Abstract
The Neurospora crassa gene NcZrg-17 encodes a membrane protein with homology to the cation diffusion facilitator (CDF) family of transporters. We analyzed the phenotypic and functional characteristics of ΔNcZrg-17 and the implications of these characteristics in vivo. The ΔNcZrg-17 mutant showed several phenotypes that are zinc suppressible such as reduced growth rate, short aerial hyphae, increased hyphal branching, early and enhanced conidiation and delayed conidial germination. Furthermore, the NcZrg-17 gene was found to be crucial for survival in the presence of endoplasmic reticulum (ER) stress inducing chemical agents. In addition, we found that ΔNcZrg-17 mutant is defective in protein secretion on cellulose media under low zinc conditions, pointing towards a physiological role for NcZrg-17 in N. crassa. A gradual and delayed transcriptional upregulation (~ threefold) of NcZrg-17 on exposure to low zinc suggests its role in adaptation to low zinc rather than zinc homeostasis. Together our findings support a function of NcZrg-17 in normal vegetative growth, tolerance to ER stress and degradation of cellulose under low zinc conditions in N. crassa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zinc is a micronutrient serving both a structural and catalytic roles in several important biological processes, including gene expression, mitosis and cell division, DNA and protein synthesis. Thus, stringent regulation of cellular zinc transport systems is vital for cell survival (Fukada and Kambe 2011). The zinc-regulated transporter (ZRT)/iron-regulated transporter (IRT)-like protein (ZIP) and the cation diffusion facilitator (CDF) are two distinct zinc-specific transporter families which co-ordinate cellular Zn2+ homeostasis (Eide et al. 1996; Guerinot 2000; Zhao and Eide 1996). In contrast to the ZIP family members transporting zinc across the plasma membrane or intracellular organelles into the cytoplasm, the CDF family of transporters exports Zn2+ from the cytoplasm to the extracellular space or into intracellular organelles.
The cation diffusion facilitator (CDF, TC 2.A.4) transporters span all the three kingdoms of life (Nies 2003). The CDF family members are mostly transition metal cation Me2+ (Zn2+, Co2+, Fe2+, Cd2+ or Ni2+)/H+(K+) antiporters. Early phylogenetic analysis of CDF family members sorted the transporters into three major clusters, Zn-CDF, Fe/Zn-CDF and Mn-CDF, and an out-group, Zrg17-like cluster (Montanini et al. 2007). The systematic study of zinc transport in the yeast leads to the identification of several zinc transporters in plants and animal systems. In yeast, several CDF members have been characterized including Msc2 (meiotic-sister chromatid recombination 2), Cot1 (Cobalt Toxicity 1), Zrc1 (zinc resistance conferring 1) and Zrg17 (zinc-regulated gene 17). Zrc1 and Cot1 localize to the vacuolar membrane and are believed to sequester zinc in the vacuole (MacDiarmid et al. 2003) and both are essential for ER function (Ellis et al. 2004).
Among the various zinc-regulated genes (ZRGs) identified using a genetic screen in budding yeast, Zrg17 was hypothesized to be involved in zinc uptake (Yuan 2000). ZRG17 is a direct Zap1 target gene and its regulation is vital for maintaining ER function (Wu et al. 2011). In addition, the Zrg17p and Msc2p form a heteromeric zinc transport complex in the ER membrane and are involved in supply of zinc to the secretory pathway (Ellis et al. 2005). In fission, yeast Cis4 forms a heteromeric functional complex with Zrg17 to regulate Golgi membrane trafficking by controlling the zinc homeostasis (Fang et al. 2008).
Although the yeast model system is extensively used for the study of metal ion transporters, N. crassa offers several additional opportunities due to its multicellularity and various life cycle stages. Out of the seven putative zinc transporter genes of N. crassa belonging to the ZIP family, two genes, tzn-1 and tzn-2 have been investigated so far (Kiranmayi et al. 2009). Mutation of tzn-1 gene resulted in an aconidiation phenotype. However, none of the nine predicted CDF family transporter genes of N. crassa has been studied till date. In the N. crassa genome, four putative genes, trm-25 (NCU03145), trm-29 (NCU07262), trm-32 (NCU07709) and NcZrg-17 (NCU01254) encode CDF proteins that are structurally related to zinc transporters in other species (Kiranmayi and Mohan 2006). The Zrg17 cluster appears to be specific to the Ascomycetes (Montanini et al. 2007). In this study, we explored the role of the NcZrg-17 gene in the development and physiology of the model filamentous fungi N. crassa. We report in this paper that the NcZrg-17 gene has a role in vegetative growth, ER stress tolerance and cellulose degradation under low zinc conditions in N. crassa.
