Abstract
The precise and controlled regulation of gene expression at transcriptional and post-transcriptional levels is crucial for the eukaryotic cell survival and functions. In eukaryotes, more than 100 types of post-transcriptional RNA modifications have been identified. The N6-methyladenosine (m6A) modification in mRNA is among the most common post-transcriptional RNA modifications known in eukaryotic organisms, and the m6A RNA modification can regulate gene expression. The role of yeast m6A methyltransferase (Ime4) in meiosis, sporulation, triacylglycerol metabolism, vacuolar morphology, and mitochondrial functions has been reported. Stress triggers triacylglycerol accumulation as lipid droplets. Lipid droplets are physically connected to the different organelles such as endoplasmic reticulum, mitochondria, and peroxisomes. However, the physiological relevance of these physical interactions remains poorly understood. In yeast, peroxisome is the sole site of fatty acid β-oxidation. The metabolic status of the cell readily governs the number and physiological function of peroxisomes. Under low-glucose or stationary-phase conditions, peroxisome biogenesis and proliferation increase in the cells. Therefore, we hypothesized a possible role of Ime4 in the peroxisomal functions. There is no report on the role of Ime4 in peroxisomal biology. Here, we report that IME4 gene deletion causes peroxisomal dysfunction under stationary-phase conditions in Saccharomyces cerevisiae; besides, the ime4Δ cells showed a significant decrease in the expression of the key genes involved in peroxisomal β-oxidation compared to the wild-type cells. Therefore, identification and determination of the target genes of Ime4 that are directly involved in the peroxisomal biogenesis, morphology, and functions will pave the way to better understand the role of m6A methylation in peroxisomal biology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The precise transcriptional and post-transcriptional regulations of gene expression are crucial for the eukaryotic cell survival and functions. The RNA-binding proteins play an important role in post-transcriptional regulation by controlling the mRNA levels (Jin and Neiman 2016; Berchowitz et al. 2013). In eukaryotes, availability of specific metabolites such as S-adenosylmethionine (SAM) and cellular growth phase also regulates metabolic status and cellular functions (Saint-Marc et al. 2015; Chavez et al. 2016). SAM is the sole methyl donor for the methylation of histones, nucleic acids, and phospholipids (Ding et al. 2015). In eukaryotes, unlike protein and DNA post-synthetic modifications, RNA modifications are not well studied, and our knowledge about their function and biological relevance is limited (Schwartz et al. 2013; Blanco and Frye 2014). The deposition of N6-methyladenosine (m6A) into mRNA is among the most common post-transcriptional RNA modifications known in eukaryotes (Schwartz et al. 2013; Yue et al. 2015). N6-Adenosyl methyltransferases that introduce m6A into mRNA are found to be present in almost all kingdoms of eukaryotic life (Yue et al. 2015; Dominissini et al. 2012). Since the m6A methyltransferases are fundamentally conserved, the budding yeast Saccharomyces cerevisiae is used as a model organism to understand the physiological significance of m6A methylation.
Like protein and DNA modifications, m6A RNA modification is also governed by the growth phase and metabolic status of the cells. Maintenance of metabolic status of the cells under low-glucose or stationary-phase conditions requires energy homeostasis. As fatty acid catabolism via β-oxidation provides energy in response to low-glucose or stationary-phase conditions. It is obvious that biogenesis and proliferation of organelle involved in β-oxidation may increase under glucose-limiting or stationary-phase conditions. In yeast, peroxisomes are the sole site of fatty acid β-oxidation (Hiltunen et al. 2003; Manzanares-Estreder et al. 2017; Pascual-Ahuir et al. 2017). In yeast, the metabolic status of the cell readily governs the number and physiological function of peroxisomes. Under low-glucose or stationary-phase conditions, peroxisome biogenesis and proliferation increase in cells (Rajvanshi et al. 2017; Lefevre et al. 2013). Under high-glucose conditions, both number and functions of yeast peroxisomes are low, while in the presence of a non-fermentable carbon source, peroxisomal proliferation takes place rapidly (Sibirny 2016). Peroxisomal function and adaptation in response to different stresses such as salt stress and sugar limitation are crucial for the cell survival (Manzanares-Estreder et al. 2017; Pascual-Ahuir et al. 2017). Sugar limitation triggers a metabolic shift from fermentation to respiration that is fuelled by the elevated peroxisomal oxidation of internally stored fatty acids.
