Abstract
Mitochondrial biogenesis and functions rely on transport of their resident proteins as well as small molecules/ions across their membranes. The TOM complex functions as a protein entry gate for most mitochondrial proteins and mitochondrial porin facilitates transport of small-molecule metabolites and ions. We recently found a novel role of porin in regulation of the TOM complex assembly, the dynamic exchange between the dimer and trimer, and different substrate specificities of the dimer and trimer. Using distinct assembly forms customized for different client proteins, the TOM complex can handle ~ 1000 different mitochondrial protein for their import into mitochondria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
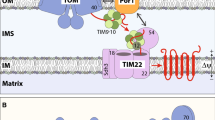
Most mitochondrial proteins are synthesized in the cytosol, and are imported into mitochondria via the entry gate, the TOM complex in the mitochondrial outer membrane (OM). The TOM complex consists of the channel-forming β-barrel protein Tom40 and α-helical proteins, i.e. the receptor subunits (Tom20, Tom22 and Tom70) and small TOM proteins (Tom5, Tom6 and Tom7). The TOM complex forms primarily the trimeric structure, which has three pores of Tom40 tethered by the three transmembrane helices of Tom22 (Shiota et al. 2015). The TOM complex trimer undergoes a dynamic conversion or exchange with a minor population of the dimer containing two Tom40 pores, but lacking Tom22 (Fig. 1).
Model of the trimer–dimer conversion of the TOM complex by porin. The TOM complex dimer contains two pores of Tom40 without Tom22, and the TOM complex trimer contains three pores of Tom40 tethered by three Tom22 molecules. Free Tom22 dissociated from the TOM complex trimer is stabilized by Por1, so that Por1 promotes the trimer to dimer conversion. Tom6 promotes the dimer-to-trimer conversion of the TOM complex. Most preproteins use the TOM complex trimer to cross the OM, while the TIM40/MIA pathway substrates cross the OM via the TOM complex dimer
The TOM complex dimer is identical to the previously identified assembly intermediate of newly synthesized and imported Tom40 (Model et al. 2001). After crossing the OM via the TOM complex, Tom40 is first engaged with the TOB/SAM complex in the OM from the intermembrane space (IMS) to form a β-barrel structure. Then Tom40 is replaced by another β-barrel OM protein Mdm10 to form a pre-assembly TOM complex (Yamano et al. 2010), which corresponds to the TOM complex dimer lacking Tom22. This intermediate becomes matured to the trimeric complex by incorporating Tom22. Therefore, the TOM complex dimer formed by the trimer–dimer exchange can function as a template or scaffold to replace the defective Tom40 molecule in the trimeric complex by the newly imported functional Tom40 molecule, thereby contributing to the quality control of the multi-subunit TOM complex (Shiota et al. 2015).
Mitochondrial porin is a conserved voltage-dependent anion channel (VDAC), which in yeast is called Por1. Although the TOM complex mediates vectorial import of mitochondrial preproteins and Por1 facilitates bidirectional transport of small-molecule metabolites and ions, the relationship between the TOM complex and porin was not obvious. Two recent studies including ours revealed the novel functional cooperation of the two channels, the TOM complex and porin (Sakaue et al. 2019; Ellenrieder et al. 2019). We found that, upon overexpression of Tom22 in vivo, unassembled Tom22 was associated with Por1 and that in vitro assembly of imported Tom22 into the TOM complex was accelerated in the absence of Por1 (Sakaue et al. 2019). These observations suggest that Por1 transiently “chaperones” free Tom22, so that trimer formation of the TOM complex is partially suppressed, and the presence of a minor but distinct fraction of the dimer is secured.