Materials and methods
N. crassa strains and culture conditions
Growth, crossing, and maintenance of Neurospora strains were essentially as described previously (Davis and de Serres 1970). The N. crassa strains used in this study are listed in Table 1. The NcZrg-17 knockout strain was generated by the Neurospora genome project (Colot et al. 2006; McCluskey et al. 2010) and was obtained from the Fungal Genetics Stock Center (FGSC, Manhattan, KS). The strain was confirmed by PCR amplification (Fig S5). The N. crassa cultures were grown in basal medium (BM) (Mohan and Sastry 1983) for vegetative growth and synthetic crossing medium (SCM) was used for crossing (Westergaard and Mitchell 1947). Strains were grown in BM liquid or BM solid medium supplemented with 1.5% agar. Zinc was supplied as ZnCl2 in BM at a concentration of 3 µM unless otherwise mentioned. Low zinc basal medium (LZBM) was prepared by supplementation of 1× BM salts devoid of zinc with 1 mM EDTA. LZBM supplemented with 1, 10 or 100 µM ZnCl2 are represented as LZBM1, LZBM10 or LZBM100, respectively. All chemicals were of analytical grade and were purchased from Sigma Aldrich (Bangalore, India).
The radial colony extension rate of the strains was measured by inoculating conidial suspension in the center of the BM-agar plates (90 mm diameter petri dish) and incubated at 30 °C. The colony edge was marked every 2 h between 12 and 24 h. The colony area occupied by wild type and mutant strain was measured using ImageJ (v.1.50i) (Schneider et al. 2012). For each time point extension rate was determined and mean extension rate of duplicate samples calculated (n = 4 for each test condition). Growth rate is represented as percentage growth of wild type on BM. To study the growth of aerial hyphae, 2 µl of ~ 1 × 106 conidia of N. crassa strains was inoculated in test tubes containing 1 ml of the indicated media with 2% agar and incubated for 2 days in dark followed by 1 day in light at 30 °C, cultures were then photographed, and aerial hyphae height was measured. For cell wall and septa visualization, wild type and ΔNcZrg-17 strains were grown on thin layer of BM solidified with 1.5% agarose on a glass coverslip and a drop of Calcofluor White M2R solution (Sigma) was applied to the samples and viewed with a Zeiss Axioscope epifluorescence microscope. For the time course study of conidial development, 1 ml BM-agar slants were inoculated with ~ 1 × 106 cells/ml conidia in a 7-ml tube and incubated at 30 °C for 132 h. At 12 h intervals after inoculation in BM slants, conidia were harvested using sterile water followed by the conidial counting using a hemocytometer. The time length (GT50) required for conidia to achieve 50% germination was calculated by the examination of the percent germination over the time of incubation, which were spread with the aliquots of 100 μl conidial suspension and examined at 2 h intervals during 24 h incubation at 30 °C.
Complementation of ΔNcZrg-17
The 2016 bp DNA fragment of the NcZrg-17 open reading frame was amplified by PCR using genomic DNA as a template. The N. crassa genomic DNA was extracted using a QIAamp DNA mini kit (QIAGEN). The PCRs were performed using Phusion high fidelity DNA polymerase (Thermo Fisher Scientific, MA, USA) with the SmaI site-conjugated primers (forward primer, 5′-ATGGAGCCCCATACTGGCGC-3′; reverse primer, 5′-CGTAGAATCAGTACTCATCTGC-3′). The resulting PCR product was digested using SmaI and cloned into the SmaI restriction sites of pRS426-pan2-V5-GFP (Pnit-6::5xGly::V5::gfp) vector (Ouyang et al. 2015) that resulted in pSN-5. This construct was transformed into the ΔNcZrg-17 recipient using standard procedure (Vann 1995). Transformants were selected on sorbose-containing medium (FGS) supplemented with basta (400 µg/ml) and pantothenate (10 µg/ml), and initial heterokaryotic transformants were crossed with the opposite mating type of the ΔNcZrg-17 mutant strain to isolate the homokaryotic strain Pn-NcZrg-17. The homokaryotic strain was verified using primer pairs to verify the presence of the NcZrg-17 allele at both the endogenous and exogenous locus. The exogenous integration of NcZrg-17 was verified using the forward primers Zrg-17-KOC-F specific for upstream of the open reading frame of gene NcZrg-17 and with the common reverse primer 5HPHR that is specific for the hph cassette used to generate the knockout mutants (Colot et al. 2006; Deka et al. 2011). Similarly, the endogenous copy of the NcZrg-17 was verified using allele-specific primer pairs Zrg-17-F and Zrg-17-R. Amplification of PCR products of size 1301 and 2016 bp indicates the presence of the NcZrg-17 allele in the homokaryotic strain at the endogenous and exogenous locus, respectively (Fig S6).
