Abstract
The majority of the cell wall of a plant is composed of cellulose. Cellulose is an outstanding abundant, fibrous, and water-insoluble polymer on earth. The excellent hierarchical structure and semicrystalline nature of plant cellulose permit the easy isolation of nanofibers and nanocrystals through mechanically and chemically applied top-down destruction strategies. The cellulose molecules in nanocomposites can be separated into types such as bacterial nanocellulose (BNC), crystalline nanocellulose (CNC), and cellulose nanofibrils (CNF), which are biodegradable, environmentally friendly, and possess remarkably improved properties compared to conventional materials. Generally, they are deliberated as second-generation renewable resources, which assist as a superior replacement for petroleum-based materials. Research studies on nanocellulose are extensively accelerating due to petroleum-based materials issues like CO2 emissions, plastic based-pollution, and the absence of renewable energy. Research studies regarding these materials are interestingly increasing due to their outstanding properties such as biocompatibility, renewability, higher mechanical and lower density values, while sustainable production still associated with various challenges. Here, we comprehensively review the recent developments in nanocellulose production structural dimensions, properties, and applications, dedicated to drug delivery system, food industry, piezoelectric sensors, actuators, energy generators biosensing and bioimaging electronic devices.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The extensive and abundant polymer on Earth “Cellulose” is converted as new morphological and structural changes in the form of nanocellulose with the help of advanced and developed nanotechnology. Nanocellulose is a nanosized material found in a wide-range of diversity owing to excellent characteristics, produced from plants, bacteria, or animals [1]. Generally, nanocellulose is characterized into three forms such as cellulose nanocrystals (CNC), cellulose nanofibers (CNF), and bacterial nanocellulose (BNC) [2]. Cellulose is the primary part of the plant's cell wall, considered the world's supreme prevalent organic material because of its structural strength [3]. Usually, its extracted from trees such as flax, wheat straw, rice husk, cotton stalk, cotton fibers, hemp, bamboo, and jute [4, 5]. Cellulose comprises amorphous and crystalline regions of different ratios based on the sources of the raw material. Research shows that the surface morphology of native cellulose exists as a combination of two crystalline allomorphs, namely cellulose I alpha and II β [6, 7]. The existence and ability of hydroxyl groups to develop hydrogen bonds play a significant role in leading the processing of crystalline packages and concerned with cellulose's physical characteristics [8]. It has excellent properties, such as a larger surface area, a lower density, a higher aspect ratio, better mechanical properties, a low cost, and the ability to adapt to different surface characteristics, which make it an ideal material. The abundance of hydroxyl groups in nanocellulose makes surface modification easy. Since CNC have an excellent adsorptive property due to their high surface area, electrostatic interactions between nanoparticles and oxygen atoms of hydroxyl groups of cellulose caused nanoparticles to be adsorbed on CNC surfaces [9]. Nanocellulose can be functionalized in two ways: first, by oxidizing or cationizing its hydroxyl group in order to achieve UV barrier, antibacterial, high thermal, and antioxidant properties [10]. Additionally, a second technique uses nanocellulose as a template for forming nanoparticle hybrids, such as ZnO-NPs, Ag-NPs, CuO-NPs and Fe3O4-NPs that impart functional properties. Nanocellulose serves both as a template and as a capping agent for the preparation of hybrid nanocellulose/nanoparticles [11, 12]. Cellulose indirectly plays a big part in the human food chain. Various businesses, such as veterinary foods, wood and paper, strands and clothing, skincare items, and pharmaceuticals, also allow the versatile usage of this polymer [13, 14]. A number of current and existing nanocellulose uses in nanocomposites such as emulsifiers, wood adhesives, and evolving biomedical applications [15, 16]. The use of cellulose-based nanomaterials in wastewater treatment and environmental conservation has received considerable interest. [15]. In addition, in water remediation, primarily due to its reasonable cost, high availability, harmless handling process, large surface area, and high affinity for absorbing various contaminants [17]. The fact that cellulose is a safe, biodegradable material that has no harmful effects on humans or the environment is also noteworthy for security concerns, especially for extensive usage. Therefore cellulose-based nanomaterials have been extensively researched for their usage in many water treatments to fulfill water shortages across the globe [18]. A notable characteristic of nanoscale cellulose structure material is its highly explicit mechanical characteristics, superior hydrophilicity, and chemically modified surface functionality for enhanced adsorption. Nanocellulose also contains strong hydroxyl groups (–OH), which makes it a highly efficient surface engineering material. Several applications of this material include template support, self-governing functional material, and strengthening unit in hybrid materials have demonstrated its capacity to usage in environmental health sector. Further, covalent bonding, surface graft polymerization, and physical adsorption have been used to enhance its performance due to the presence of a large amount of reactive groups [19].
