of the Ty1 retrotransposon in S. cerevisiae. Genes Dev 22:615–626. https://doi.org/10.1101/gad.458008
Blower MD (2016) Centromeric transcription regulates aurora-B localization and activation. Cell Rep 15:1624–1633. https://doi.org/10.1016/j.celrep.2016.04.054
Bobkov GOM, Gilbert N, Heun P (2018) Centromere transcription allows CENP-A to transit from chromatin association to stable incorporation. J Cell Biol 217:1957–1972. https://doi.org/10.1083/jcb.201611087
Bram RJ, Kornberg RD (1987) Isolation of a Saccharomyces cerevisiae centromere DNA-binding protein, its human homolog, and its possible role as a transcription factor. Mol Cell Biol 7:403–409
Buehl CJ, Kuo MH (2018) Critical roles of Shugoshin and histones as tension sensors during mitosis. Curr Genet 64:1215–1219. https://doi.org/10.1007/s00294-018-0846-4
Cai M, Davis RW (1990) Yeast centromere binding protein CBF1, of the helix-loop-helix protein family, is required for chromosome stability and methionine prototrophy. Cell 61:437–446
Camblong J, Iglesias N, Fickentscher C, Dieppois G, Stutz F (2007) Antisense RNA stabilization induces transcriptional gene silencing via histone deacetylation in S. cerevisiae. Cell 131:706–717. https://doi.org/10.1016/j.cell.2007.09.014
Candelli T, Challal D, Briand JB, Boulay J, Porrua O, Colin J, Libri D (2018) High-resolution transcription maps reveal the widespread impact of roadblock termination in yeast. EMBO J. https://doi.org/10.15252/embj.201797490
Catania S, Pidoux AL, Allshire RC (2015) Sequence features and transcriptional stalling within centromere DNA promote establishment of CENP-A chromatin. PLoS Genet 11:e1004986. https://doi.org/10.1371/journal.pgen.1004986
Chan FL, Marshall OJ, Saffery R, Kim BW, Earle E, Choo KH, Wong LH (2012) Active transcription and essential role of RNA polymerase II at the centromere during mitosis. Proc Natl Acad Sci USA 109:1979–1984. https://doi.org/10.1073/pnas.1108705109
Chen CC, Bowers S, Lipinszki Z, Palladino J, Trusiak S, Bettini E, Rosin L, Przewloka MR, Glover DM, O’Neill RJ, Mellone BG (2015) Establishment of centromeric chromatin by the CENP-A assembly factor CAL1 requires FACT-mediated transcription. Dev Cell 34:73–84. https://doi.org/10.1016/j.devcel.2015.05.012
Choi ES, Stralfors A, Castillo AG, Durand-Dubief M, Ekwall K, Allshire RC (2011) Identification of noncoding transcripts from within CENP-A chromatin at fission yeast centromeres. J Biol Chem 286:23600–23607. https://doi.org/10.1074/jbc.M111.228510
Dhatchinamoorthy K, Mattingly M, Gerton JL (2018) Regulation of kinetochore configuration during mitosis. Curr Genet 64:1197–1203. https://doi.org/10.1007/s00294-018-0841-9
Du Y, Topp CN, Dawe RK (2010) DNA binding of centromere protein C (CENPC) is stabilized by single-stranded RNA. PLoS Genet 6:e1000835. https://doi.org/10.1371/journal.pgen.1000835
Ferri F, Bouzinba-Segard H, Velasco G, Hube F, Francastel C (2009) Non-coding murine centromeric transcripts associate with and potentiate Aurora B kinase. Nucleic Acids Res 37:5071–5080. https://doi.org/10.1093/nar/gkp529
Furuyama S, Biggins S (2007) Centromere identity is specified by a single centromeric nucleosome in budding yeast. Proc Natl Acad Sci USA 104:14706–14711. https://doi.org/10.1073/pnas.0706985104
Greaves IK, Rangasamy D, Ridgway P, Tremethick DJ (2007) H2A.Z contributes to the unique 3D structure of the centromere. Proc Natl Acad Sci USA 104:525–530. https://doi.org/10.1073/pnas.0607870104