The expression of lipid droplets- and peroxisomes-localized long-chain fatty acyl–CoA synthetase (Faa1) increases upon stress conditions (Manzanares-Estreder et al. 2017). Among four long-chain acyl–CoA synthetases Faa1–Faa4, Faa1 is the major contributor, accounting for ≥ 90% of the activity (Black and DiRusso 2007). Faa1 supplies the acyl–CoA for triacylglycerol (TAG) biosynthesis; besides, Faa1 is required for the mobilization of fatty acids from lipid droplets (LDs). In the previous study, we have shown that yeast m6A methyltransferase Ime4 epitranscriptionally regulates TAG accumulation and formation of LDs through its target FAA1. IME4 gene deletion causes a significant increase in the expression of the FAA1 gene (Yadav and Rajasekharan 2017a). Different stresses such as nutrient stress, glucose limitation, and stationary-phase conditions cause TAG accumulation in LDs (Yadav and Rajasekharan 2016a; Horvath et al. 2011). Several studies have shown that LDs are physically connected to the endoplasmic reticulum, mitochondria, and peroxisomes (Martin and Parton 2006; Goodman 2008; Murphy et al. 2009). However, the physiological relevance of these contacts remains poorly understood. Recently, we have shown that IME4 gene deletion produces mitochondrial dysfunction (Yadav and Rajasekharan 2017b). Upon stress, the number of peroxisomes attached to the mitochondria increases (Manzanares-Estreder et al. 2017). In addition, studies have shown that Ime4 has stationary-phase-related functions when glucose is used up (Yadav and Rajasekharan 2017a; Hongay et al. 2006). Therefore, keeping the above points in mind, we hypothesize a possible role of Ime4 in the peroxisomal functions.
In the present study, we report that IME4 gene deletion causes a significant decrease in number of peroxisomes. Our data showed that IME4 gene deletion causes reduced expression of important genes involved in peroxisomal fatty acid β-oxidation under stationary growth phase conditions. In addition, we have demonstrated that like transcriptional regulators (Msn2/Msn4) of peroxisome biogenesis and function (Rajvanshi et al. 2017), deletion of epitranscriptional regulator Ime4 causes the accumulation of free fatty acids (FFA) due to the defective peroxisomal β-oxidation under stationary-phase conditions. Like peroxisomal β-oxidation-defective strains, the ime4Δ strain is found to be sensitive to fatty acid toxicity. It will be interesting to identify the target genes of Ime4 that are directly involved in the peroxisomal biogenesis, morphology, and functions.