In spite of petroleum-based materials application in various industrial sectors, petro-derivatives don’t have biodegradable properties which limit their applications as compared to bio-based materials. So the usage of environmental friendly, compatible, biodegradable, and renewable polymers can assist the industries in a promising way due to their outstanding characteristics and advantages in contrast to petro-based materials as Table 1, giving a clear comparison. Recently, nanocelluloses are considered as an extensively used green material because of their fundamental characteristics, renewability, and abundance. Nanocellulose surface modification enables to the transformation of simple molecules into more complex polymer blends or composites for an outstanding utilization in several fields. Such as nanocellulose surface modification by hydroxyl groups has considerably improved its prospective to an inclusive range of applications. Nanocellulose based functional materials are produced by various modification techniques and have been used in food packaging, biosensing, and biomedical applications. Moreover, due to its renewability, biocompatibility, bioavailability, and different remarkable properties, nanocellulose (NC) has gained wide attention in food industry to save the food stuffs. Its rheological behavior and water absorption ability, crystallinity, and tunable surface chemistry, as well as its non-cytotoxicity and non-genotoxicity, make it suitable for food use. There are various commercial markets where nanocellulosic materials are extremely useful, such as packaging. These materials have distinctive characteristics which can substantially boost those markets. As a food stabilizing agent, dietary fiber, thickener, flavor carrier, suspension stabilizer, and calorie reducer, NC has a wide range of applications in food [20]. In addition to fillings, crushes, biscuits, cream, ice cream, chips, wafers, soups, and puddings, it can also be used to produce desserts and fillings. A food application could benefit from NC gel's good rheological properties. Due to its high viscosity, NC is an excellent food gelling agent and a non-caloric stabilizing agent. While used as nanofillers in packaging films, NC materials have great potential for sustainable improvement in tensile and barrier properties [21]. In addition to applications of nanocellulose in biosensing and electronics, its promising hybrids showed unique characteristics, including high mechanical strength, flexibility, stretching, shape memory effect, photo dynamics, photothermal activity, electrical conductivity, semi conductivity, thermal conductivity, optical transparency, intrinsic fluorescence and luminescence, and high filtration and adsorption. In recent studies, CNs have been advocated as green electrical components and their potential has been examined in organic diodes, smart papers, rechargeable lithium ion batteries, supercapacitors, and photovoltaic cells. Comparatively to plastic or silicone-based counterparts, they offer adequate pliability, low costs, light weight, and recyclability. There have been several studies on CNs' potential contribution to the construction of high surface area two-dimensional nanomembranes for storage devices and fuel cells. Therefore as a multifunctional nanocellulosic materials have the potential in several applications including wound dressings, tissue engineering, electrical stimulation of damaged tissues, biological molecule isolation, and drug delivery [22]. Many articles, book chapters, and reviews have been published on cellulose sources, nanocellulose extraction, properties, and applications. [23, 24]. In contrast, there is still a lack of literature on nanocellulose-based materials used in drug delivery systems (DDS), food packaging, and biosensing to diagnose various diseases [25]. Nanocellulose is effective in drug delivery system due to having a large surface area and high polymerization power, enabling it to provide maximum packaging and attaching potential for chemotherapeutic agents to monitor drug release [26]. For drug delivery systems, all forms of nanocellulose have great potential [2]. However, the selection of practical, organic, non-toxic, and cheap materials is crucial while preserving bioactivity and reducing unwanted side effects [27]. In recent times, significant progress in exploring different natural polymer’ uses has developed in encouraging biomaterials such as collagen, starch, alginate, gelatin, chitosan, elastin, and cellulose, due to their inexpensive, renewable, and environmentally friendly properties Among several other aspects, cellulosic nanomaterials have attracted considerable industrial and scientific attention due to their extraordinary biochemical, functional, geometric, and biological features, along with their high degree of biocompatibility, biodegradability, and bioavailability. Several studies have been published in past few years on the synthesis of nanocellulose and its hybrid products as metal-based composites by modification procedures. [28, 29]. This review paper would concentrate on preparing and applying various forms of nanocellulose for drug delivery system, food packaging, and biosensing applications.