Stress tolerance assays
The response of ∆NcZrg-17 to abiotic stress was analyzed on BM supplemented with known chemical stress inducers. Cell wall stress was induced using ionic detergent sodium dodecyl sulfate (SDS) at 0.01% (w/v), glycan-binding agent Congo red (CR) at 400 µg/ml or glycan and chitin binding agent Calcofluor white (CFW) at 300 µg/ml (Hill et al. 2006; Arias et al. 2011). Two kinds of ER stress-causing agents were used: either 5 mM dithiothreitol (DTT) that prevents disulfide bond formation, or 50 µg/ml tunicamycin (TN) that inhibits N-linked glycosylation (Fan et al. 2015; Montenegro-Montero et al. 2015). Oxidative stress was induced using 24 mM hydrogen peroxide. Salt stress was induced using 1 M sodium chloride (NaCl) and osmotic stress was induced using 1 M sorbitol and percent survival was determined. Conidia of wild type and ΔNcZrg-17 were inoculated at a concentration of ~ 1 × 106 conidia/ml into liquid BM and germinated with shaking at 180 rpm for 2 h in the dark at 30 °C. Germlings were supplemented with indicated chemicals (test) or without (control) and further germinated for 1 h at 30 °C. Germlings were plated on sorbose-BM (to induce colonial growth) and incubated at 30 °C for 24 h, and percent survival was scored using the formula (the number of viable colonies from test plates)/(the number of colonies from control plates) × 100. The percentage survival of ∆NcZrg-17 was expressed relative to wild-type survival under individual test conditions. Thermotolerance assays were performed as described previously (Barman and Tamuli 2015).
Isolation of RNA and real-time PCR
RNA was isolated from N. crassa as described previously (Korripally et al. 2010) and was used to synthesize cDNA using cDNA Synthesis Kit (Thermo Scientific, MA, USA) according to the manufacture’s protocol. Real-time PCR was performed using the gene-specific primers (Table S1) with the SYBR® Select Master Mix (Applied Biosystems, CA, USA) in an ABI 7500 Fast Real-Time PCR System (Applied Biosystems, CA, USA). The \({{\text{2}}^{ - \Delta \Delta {{\text{C}}_T}}}\) quantification method (Livak and Schmittgen 2001) was used to calculate the fold change expression of the genes, using the gpd-1 gene as an endogenous control and wild type as calibrator.
Protein estimation
The amount of proteins in culture supernatants per condition was measured using the Bradford assay (HiMedia), using 100 μl of culture supernatant. The total amount of mycelial proteins was determined as described earlier (Tiwari et al. 2011).
Statistical analysis
Raw data were initially processed in Excel and then imported to RStudio (v. 1.1.383, Rstudio Team 2015) and plotted using ggplot2 (Wickham 2009). One-way ANOVA and post hoc analysis were performed using the Multcomp package (http://cran.r-project.org/web/packages/multcomp/index.html).
Results
NcZRG-17 belongs to the CDF family of heavy metal transporters
The NcZrg-17 gene encodes a 671-amino acid protein that exhibited approximately 30% amino acid identity in a BLAST analysis (https://blast.ncbi.nlm.nih.gov/Blast.cgi; Altschul et al. 1990) to the Saccharomyces cerevisiae ScZrg17. Despite the lack of primary sequence homology, NcZRG-17 and ScZrg17 share similar hydropathic plots (Fig. 1a). Some of the predicted topological and conserved features of CDF family (Montanini et al. 2007) shared between NcZRG-17 and ScZrg17 include: six transmembrane domains (TMDs), localization of both the amino- and carboxyl-terminal ends on the cytosolic surface of cellular membranes, conserved LxxxD and TxxxS/T/A motifs in the TMD2 and TMD5 for the metal transport and a conserved glycine residue close to the beginning of TMD3 (Fig. 1b). NcZRG-17 does not share the distinct histidine-rich domain (...HDHDEINEQIPHSH…) of ScZrg17 present in between TMDs III and IV. Instead, NcZRG-17 has an overall higher content of histidine residues (15%) in the loop than the ScZrg17 (9%). The percent of total charged and polar amino acids in the loop between TMDs III and IV in NcZRG-17 and ScZrg17 is 52 and 63%, respectively (Fig. 1c).