The yeast m6A methyltransferase and peroxisomal functions
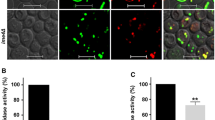
As glucose is being used up, the diauxic shift occurs followed by the stationary phase, involving an increase in the expression of nuclear genes responsible for peroxisome biogenesis and proliferation increase in cells (Rajvanshi et al. 2017; Lefevre et al. 2013). In yeast cells, fully developed and functional peroxisomes are characteristic features of stationary phase (Rajvanshi et al. 2017; Lockshon et al. 2007). Therefore, in the present work, the stationary-phase-grown yeast cells were harvested to understand the role of Ime4 in peroxisomal functions. The yeast deletion strains used in this study were procured from Euroscarf. The peroxisomal targeting pUG36-RFP-PTS1 plasmid used in this study is a kind gift from Professor Jeffrey E. Gerst, Department of Molecular Genetics, Weizmann Institute of Science, Israel. The primers used here, POX1 qRT (forward 5′ GCAGGAGAGAGGTGCCACTT 3′ and reverse 5′ CAGCCCTAGTCTGCAGCTGG 3′), FOX2 qRT (forward 5′ CTCCCAATGAACAAGGCTCAG 3′ and reverse 5′ CCAGATTGCATGAGACTTCCC′), and POT1 qRT (forward 5′ GGAGAGCGCCATGGGTAAG 3′ and reverse 5′ CGATGGCAGACCTGTTAGCA 3′) were the same as reported previously (Rajvanshi et al. 2017). The plasmid constructs, YEp357-POX1pro, YEp357-FOX2pro, and YEp357-POT1pro, were the same as reported previously (Rajvanshi et al. 2017). The growth and culture conditions were the same as reported previously (Rajvanshi et al. 2017; Yadav and Rajasekharan 2016b). Briefly, a single colony of the yeast strains was precultured in 5 ml of yeast extract peptone dextrose (YPD) liquid medium at 30 °C and subcultured in the required volume of synthetic minimal medium (SM) at 30 °C. The stationary-phase yeast cells grown in SM were harvested to assess the peroxisomal morphology and functions. For peroxisomal microscopic imaging, wild-type and ime4∆ cells were transformed with the pUG36-RFP-PTS1 construct, which has a PTS1-RFP sequence for peroxisomal localization. For microscopic imaging, a Zeiss LSM 700 confocal laser scanning microscope was used. Our RFP-PTS1 localization study showed that like peroxisomal-defective msn2Δmsn4Δ strain, the ime4Δ strain has a low number of peroxisomes (Fig. 1a). The quantitative real-time (qRT) analyses and β-galactosidase assays showed that IME4 gene deletion causes a significant decrease in the expression of key genes POX1, FOX2, and POT1 involved in peroxisomal β-oxidation (Fig. 1b–d). As in peroxisome-defective strains, the peroxisomal oxidation of internally stored fatty acids is reduced, and we hypothesized that peroxisome-defective strains might cause accumulation of FFA. Thin layer chromatographic (TLC) analyses showed that like peroxisomal-defective msn2Δmsn4Δ strain, the ime4Δ strain has an increased level of FFA compared to wild-type cells (Fig. 2a, b). The qRT analyses, β-galactosidase assays, and TLC analyses were performed as reported earlier (Yadav and Rajsekharan 2017a). Yeast strains that are defective in peroxisome functions and fatty acid metabolism are unable to grow fatty acid-supplemented medium (Rajvanshi et al. 2017; Lockshon et al. 2007; Faergeman et al. 2001). Therefore, we checked the effect of fatty acid toxicity on the ime4Δ strain. For fatty acid toxicity test, a single colony of wild-type or the ime4∆ strain was precultured in 5 ml of YPD medium and allowed to grow at 30 °C for 24 h with constant shaking. An equal amount of cells was taken from preculture and subcultured in 5 ml of SM medium containing 0.5 mM oleic acid and 0.4% glucose at 30 °C for 18 h. After adaptation in the liquid medium, an equal amount of cells (A 600 = 1) was taken, and cells were serially diluted and spotted on a SM agar plate having 0.5 mM oleic acid and 0.4% glucose. A 600 = 0.1, cells were used as neat and other dilutions were 1:10, 1:100, 1:1000, and 1:10,000. The plates were kept at 30 °C and images were taken on the 3rd and 8th day for glucose-grown and oleic acid + glucose-grown cells, respectively. Our data showed that ime4Δ strain is sensitive to fatty acid toxicity and exhibited a significant growth defect on oleic acid containing SM agar plate (Fig. 2c).