Biomass sources
Cellulose materials are isolated from several plant fibers [38], as well as bacterial sources. Scientists are interesting to produce cellulose and nanocellulose from valuable sources using biowastes. Agricultural residues and forest crops are stated as lignocellulosic biomass [39]. As the second richest source of nanocellulose, agricultural sources come from waste materials from farming fields. Corn husk, wheat straw, rice husk, corncob, and banana rachis are some of the most commonly used agricultural biomass sources. In addition to tomato waste, sugarcane bagasse, carrot pulp, garlic peels, and other industrial waste contain nanocellulose. Nanocellulose can also be found in marine animals, such as tunicate, and microorganisms like bacteria and fungi [40]. There are several components present besides cellulose in these sources, including lignin, hemicellulose, pectin, waxes, etc. [41]. The dry mass analysis of lignin, hemicellulose, and cellulose from different sources is shown in Table 2.
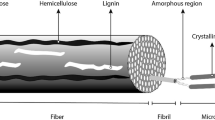
Nanocellulose can be acquired from biomass (wood) generally in two steps like the first step can be the deconstruction of lignocellulose material to obtain cellulose as pure as possible, the second step is conducted to produce nanocellulose as it can be seen in Figs. 1 and 4. The structure of lignocellulose is defined as a rigid material linking layers of lignin, hemicellulose, and cellulose with the help of strong covalent and hydrogen bonds, so enabling the wood and plants to resist pest and chemical degradation attacks [42]. Generally, an extensive multistep refining process is employed on biomass to reduce non-cellulosic material while cellulose is preserved. As lignin is cross-linked covalently with cellulose and hemicellulose through ester and ether linkages, the cross-linked structure resists structural breakdown, which is mentioned as lignocellulose biomass resistance [43]. So commonly, special chemical and mechanical treatments are applied to obtain nanocellulose by rupturing the lignocellulosic biomass structure. On an industrial scale, this process is done by a technique named kraft pulping which is a combination of mechanical and chemical treatments of biomass to obtain nearly pure cellulose [44]. The most recent study revealed the usage of an environmental friendly technique in which poplar wood powder is assorted with various harsh eutectic solvents and later on exposed to microwave irradiation for almost 3 min as shown in Fig. 1 [42]. As a result, lignin was removed almost 80% and cellulosic material remained in 75% crystalline cellulose form. Similarly, authors claimed it an outstanding cellulose extraction method involved in utilization of reusable bio-sourced harsh eutectic solvents as well as low energy microwave consumption treatment. After pure cellulose extraction, nanocellulose is recovered by the further treatment process. Cellulose-derived substances within the nanometer scale are defined as nanocellulose [45]. Nanofibers primarily incorporate nanocrystalline cellulose, cellulose nanofibers/nanofibrillated cellulose, and bacterial nanocellulose [15, 46]. These forms of nanocellulose are due to various origins and extraction processes that different in structure, particle density, or crystalline nature but almost similar in composition [46].