Hydropathic analysis and predicted topology of NcZRG-17. a Aligned hydropathic plots of ScZrg17 and NcZRG-17 generated in the BioEdit Sequence Alignment Editor software, version 7 (Hall 1999) using the Kyte–Doolittle algorithm (Kyte and Doolittle 1982) with a window size of 15. b Predicted topology map of NcZRG-17 obtained with the Protter program (Omasits et al. 2013). The putative TMDs are labeled with Roman numerals. The conserved LxxxD and TxxxS/T/A motifs in the TMD2 and TMD5 as well as a conserved glycine residue close to the beginning of TMD3 are represented as white letters on a black background. Polar amino acids in the loop present in between TMDs III and IV toward the ER lumen are shown in gray background. c The amino acid sequence of loop present in between TMDs III and IV of ScZrg17 and NcZRG-17 by ClustalW algorithm (Larkin et al. 2007). The histidine-rich domain of ScZrg17 is shown in italic. Identical residues are highlighted in gray background. Red, blue and black texts indicate charged, polar and hydrophobic amino acids, respectively
Effect of NcZrg-17 in the developmental phases of N. crassa
On minimal medium (BM-agar plate), ∆NcZrg-17 forms a compact slow growing; crenulated mycelial mat (top panel; Fig. 2a). After about 24 h fluffy aerial hyphae appear along the colony edges, which fill the plate rapidly and yield a dense conidiation pattern (bottom panel; Fig. 2a). Under zinc-limiting condition (LZBM1), ∆NcZrg-17 growth is severely retarded in comparison to the wild type. However, this phenotype could be suppressed by supplementation with 100 µM ZnCl2 (Fig. 2a, b). Further supplementation with ZnCl2 restores the ∆NcZrg-17 growth to a maximum of ~ 50% of wild type (not shown). In addition, adding other metals up to 1000 µM concentrations (Fe, Cu, Mn, Co, Cd) did not increase growth rate of either wild type or ∆NcZrg-17 in LZBM1 indicating that these cells are specifically limited for zinc (not shown). Similar results were obtained when the strains were grown in yeast extract-peptone-dextrose (YPD) medium, a complex medium (not shown). In LZBM1, ∆NcZrg-17 fails to form aerial hyphae; supplementation with 100 µM ZnCl2 partially restores the height of ∆NcZrg-17 aerial hyphae to wild type levels (Fig. 2c). The lateral branching frequency of ∆NcZrg-17 hyphae at the colony periphery was ~ fourfold higher than wild type and the branching angles were reduced in the extension zone of mutant colonies (Fig. 2d). Also, the ∆NcZrg-17 was less likely to develop the branching pattern seen in the wild type, which involves a few large leading hyphae with subsidiary branches. The ∆NcZrg-17 conidiate prematurely and also conidiation is twofold of wild type (Fig. 2a, e). The conidial germination of ∆NcZrg-17 in LZBM1 is delayed (~ threefold of wild type); supplementation with 100 µM ZnCl2 restores the growth rate to wild type levels (Fig. 2f). The role of the NcZrg-17 gene in the sexual development of N. crassa was evaluated by testing the ability of ∆NcZrg-17 mutant to form perithecia. A confrontation cross between opposite mating types of the ∆NcZrg-17 mutant produced perithecia comparable to the wild-type cross. Also, ∆NcZrg-17 mutant was fertile both as a female and male parent (not shown). The complemented strain exhibited wild-type phenotypes for all functions tested.
Phenotypic characterisation of NcZrg-17 mutant and the complemented strain, Pn-NcZrg-17. a Colony morphology of the wild type, the NcZrg-17 gene knockout mutant (ΔNcZrg-17) and the homozygous ΔNcZrg-17 mutant complemented with a NcZrg-17-gfp gDNA under the control of the nit-6 promoter (Pn-NcZrg-17). The photographs were taken after 24 and 96 h (upper and lower panels, respectively) of incubation on LZBM1, LZBM100 and BM plates at 30 °C. b Growth rate of the wild type, ΔNcZrg-17 and Pn-NcZrg-17 strains (black, gray and light gray bars, respectively) on indicated media at 30 °C are expressed as percent control of wild-type growth on BM. c Aerial hyphae of the strains in the indicated media. Strains were incubated in dark at 30 °C for 3 days and under light at room temperature for 4 day. d Low magnification Calcofluor stained fluorescent images of the colony edge of the indicated strains. e Time length required for 50% conidial germination (GT50) on BM plates. Black, gray and light gray bars represent wild type, ΔNcZrg-17 and Pn-NcZrg-17 strains, respectively. f Time course of conidial development of the indicated strains. For a clearer visualization, conidial development till 60 h is shown as an inset. All phenotypic assays were performed at least three independent times with similar results. Error bars indicate standard deviations calculated from the data for three independent experiments (n = 3) with P values < 0.05 (asterisk), < 0.01 (double asterisk), and < 0.001 (triple asterisk) compared with the wild type strain as measured by one-way ANOVA test
Functional evaluation of NcZrg-17
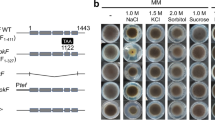
To examine whether ∆NcZrg-17 was defective in response to abiotic stress, the wild type and ∆NcZrg-17 strains were exposed to cell wall, ER, oxidative, salt, osmotic and thermal stress in BM. The survival of ∆NcZrg-17 was reduced by ~ 35% of wild type on exposure to ER stress inducing chemicals DTT and tunicamycin (Fig. 3a). A moderate decrease in survival of ∆NcZrg-17 (~ 25% of wild type) was observed on exposure to hydrogen peroxide. Other stress conditions including thermal stress appear to influence ∆NcZrg-17 survival similar to wild type (Fig. 3a, b). To further establish the role of NcZrg-17 in coping with ER stress in the natural environment, we evaluated the growth ∆NcZrg-17 on cellulose, a natural substrate. We found that the growth of ∆NcZrg-17 on cellulose as the sole carbon source is dramatically impaired under zinc-limiting conditions (Fig. 3c). Also when wild type and ∆NcZrg-17 mycelia pre-grown in BM were transferred to cellulose media supplemented with a range of zinc concentrations and the protein concentration from culture supernatants was quantified it revealed that ∆NcZrg-17 was severely impaired in protein secretion under zinc-limiting conditions (Fig. 3d). The amount of protein secreted by ∆NcZrg-17 on glucose media appeared similar to the wild type (not shown).