What is the physiological role of Por1-mediated stabilization of free Tom22 and resultant and substantial shift of the TOM complex to the dimer? Although the TOM complex dimer is a minor form, depletion of Por1 further shifted the dimer to the trimer. Under this por1Δ and culturing in fermentation medium conditions, while in vitro import of most mitochondrial preproteins was not affected, import of cysteine-rich IMS preproteins was markedly impaired (Sakaue et al. 2019). It was previously reported that these IMS proteins, or TIM40/MIA import pathway substrates, cross the OM via the TOM complex containing Tom40 but not Tom22 (Gornicka et al. 2014), and then undergo oxidative folding or disulfide-bond formation in the IMS with the aid of Tim40/Mia40. Given this, as Por1 depletion leads to complete loss of free Tom22 and significant decrease in the dimeric TOM complex, the import inhibition of the TIM40/MIA pathway substrates would be due to the substantial failure in the dynamic exchange of the trimeric TOM complex with the dimeric complex. In other words, the dimeric TOM complex could function as the entry gate specially customized for a subset of mitochondrial preproteins, TIM40/MIA pathway substrates. We speculate that, since Tom22 interacts with the TIM23 complex via Tim50 to achieve efficient transfer of the TIM23 pathway substrates, disengaging the TIM23 complex, by release of Tom22 from the TOM complex upon the trimer-to-dimer conversion, would remove a steric hindrance to the passage of proteins from the TOM complex to Tim40/Mia40 in the IMS (Fig. 1).
Our finding gave a clue to understanding of how the TOM complex is capable of handling ~ 1000 different mitochondrial precursors with different chemical as well as structural properties. By making on-demand use of the two different TOM complex assemblies, the dimer and trimer, customized for different client proteins, the TOM complex can achieve efficient transfer of proteins to different intra-mitochondrial sorting pathways. How TIM40/MIA pathway substrates discriminate the dimer and trimer of the TOM complex is an essential question to be addressed in future studies. Query ID="Q1" Text="No queries."
References
Ellenrieder L, Dieterle MP, Doan KN, Mårtensson CU, Floerchinger A, Campo ML, Pfanner N, Becker T (2019) Dual role of mitochondrial Porin in metabolite transport across the outer membrane and protein transfer to the inner membrane. Mol Cell 73:1056–1065
Gornicka A, Bragoszewski P, Chroscicki P, Wenz L-S, Schultz C, Rehling P, Chacinska A (2014) A discrete pathway for the transfer of intermembrane space proteins across the outer membrane of mitochondria. Mol Biol Cell 25:3999–4009
Model K, Meisinger C, Prinz T, Wiedemann N, Truscott KN, Pfanner N, Ryan MT (2001) Multistep assembly of the protein import channel of the mitochondrial outer membrane. Nat Struct Biol 8:361–370
Sakaue H, Shiota T, Ishizaka N, Kawano S, Tamura Y, Tan KS, Imai K, Motono C, Hirokawa T, Taki K, Miyata N, Kuge O, Lithgow T, Endo T (2019) Porin associates with Tom22 to regulate the mitochondrial protein gate assembly. Mol Cell 73:1044–1055
Shiota T, Imai K, Qiu J, Hewitt VL, Tan K, Shen H-H, Sakiyama N, Fukasawa Y, Hayat S, Kamiya M, Elofsson A, Tomii K, Horton P, Wiedemann N, Pfanner N, Lithgow T, Endo T (2015) Molecular architecture of the active mitochondrial protein gate. Science 349:1544–1548
Yamano K, Tanaka-Yamano S, Endo T (2010) Mdm10 as a dynamic constituent of the TOB/SAM complex directs coordinated assembly of Tom40. EMBO Rep 11:187–193
Acknowledgements
This work was supported by JSPS KAKENHI to T.E. (15H05705 and 2222703) and a JST CREST grant to T.E. (JPMJCR12M1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kupiec.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sakaue, H., Endo, T. Regulation of the protein entry gate assembly by mitochondrial porin. Curr Genet 65, 1161–1163 (2019). https://doi.org/10.1007/s00294-019-00979-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-019-00979-7