Effect of IME4 gene deletion on peroxisomal status and functions. a IME4 gene deletion and peroxisomal status. Stationary-phase yeast cells expressing peroxisomal targeting PTS1-RFP protein were imaged by a confocal microscope. The optimum brightness and contrast were adjusted in the images as per requirement. Merged superimposed panel of fluorescence and DIC (differential interference contrast), bar 5 μm, WT wild-type, PTS1-RFP pUG36-RFP-PTS1. b A schematic diagram representing the key genes POX1, FOX2, and POT1 involved in peroxisomal β-oxidation. c Compared to the WT strain, an analysis of the expression of the key genes involved in peroxisomal fatty acid β-oxidation in the ime4Δ strain. Yeast cells were harvested from the stationary phase, and gene expression analyses were performed. The horizontal dotted line represents the expression in the WT strain. The values are represented as fold changes. d Compared to the WT strain, β-galactosidase activities for the key genes involved in peroxisomal β-oxidation in the ime4Δ strain. Cells were collected, and β-galactosidase activity assays were performed. The obtained ime4Δ strain values were represented in comparison with the obtained WT values which were set to 100% (represented by the horizontal dotted line in the graph). The values are presented as the mean ± SEM (n = 3). Significance was determined at **p < 0.01
Effect of the IME4 gene on the free fatty acids accumulation and fatty acids toxicity. a, b Effect of peroxisomal fatty acid β-oxidation defects on the free fatty acids (FFA) content of the cells. In each TLC analysis, the lipids were extracted from the stationary-phase cells grown in the presence of [14C]acetate and were analyzed on a silica-TLC plate using the nonpolar lipids solvent system (petroleum ether: diethyl ether: acetic acid, 70:30:1, v/v) followed by phosphorimaging. The amount of FFA was determined relative to the obtained WT value which was set to 100%. WT wild-type, SE steryl esters, TAG triacylglycerols, FFA free fatty acids. The values are presented as the mean ± SEM (n = 3). Significance was determined at **p < 0.01. c Effect of lipotoxicity on the ime4Δ strain. For the spotting experiment, after adaptation, the equal amount of cells were collected, serially diluted, and spotted onto SM agar plate having 0.5 mM oleic acid and 0.4% glucose. d Schematic diagram depicting the role the of m6A methyltransferase in organelle functions. We propose that the m6A methyltransferases may play a key role in organelle biogenesis, morphology, and functions
Discussion and future perspectives
In the present study, we report that IME4 gene deletion causes peroxisomal dysfunction under stationary-phase conditions in S. cerevisiae. Identification and determination of the target genes of Ime4 that are directly involved in the peroxisomal biogenesis, morphology, and functions will pave the way to understand the role of m6A methylation in peroxisomal biology. The peroxisomes are dynamic organelles of the living eukaryotic cells. Low-glucose or stationary-phase conditions trigger peroxisome biogenesis and proliferation in yeast cells (Rajvanshi et al. 2017; Lefevre et al. 2013). Peroxisomal activity is an important event for the successful adaptation to different stresses such as salt stress, glucose limitation, and stationary-phase conditions. Glucose is the preferred carbon source and is required for exponential cell division. Glucose depletion triggers the yeast cells to enter into the diauxic shift followed by the stationary phase. Our work provides an evidence for the role of ime4 in maintaining energy homeostasis during carbon source starvation stress via regulation of the genes involved in peroxisomal fatty acid metabolism. Fatty acid catabolism via β-oxidation is an important process to maintain fatty acid homeostasis, but an excess free fatty acids are toxic to the cells. Defective fatty acid metabolism causes lipotoxicity in yeast (Rajvanshi et al. 2017; Lockshon et al. 2007; Faergeman et al. 2001). Therefore, the cells direct free fatty acids toward the synthesis of nonpolar lipids such as TAG and steryl esters (SE) to avoid fatty acid toxicity (Kohlwein 2010). Previously, we have reported that ime4Δ and msn2Δmsn4Δ strains are nonpolar lipids accumulators (Yadav and Rajsekharan 2017a; Rajvanshi et al. 2017). The present study also shows that fatty acid oxidation-defective strains are TAG and SE accumulators (Fig. 2a, b), which further validates the above finding.