Functional evaluation of NcZrg-17 in the physiology of N. crassa. a Chemical-induced stress assays were performed as described in Materials and Methods and percent survival determined. SDS sodium dodecyl sulfate, CR congo red, CFW Calcofluor white, DTT dithiothreitol, TN tunicamycin, PER hydrogen peroxide, NaCl sodium chloride, SBT sorbitol. b Viability of 2 h old germlings after exposure to 52 °C lethal temperature with (induced; black bars) or without (uninduced; light gray bars) pre-exposure to a sublethal heat shock temperature of 44 °C. c Conidia of wild type, ΔNcZrg-17 and Pn-NcZrg-17 (black, gray and light gray, respectively) were inoculated on BM-agar with glucose or cellulose as sole carbon source (2% w/v). Plates were placed for 3 days at 30 °C in constant dark and then grown for an additional 24 h in constant light at 30 °C before imaging, except with the cellulose plates, which were photographed after 6 days under the same conditions. d The protein concentration from wild type, ΔNcZrg-17 and Pn-NcZrg-17 (black, gray and light gray, respectively) culture supernatants grown for 5 days under a range of zinc concentrations in LZBM with cellulose as a sole carbon source, was normalized to the total amount of mycelial proteins per condition, and is expressed relative to the ratio exhibited by the wild-type growing on BM-glucose. All phenotypic assays were performed at least three independent times with similar results. Error bars indicate standard deviations calculated from the data for three independent experiments (n = 3) with P values < 0.05 (asterisk), < 0.01 (double asterisk), and < 0.001 (triple asterisk) compared with the wild-type strain as measured by one-way ANOVA test
NcZrg-17 is regulated in a zinc-dependent manner
The level of expression of NcZrg-17 by real-time PCR revealed that NcZrg-17 was expressed at similar levels across the various lifecycle stages (Fig. 4a). To determine further the function and regulation of NcZrg-17, the effect of Zn2+ availability on the level of NcZrg-17 mRNA was investigated. Compared with cells grown under control conditions (BM), NcZrg-17 transcript levels were increased in response to zinc deficiency (> threefold increase) and was restored to basal levels on further zinc supplementation (Fig. 4b). Moreover, the increase in expression of NcZrg-17 in response to low zinc occurred only after prolonged incubation in low zinc (Fig. 4c).
Quantitative real-time PCR analysis of NcZrg-17. a Expression of NcZrg-17 during the N. crassa lifecycle. Total RNA was isolated from cultures of wild type strain and subjected to quantitative RT-PCR analysis as described in Materials and Methods. The tissues used were the following: G, 5-day-old conidia inoculated into liquid BM at a concentration of 1 × 106 cells/ml and incubated for 3 h days with shaking at 200 rpm in dark at 30 °C (germlings); V: conidia were inoculated in 50 ml conical flask containing 10 ml of basal medium and cultured in the dark at 30 °C with agitation at 160 rpm for 2 days (vegetative mycelia); S, cultures grown for 7 days on SCM plates at 25 °C under light (protoperithecial cultures); and P, SCM plate cultures 3 days after fertilization with wild-type conidia (perithecial cultures). b NcZrg-17 gene expression under a long-term exposure to zinc. Total RNAs were extracted from wild-type mycelia developed in LZBM medium supplemented with 1, 10, 100 or 1000 µM Zn2+. c NcZrg-17 gene expression under a short-term exposure to zinc. Total RNAs were extracted from wild-type mycelia cultured in standard conditions for 24 h, collected, washed and resuspended in LZBM1 for 10, 60, 180, and 360 min to LZBM1. The fold change indicates the NcZrg-17 genes expression in test condition over the expression of genes in dormant fresh conidia
Discussion
In the CDF family of transporters only, the Zrg17 cluster is specific for Ascomycetes (Montanini et al. 2007). Most of our understanding about the Zrg17 cluster comes from studies of ScZrg17 of S. cerevisiae. The present study is the first report elucidating the role of a CDF family member of N. crassa (Fig. S1) and indeed of a filamentous fungal member of the Zrg17 cluster. Three lines of evidence support the hypothesis that NcZrg-17 belongs to zinc-CDF family. First, NcZRG-17 is structurally similar to the budding yeast zinc transporter ScZrg17. Second, the deletion of NcZrg-17 resulted in several phenotypes that are zinc suppressible. Third, the expression of NcZrg-17 is regulated by zinc levels.