Hill A, Bloom K (1987) Genetic manipulation of centromere function. Mol Cell Biol 7:2397–2405
Hou H, Wang Y, Kallgren SP, Thompson J, Yates JR 3rd, Jia S (2010) Histone variant H2A.Z regulates centromere silencing and chromosome segregation in fission yeast. J Biol Chem 285:1909–1918. https://doi.org/10.1074/jbc.M109.058487
Houseley J, Kotovic K, El Hage A, Tollervey D (2007) Trf4 targets ncRNAs from telomeric and rDNA spacer regions and functions in rDNA copy number control. EMBO J 26:4996–5006. https://doi.org/10.1038/sj.emboj.7601921
Ideue T, Cho Y, Nishimura K, Tani T (2014) Involvement of satellite I noncoding RNA in regulation of chromosome segregation. Genes Cells 19:528–538. https://doi.org/10.1111/gtc.12149
Jin QW, Fuchs J, Loidl J (2000) Centromere clustering is a major determinant of yeast interphase nuclear organization. J Cell Sci 113(Pt 11):1903–1912
Kamakaka RT, Biggins S (2005) Histone variants: deviants? Genes Dev 19:295–310. https://doi.org/10.1101/gad.1272805
Kitamura E, Tanaka K, Kitamura Y, Tanaka TU (2007) Kinetochore microtubule interaction during S phase in Saccharomyces cerevisiae. Genes Dev 21:3319–3330. https://doi.org/10.1101/gad.449407
Kolaczkowski M, Kolaczkowska A, Gaigg B, Schneiter R, Moye-Rowley WS (2004) Differential regulation of ceramide synthase components LAC1 and LAG1 in Saccharomyces cerevisiae. Eukaryot Cell 3:880–892. https://doi.org/10.1128/EC.3.4.880-892.2004
Kulaeva OI, Gaykalova DA, Studitsky VM (2007) Transcription through chromatin by RNA polymerase II: histone displacement and exchange. Mutat Res 618:116–129. https://doi.org/10.1016/j.mrfmmm.2006.05.040
LaCava J, Houseley J, Saveanu C, Petfalski E, Thompson E, Jacquier A, Tollervey D (2005) RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell 121:713–724. https://doi.org/10.1016/j.cell.2005.04.029
Lechner J, Carbon J (1991) A 240 kd multisubunit protein complex, CBF3, is a major component of the budding yeast centromere. Cell 64:717–725
Ling YH, Yuen KWY (2019) Point centromere activity requires an optimal level of centromeric noncoding RNA. Proc Natl Acad Sci USA 116:6270–6279. https://doi.org/10.1073/pnas.1821384116
McNulty SM, Sullivan LL, Sullivan BA (2017) Human centromeres produce chromosome-specific and array-specific alpha satellite transcripts that are complexed with CENP-A and CENP-C. Dev Cell 42(226–240):e226. https://doi.org/10.1016/j.devcel.2017.07.001
Mellor J, Jiang W, Funk M, Rathjen J, Barnes CA, Hinz T, Hegemann JH, Philippsen P (1990) CPF1, a yeast protein which functions in centromeres and promoters. EMBO J 9:4017–4026
Meneghini MD, Wu M, Madhani HD (2003) Conserved histone variant H2A.Z protects euchromatin from the ectopic spread of silent heterochromatin. Cell 112:725–736
Nakagawa T, Okita AK (2019) Transcriptional silencing of centromere repeats by heterochromatin safeguards chromosome integrity. Curr Genet. https://doi.org/10.1007/s00294-019-00975-x
Neil H, Malabat C, d’Aubenton-Carafa Y, Xu Z, Steinmetz LM, Jacquier A (2009) Widespread bidirectional promoters are the major source of cryptic transcripts in yeast. Nature 457:1038–1042. https://doi.org/10.1038/nature07747
Ohkuni K, Kitagawa K (2011) Endogenous transcription at the centromere facilitates centromere activity in budding yeast. Curr Biol 21:1695–1703. https://doi.org/10.1016/j.cub.2011.08.056
Pezer Z, Ugarkovic D (2008) RNA Pol II promotes transcription of centromeric satellite DNA in beetles. PLoS One 3:e1594. https://doi.org/10.1371/journal.pone.0001594