Acylation of free fatty acids is an evolutionarily conserved process to avoid fatty acid toxicity (Petschnigg et al. 2009; Kurat et al. 2006). All organisms can sense different stresses and undergo many molecular changes that allow them to adapt and survive (Nostramo and Herman 2016; Ho and Gasch 2015). Any abnormality in the cell homeostasis triggers nutrient sensing and signaling pathways. Energy extraction and mobilization from different sources are vital processes for any organism; in fact, dedicated, evolutionary conserved pathways exist across eukaryotic organisms (Conrad et al. 2014; Efeyan et al. 2015). Any abnormalities in these pathways cause metabolic disorders. For example, any defect in glucose sensing and signaling processes causes diabetes and related diseases (Resnick and Howard 2002). Abnormalities in lipid sensing and signaling processes cause obesity and related diseases in eukaryotes. An abnormality in peroxisomal fatty acid β-oxidation causes peroxisomal disorders (Steinberg et al. 2006; Wierzbicki et al. 2002).
In our earlier studies, we have reported that IME4 gene deletion causes organelle dysfunction such as LDs-, vacuole-, and mitochondrial dysfunction (Yadav and Rajasekharan 2017a, b), while in the present study, IME4 gene deletion produces the peroxisomal dysfunction. Therefore, we propose that the m6A methyltransferases may play a key role in organelle biogenesis, morphology, and functions (Fig. 2d). The determination of the role of Ime4 and its targets in organelle biogenesis, morphology, and functions probably could help in the development of therapeutic strategies focused on the m6A methyltransferases.
References
Berchowitz LE, Gajadhar AS, van Werven FJ, De Rosa AA, Samoylova ML, Brar GA, Xu Y, Xiao C, Futcher B, Weissman JS, White FM, Amon A (2013) A developmentally regulated translational control pathway establishes the meiotic chromosome segregation pattern. Genes Dev 27:2147–2163. doi:10.1101/gad.224253.113
Black PN, DiRusso CC (2007) Yeast acyl-CoA synthetases at the crossroads of fatty acid metabolism and regulation. Biochim Biophys Acta 1771:286–298. doi:10.1016/j.bbalip.2006.05.003
Blanco S, Frye M (2014) Role of RNA methyltransferases in tissue renewal and pathology. Curr Opin Cell Biol 31:1–7. doi:10.1016/j.ceb.2014.06.006
Chavez S, Garcia-Martinez J, Delgado-Ramos L, Perez-Ortin JE (2016) The importance of controlling mRNA turnover during cell proliferation. Curr Genet 62:701–710. doi:10.1007/s00294-016-0594-2
Conrad M, Schothorst J, Kankipati HN, Van Zeebroeck G, Rubio-Texeira M, Thevelein JM (2014) Nutrient sensing and signaling in the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev 38:254–299. doi:10.1111/1574-6976.12065
Ding W, Smulan LJ, Hou NS, Taubert S, Watts JL, Walker AK (2015) s-Adenosylmethionine levels govern innate immunity through distinct methylation-dependent pathways. Cell Metab 22:633–645. doi:10.1016/j.cmet.2015.07.013
Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, Sorek R, Rechavi G (2012) Topology of the human and mouse m6A RNA methylomes revealed by m6A-sEq. Nature 485:201–206. doi:10.1038/nature11112
Efeyan A, Comb WC, Sabatini DM (2015) Nutrient-sensing mechanisms and pathways. Nature 517:302–310. doi:10.1038/nature14190