The vegetative phase of NcZrg-17 mutant exhibits various phenotypic defects, whereas the sexual phase remains largely unaffected. The various morphological phenotypes of ∆NcZrg-17 such as reduced growth rate, short aerial hyphae, increased hyphal branching, increased and early conidiation and delayed conidial germination suggest the importance of NcZrg-17 in the various developmental stages of the model filamentous fungus. The phenotypic defects of the ∆NcZrg-17 mutant could be complemented by constitutive activation of NcZrg-17, but only partially suppressed by supplementation of the media with zinc, suggesting that NcZrg-17 is functionally non-redundant with other closely related zinc transporters such as NcMsc-2.
The NcZrg-17 mutant exhibited an increased sensitivity to hydrogen peroxide-induced oxidative stress (~ 25% reduced survival compared to wild type). Further, supplementation of cellulose media with N-acetyl-cysteine (NAC; antioxidant which can diminish oxidative stress) improved the protein secretion across a range of zinc concentrations (Fig. S3). It is known that ER stress and oxidative stress are closely linked events (Malhotra and Kaufman 2007), and therefore antioxidants could decrease ER stress and improve protein secretion (Malhotra et al. 2008). The NcZrg-17 mutant is not sensitive to cell wall stress inducing agents which appear to agree with an earlier report, which proposed that cell wall and ER stress responses are uncoupled in N. crassa (Montenegro-Montero et al. 2015), unlike studies in other filamentous fungi (Malavazi et al. 2014).
Transcript levels of NcZrg-17 did not exhibit any stage-specific peaks of expression though phenotypic defects appeared to be prominent in vegetative stages rather than in the sexual stages. The expression of NcZrg-17 responded to zinc levels, being upregulated under zinc-deplete conditions and down-regulated under zinc-replete conditions. Further, transcript levels of NcZrg-17 were specifically induced by Zn2+, since the addition of other metals (Mn, Fe, Cu and Cd) did not significantly alter the transcript levels of NcZrg-17 (not shown). A significant upregulation of NcZrg-17 expression was observed only after 3 h of shifting the culture from high zinc to low zinc condition, suggesting role of NcZrg-17 in adaptation to zinc deficiency rather than playing a role in zinc homeostasis. These results suggest that NcZrg-17 mRNA levels may be regulated in a manner similar to ScZrg17 (Wu et al. 2008) in a Zap1-dependent manner (Wu et al. 2011). However, analysis of the upstream region of NcZrg-17 did not reveal any 11 bp-binding sites known as zinc-responsive elements (ZREs) for Zap1 (Fig. S2), suggesting a different mechanism for regulation of NcZrg-17 expression. Some of the predicted upstream regulatory elements in NcZrg-17 include antioxidant response element (ARE), stress response element (STRE) and calcineurin-dependent response element (CDRE) (Fig. S2).
Although various mutually non-exclusive hypotheses may be suggested to account for the mechanistic basis for the secretory defect of ∆NcZrg-17 in cellulose but not glucose under low zinc, the most intuitive explanation would be that a secretory defect would only be observable when a particular threshold in ER secretory load is exceeded and under our experimental conditions, such a threshold appears during growth on cellulose under low zinc conditions, a normal growth condition encountered by N. crassa in its natural environment. Support for this hypothesis come from our results that the growth on glucose-containing media under low or high zinc condition or growth on cellulose media under high zinc conditions stimulated a mild-to-moderate UPR induction in both the wild type and ∆NcZrg-17 strains, whereas growth on cellulose media under low zinc conditions resulted in a marked induction of UPR genes in ∆NcZrg-17 (Fig S4). These results are consistent with previous reports suggesting that four CDF transporters Msc2, Zrc1, Cot1 and Zrg17 are associated with ER zinc acquisition in yeast (Ellis et al. 2004, 2005), zinc is required for ER function in eukaryotes (Ellis et al. 2004) and that growth on cellulose triggers the UPR in N. crassa (Benz et al. 2014; Montenegro-Montero et al. 2015).
Although the precise subcellular localization of NcZrg-17 could not be determined, a significant fraction of the GFP fluorescence appears to co-localize with the nuclear stain (Fig S7), a pattern reminiscent of the ER localization. In S. cerevisiae, immunoblotting analysis of subcellular fraction suggested that ScZrg17 protein was primarily localized to the ER (Ellis et al. 2005).