Pluta AF, Mackay AM, Ainsztein AM, Goldberg IG, Earnshaw WC (1995) The centromere: hub of chromosomal activities. Science 270:1591–1594
Quenet D, Dalal Y (2014) A long non-coding RNA is required for targeting centromeric protein A to the human centromere. Elife 3:e03254. https://doi.org/10.7554/eLife.03254
Rosic S, Kohler F, Erhardt S (2014) Repetitive centromeric satellite RNA is essential for kinetochore formation and cell division. J Cell Biol 207:335–349. https://doi.org/10.1083/jcb.201404097
Stephens AD, Snider CE, Haase J, Haggerty RA, Vasquez PA, Forest MG, Bloom K (2013) Individual pericentromeres display coordinated motion and stretching in the yeast spindle. J Cell Biol 203:407–416. https://doi.org/10.1083/jcb.201307104
Talbert PB, Henikoff S (2018) Transcribing centromeres: noncoding RNAs and kinetochore assembly. Trends Genet 34:587–599. https://doi.org/10.1016/j.tig.2018.05.001
Thomas D, Jacquemin I, Surdin-Kerjan Y (1992) MET4, a leucine zipper protein, and centromere-binding factor 1 are both required for transcriptional activation of sulfur metabolism in Saccharomyces cerevisiae. Mol Cell Biol 12:1719–1727
Topp CN, Zhong CX, Dawe RK (2004) Centromere-encoded RNAs are integral components of the maize kinetochore. Proc Natl Acad Sci USA 101:15986–15991. https://doi.org/10.1073/pnas.0407154101
Vanacova S, Wolf J, Martin G, Blank D, Dettwiler S, Friedlein A, Langen H, Keith G, Keller W (2005) A new yeast poly(A) polymerase complex involved in RNA quality control. PLoS Biol 3:e189. https://doi.org/10.1371/journal.pbio.0030189
Westhorpe FG, Straight AF (2014) The centromere: epigenetic control of chromosome segregation during mitosis. Cold Spring Harb Perspect Biol 7:a015818. https://doi.org/10.1101/cshperspect.a015818
Wong LH, Brettingham-Moore KH, Chan L, Quach JM, Anderson MA, Northrop EL, Hannan R, Saffery R, Shaw ML, Williams E, Choo KH (2007) Centromere RNA is a key component for the assembly of nucleoproteins at the nucleolus and centromere. Genome Res 17:1146–1160. https://doi.org/10.1101/gr.6022807
Wyers F, Rougemaille M, Badis G, Rousselle JC, Dufour ME, Boulay J, Regnault B, Devaux F, Namane A, Seraphin B, Libri D, Jacquier A (2005) Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell 121:725–737. https://doi.org/10.1016/j.cell.2005.04.030
Xu Z, Wei W, Gagneur J, Perocchi F, Clauder-Munster S, Camblong J, Guffanti E, Stutz F, Huber W, Steinmetz LM (2009) Bidirectional promoters generate pervasive transcription in yeast. Nature 457:1033–1037. https://doi.org/10.1038/nature07728
Zhang H, Roberts DN, Cairns BR (2005) Genome-wide dynamics of Htz1, a histone H2A variant that poises repressed/basal promoters for activation through histone loss. Cell 123:219–231. https://doi.org/10.1016/j.cell.2005.08.036
Acknowledgements
This research was funded by the Hong Kong Research Grants Council General Research Fund (Project Number: 17113418) and Collaborative Research Fund (Project Number: C7058-18GF), and the University of Hong Kong Seed Funds for Basic Research (Project Numbers: 201311159169 and 201509159021).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kupiec.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ling, Y.H., Yuen, K.W.Y. Centromeric non-coding RNA as a hidden epigenetic factor of the point centromere. Curr Genet 65, 1165–1171 (2019). https://doi.org/10.1007/s00294-019-00988-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-019-00988-6