Faergeman NJ, Black PN, Zhao XD, Knudsen J, DiRusso CC (2001) The Acyl–CoA synthetases encoded within FAA1 and FAA4 in Saccharomyces cerevisiae function as components of the fatty acid transport system linking import, activation, and intracellular Utilization. J Biol Chem 276:37051–37059. doi:10.1074/jbc.M100884200
Goodman JM (2008) The gregarious lipid droplet. J Biol Chem 283:28005–28009. doi:10.1074/jbc.R800042200
Hiltunen JK, Mursula AM, Rottensteiner H, Wierenga RK, Kastaniotis AJ, Gurvitz A (2003) The biochemistry of peroxisomal beta-oxidation in the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev 27:35–64. doi:10.1016/S0168-6445(03)00017-2
Ho YH, Gasch AP (2015) Exploiting the yeast stress-activated signaling network to inform on stress biology and disease signaling. Curr Genet 61:503–511. doi:10.1007/s00294-015-0491-0
Hongay CF, Grisafi PL, Galitski T, Fink GR (2006) Antisense transcription controls cell fate in Saccharomyces cerevisiae. Cell 127:735–745. doi:10.1016/j.cell.2006.09.038
Horvath SE, Wagner A, Steyrer E, Daum G (2011) Metabolic link between phosphatidylethanolamine and triacylglycerol metabolism in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta 1811:1030–1037. doi:10.1016/j.bbalip.2011.08.007
Jin L, Neiman AM (2016) Post-transcriptional regulation in budding yeast meiosis. Curr Genet 62:313–315. doi:10.1007/s00294-015-0546-2
Kohlwein SD (2010) Triacylglycerol homeostasis: insights from yeast. J Biol Chem 285:15663–15667. doi:10.1074/jbc.R110.118356
Kurat CF, Natter K, Petschnigg J, Wolinski H, Scheuringer K, Scholz H, Zimmermann R, Leber R, Zechner R, Kohlwein SD (2006) Obese yeast: triglyceride lipolysis is functionally conserved from mammals to yeast. J Biol Chem 281:491–500. doi:10.1074/jbc.M508414200
Lefevre SD, van Roermund CW, Wanders RJ, Veenhuis M, van der Klei IJ (2013) The significance of peroxisome function in chronological aging of Saccharomyces cerevisiae. Aging cell 12:784–793. doi:10.1111/acel.12113
Lockshon D, Surface LE, Kerr EO, Kaeberlein M, Kennedy BK (2007) The sensitivity of yeast mutants to oleic acid implicates the peroxisome and other processes in membrane function. Genetics 175:77–91. doi:10.1534/genetics.106.064428
Manzanares-Estreder S, Espi-Bardisa J, Alarcon B, Pascual-Ahuir A, Proft M (2017) Multilayered control of peroxisomal activity upon salt stress in Saccharomyces cerevisiae. Mol Microbiol 104:851–868. doi:10.1111/mmi.13669
Martin S, Parton RG (2006) Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol 7:373–378. doi:10.1038/nrm1912
Murphy S, Martin S, Parton RG (2009) Lipid droplet–organelle interactions; sharing the fats. Biochim Biophys Acta 1791:441–447. doi:10.1016/j.bbalip.2008.07.004
Nostramo R, Herman PK (2016) Deubiquitination and the regulation of stress granule assembly. Curr Genet 62:503–506. doi:10.1007/s00294-016-0571-9
Pascual-Ahuir A, Manzanares-Estreder S, Timon-Gomez A, Proft M (2017) Ask yeast how to burn your fats: lessons learned from the metabolic adaptation to salt stress. Curr Genet. doi:10.1007/s00294-017-0724-5
Petschnigg J, Wolinski H, Kolb D, Zellnig G, Kurat CF, Natter K, Kohlwein SD (2009) Good fat, essential cellular requirements for triacylglycerol synthesis to maintain membrane homeostasis in yeast. J Biol Chem 284:30981–30993. doi:10.1074/jbc.M109.024752