The formation of the heteromeric functional complex for zinc transport in the eukaryotic secretory pathway appears to be an evolutionarily conserved mechanism (Ellis et al. 2005; Suzuki et al. 2005a, b; Ishihara et al. 2006). The ∆NcMsc-2 mutant also exhibited growth defects at low zinc which were suppressible by the addition of zinc (our unpublished data). Further, molecular analysis is needed to confirm the interaction between NcZRG-17 and NcMSC-2. In conclusion, it is shown that NcZrg-17 plays a role in the developmental stages of N. crassa and is physiologically relevant in supplying zinc for ER function for cellulose degradation under low zinc. This work adds to previous studies in Neurospora and other fungi presenting the importance of membrane transporters in numerous physiological and developmental processes (Fukada and Kambe 2011; Beseli et al. 2015). The current work in the laboratory is focused on the interrelations between the CDF genes and specific zinc-dependent targets required for development.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Arias P, Díez-Muñiz S, García R, Nombela C, Rodríguez-Peña JM, Arroyo J (2011) Genome-wide survey of yeast mutations leading to activation of the yeast cell integrity MAPK pathway: novel insights into diverse MAPK outcomes. BMC Genom 12:1
Barman A, Tamuli R (2015) Multiple cellular roles of Neurospora crassa plc-1, splA2, and cpe-1 in regulation of cytosolic free calcium, carotenoid accumulation, stress responses, and acquisition of thermotolerance. J Microbiol 53:226–235
Benz JP, Chau BH, Zheng D, Bauer S, Glass NL, Somerville CR (2014) A comparative systems analysis of polysaccharide-elicited responses in Neurospora crassa reveals carbon source-specific cellular adaptations. Mol Microbiol 91:275–299
Beseli A, Amnuaykanjanasin A, Herrero S, Thomas E, Daub ME (2015) Membrane transporters in self resistance of Cercospora nicotianae to the photoactivated toxin cercosporin. Curr Genet 61:601–620
Colot HV, Park G, Turner GE, Ringelberg C, Crew CM, Litvinkova L, Weiss RL, Borkovich KA, Dunlap JC (2006) A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc Natl Acad Sci 103:10352–10357
Davis RH, de Serres FJ (1970) Genetic and microbiological research techniques for Neurospora crassa. Methods Enzymol 17:79–143
Deka R, Kumar R, Tamuli R (2011) Neurospora crassa homologue of Neuronal Calcium Sensor-1 has a role in growth, calcium stress tolerance, and ultraviolet survival. Genetica 139(7):885–894
Eide D, Broderius M, Fett J, Guerinot ML (1996) A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci 93:5624–5628
Ellis CD, Wang F, MacDiarmid CW, Clark S, Lyons T, Eide DJ (2004) Zinc and the Msc2 zinc transporter protein are required for endoplasmic reticulum function. J Cell Biol 166:325–335
Ellis CD, MacDiarmid CW, Eide DJ (2005) Heteromeric protein complexes mediate zinc transport into the secretory pathway of eukaryotic cells. J Biol Chem 280:28811–28818
Fan F, Ma G, Li J, Liu Q, Benz JP, Tian C, Ma Y (2015) Genome-wide analysis of the endoplasmic reticulum stress response during lignocellulase production in Neurospora crassa. Biotechnol Biofuels 8:1
Fang Y, Sugiura R, Ma Y, Yada-Matsushima T, Umeno H, Kuno T (2008) Cation diffusion facilitator Cis4 is implicated in Golgi membrane trafficking via regulating zinc homeostasis in fission yeast. Mol Biol Cell 19:1295–1303
Fukada T, Kambe T (2011) Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics 3:662–674
Guerinot ML (2000) The ZIP family of metal transporters. Biochim Biophys Acta (BBA) Biomembr 1465:190–198
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In: Nucleic acids symposium series, pp 95–98
Hill TW, Loprete DM, Momany M, Ha Y, Harsch LM, Livesay JA, Mirchandani A, Murdock JJ, Vaughan MJ, Watt MB (2006) Isolation of cell wall mutants in Aspergillus nidulans by screening for hypersensitivity to Calcofluor White. Mycologia 98:399–409
Ishihara K, Yamazaki T, Ishida Y, Suzuki T, Oda K, Nagao M, Yamaguchi-Iwai Y, Kambe T (2006) Zinc transport complexes contribute to the homeostatic maintenance of secretory pathway function in vertebrate cells. J Biol Chem 281:17743–17750
Kiranmayi P, Mohan PM (2006) Metal transportome of Neurospora crassa. In Silico Biol 6:169–180
Kiranmayi P, Tiwari A, Sagar KP, Haritha A, Maruthi Mohan P (2009) Functional characterization of tzn1 and tzn2-zinc transporter genes in Neurospora crassa. Biometals 22:411–420
Korripally P, Tiwari A, Haritha A, Kiranmayi P, Bhanoori M (2010) Characterization of Ctr family genes and the elucidation of their role in the life cycle of Neurospora crassa. Fungal Genet Biol 47:237–245
Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157:105–132
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R (2007) Clustal W and clustal X version 2.0. Bioinformatics 23:2947–2948
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
MacDiarmid CW, Milanick MA, Eide DJ (2003) Induction of the ZRC1 metal tolerance gene in zinc-limited yeast confers resistance to zinc shock. J Biol Chem 278:15065–15072
Malavazi I, Goldman GH, Brown NA (2014) The importance of connections between the cell wall integrity pathway and the unfolded protein response in filamentous fungi. Brief Funct Genom 13:456–470
Malhotra JD, Kaufman RJ (2007) Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal 9:2277–2294
Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, Kaufman RJ (2008) Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci 105:18525–18530
McCluskey K, Wiest A, Plamann M (2010) The fungal genetics stock center: a repository for 50 years of fungal genetics research. J Biosci 35:119
Mohan PM, Sastry KS (1983) Interrelationships in trace-element metabolism in metal toxicities in nickel-resistant strains of Neurospora crassa. Biochem J 212:205–210
Montanini B, Blaudez D, Jeandroz S, Sanders D, Chalot M (2007) Phylogenetic and functional analysis of the cation diffusion facilitator (CDF) family: improved signature and prediction of substrate specificity. BMC Genom 8:107
Montenegro-Montero A, Goity A, Larrondo LF (2015) The bZIP transcription factor HAC-1 is involved in the unfolded protein response and is necessary for growth on cellulose in Neurospora crassa. PLoS One 10:e0131415
Nies DH (2003) Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol Rev 27:313–339
Omasits U, Ahrens CH, Müller S, Wollscheid B (2013) Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics 30:884–886
Ouyang S, Beecher CN, Wang K, Larive CK, Borkovich KA (2015) Metabolic impacts of using nitrogen and copper-regulated promoters to regulate gene expression in Neurospora crassa. G3 Genes Genomes Genet 5:1899–1908
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Suzuki T, Ishihara K, Migaki H, Ishihara K, Nagao M, Yamaguchi-Iwai Y, Kambe T (2005a) Two different zinc transport complexes of cation diffusion facilitator proteins localized in the secretory pathway operate to activate alkaline phosphatases in vertebrate cells. J Biol Chem 280:30956–30962
Suzuki T, Ishihara K, Migaki H, Matsuura W, Kohda A, Okumura K, Nagao M, Yamaguchi-Iwai Y, Kambe T (2005b) Zinc transporters, ZnT5 and ZnT7, are required for the activation of alkaline phosphatases, zinc-requiring enzymes that are glycosylphosphatidylinositol-anchored to the cytoplasmic membrane. J Biol Chem 280:637–643
RStudio Team (2015) RStudio: integrated Development for R. RStudio, Inc., Boston, MA. http://www.rstudio.com/
Tiwari A, Korripally P, Adhikarla H, Patnala K, Pamarthi MM, Bhanoori M (2011) Expression and functional characterisation of TNC, a high-affinity nickel transporter from Neurospora crassa. Fungal Genet Biol 48:1020–1026
Vann DC (1995) Electroporation-based transformation of freshly harvested conidia of Neurospora crassa. Fungal Genet Newsl A 42:53
Westergaard M, Mitchell HK (1947) Neurospora V. A synthetic medium favoring sexual reproduction. Am J Bot 34:573
Wickham H (2009) Introduction. In: ggplot2. Springer, pp 1–7
Wu C-Y, Bird AJ, Chung LM, Newton MA, Winge DR, Eide DJ (2008) Differential control of Zap1-regulated genes in response to zinc deficiency in Saccharomyces cerevisiae. BMC Genom 9:1
Wu YH, Frey AG, Eide DJ (2011) Transcriptional regulation of the Zrg17 zinc transporter of the yeast secretory pathway. Biochem J 435:259–266
Yuan DS (2000) Zinc-regulated genes in Saccharomyces cerevisiae revealed by transposon tagging. Genetics 156:45–58
Zhao H, Eide D (1996) The ZRT2 gene encodes the low affinity zinc transporter in Saccharomyces cerevisiae. J Biol Chem 271:23203–23210
Acknowledgements
Funding for this research project was provided by Science and Engineering Research Board (SERB), New Delhi (Grant no YSS/2014/000174). A.T. is supported by SERB Start-up Grant (Young Scientist).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kupiec.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tiwari, A., Ngiilmei, S.D. & Tamuli, R. The NcZrg-17 gene of Neurospora crassa encodes a cation diffusion facilitator transporter required for vegetative development, tolerance to endoplasmic reticulum stress and cellulose degradation under low zinc conditions. Curr Genet 64, 811–819 (2018). https://doi.org/10.1007/s00294-017-0794-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-017-0794-4