Rajvanshi PK, Arya M, Rajasekharan R (2017) The stress-regulatory transcription factors Msn2 and Msn4 regulate fatty acid oxidation in budding yeast. J Biol Chem. doi:10.1074/jbc.M117.801704
Resnick HE, Howard BV (2002) Diabetes and cardiovascular disease. Annu Rev Med 53:245–267. doi:10.1146/annurev.med.53.082901.103904
Saint-Marc C, Hurlimann HC, Daignan-Fornier B, Pinson B (2015) Serine hydroxymethyltransferase: a key player connecting purine, folate and methionine metabolism in Saccharomyces cerevisiae. Curr Genet 61:633–640. doi:10.1007/s00294-015-0489-7
Schwartz S, Agarwala SD, Mumbach MR, Jovanovic M, Mertins P, Shishkin A, Tabach Y, Mikkelsen TS, Satija R, Ruvkun G, Carr SA, Lander ES, Fink GR, Regev A (2013) High-resolution mapping reveals a conserved, widespread, dynamic mRNA methylation program in yeast meiosis. Cell 155:1409–1421. doi:10.1016/j.cell.2013.10.047
Sibirny AA (2016) Yeast peroxisomes: structure, functions and biotechnological opportunities. FEMS Yeast Res. doi:10.1093/femsyr/fow038
Steinberg SJ, Dodt G, Raymond GV, Braverman NE, Moser AB, Moser HW (2006) Peroxisome biogenesis disorders. Biochim Biophys Acta 1763:1733–1748. doi:10.1016/j.bbamcr.2006.09.010
Wierzbicki AS, Lloyd MD, Schofield CJ, Feher MD, Gibberd FB (2002) Refsum’s disease: a peroxisomal disorder affecting phytanic acid alpha-oxidation. J Neurochem 80:727–735. doi:10.1046/j.0022-3042.2002.00766.x
Yadav KK, Rajasekharan R (2016a) The transcription factor GCN4 regulates PHM8 and alters triacylglycerol metabolism in Saccharomyces cerevisiae. Curr Genet 62:841–851. doi:10.1007/s00294-016-0590-6
Yadav PK, Rajasekharan R (2016b) Misregulation of a DDHD domain-containing lipase causes mitochondrial dysfunction in yeast. J Biol Chem 291:18562–18581. doi:10.1074/jbc.M116.733378
Yadav PK, Rajasekharan R (2017a) The m6A methyltransferase Ime4 epitranscriptionally regulates triacylglycerol metabolism and vacuolar morphology in haploid yeast cells. J Biol Chem 292:13727–13744. doi:10.1074/jbc.M117.783761
Yadav PK, Rajasekharan R (2017b) The m6A methyltransferase Ime4 and mitochondrial functions in yeast. Curr Genet. doi:10.1007/s00294-017-0758-8
Yue Y, Liu J, He C (2015) RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev 29:1343–1355. doi:10.1101/gad.262766.115
Acknowledgements
This work was supported by the Council of Scientific and Industrial Research (CSIR), New Delhi. The corresponding author is a recipient of the JC Bose National Fellowship. We are thankful to Professor Jeffrey E. Gerst, Department of Molecular Genetics, Weizmann Institute of Science, Israel for providing the pUG36-RFP-PTS1 construct. We are grateful to the Department of Biochemistry of the Indian Institute of Science in Bangalore for the use of their facility for the radioactive study.
Author information
Authors and Affiliations
Contributions
RR conceived and initiated the project. RR, PKY, and PKR designed the experiments. PKY and PKR executed the experiments and analyzed the data. PKY, PKR, and RR discussed the data and wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by M. Kupiec.
Rights and permissions
About this article
Cite this article
Yadav, P.K., Rajvanshi, P.K. & Rajasekharan, R. The role of yeast m6A methyltransferase in peroxisomal fatty acid oxidation. Curr Genet 64, 417–422 (2018). https://doi.org/10.1007/s00294-017-0769-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-017-0769-5