Schematic structural representation of deconstruction of lignocellulose from (wood) biomass [42]
Nanocellulose structure and dimensions
Organic polymers especially cellulose and its derivatives are considered as one of the extensively studied materials for various applications including biomedical applications due to their less toxicity, renewability, bioactivity, and renewable natural origin. Plants are considered fundamental origin for cellulose extraction such as rice husk, rice straw, cotton, wood-pulp, and jute. According to the plant structure, cellulose is reported as a component of the cell divider having a stimulating part of the plant [47]. Similarly, cellulose has been reported to integrate by bacterial species such as Gluconacetobacter xylinus, Oocystis apiculata, and Microcosmus fulcatus [47,48,49]. The understanding of cellulose structure inside the plants will have obvious effects on the characterization and production method of nanocellulose as well as will affect its preparation and association in manufacturing the functional materials. During cellulose isolation through green plants by scientist Anselme Payen in the year 1838, it was reported that every plant cell wall is composed of a similar cellulose substance [50]. Cellulose is composed of β1-4 glycosidic bonds which are connected to d-glucopyranosyl units. Repeatedly, an anhydrase glucose unit is pivoted 180 degrees in the plane to create a favored position for producing an acetal bond between two closing glucopyranosyl rings. Hence, the consecutive units lead to cellobiose as an auxiliary component. In general, cellulose chain length fluctuates greatly (within the range of 300–10,000 units) depending upon the source [51, 52]. Figure 2 shows TEM images of various forms of nanocellulose.
Derivation techniques of nanocellulose
Alkaline-acidic pretreatment
Sodium hydroxide or potassium hydroxide solution is used to pre-treat biomass in this pretreatment process. Natural fibers undergo chemical treatment to remove hemicellulose, lignin, extractives, and waxes in order to modify their surface. This treatment has been described in a number of publications, including our recent publication [55]. As a result of alkali treatment, microvoids are eliminated, the surface is smoother and there is better stress distribution [56]. The fiber diameter decreased on NaOH treatment, resulting in an increased aspect ratio, resulting in better fiber–matrix interactions due to a larger effective surface area [57]. Furthermore, natural fibers can also be chemically modified with silanes, by reacting with OH-groups in natural fibers through self-condensation [58]. A lignin, hemicellulose, and pectin removal step is often performed before mechanical isolation of nanocellulose by utilizing alkaline-acid pretreatments [59, 60]. Therefore, step follows as given below.
-
(i)
To increase the surface area of the cellulosic materials, biomass natural fibers are waterlogged in NaOH (12–17.5 wt%) for 2 h.
-
(ii)
A solution of hydrochloric acid (HCL) is used to hydrolyze the fibers at 60–80 °C, causing the hemicellulose to be solubilized.
-
(iii)
A solution of NaOH (2 weight percent) is applied to the fibers at about 50–75 °C to disrupt the lignin structure.
Ionic liquids pretreatments
A low melting temperature and low vapor pressure make ionic liquids nonflammable, lower vapor pressure, and more thermally stable. Aside from alkali acids and enzymes, ionic liquids [61], have also been extensively utilized for pretreatment, particularly 1-butyl-3-methylimidazolium chloride, to dissolve cellulosic materials, which was followed by high-pressure homogenization for isolation of nanocellulose fibers. A variety of factors influence the solubilization of cellulose, including microwave power, reaction time, temperature, and the ratio of cellulose to ionic liquid [62].
Enzymes pretreatments
Pretreatment with enzymes is a biological process that degrades the non-cellulosic materials (lignin and hemicellulose) in biomass cellulose fibers. Pretreatment involves cellobiohydrolase, endoglucanase, and cellulase enzymes. Various particles in the cellulose fibers are hydrolyzed selectively or restrictively by enzymes in this pretreatment process [63]. Despite its complexity, enzyme action involves catalyzing H-bonding between cellulose fibers [64]. Since multiple organic compounds are found in cellulosic fibers [22], there is a set of selective enzymes that attack crystalline cellulose, i.e. the A- and B-type cellulases. Also, the C-type and D-type cellulases are targeted at disordered cellulose structures [65]. Enzymatic pretreatment generally requires a longer reaction time than acid hydrolysis under mild conditions [63]. Therefore enzymatic pretreatment has been used in several reports for CNF production [66, 67]. A mono-component enzyme endoglucanase was used in selective and mild hydrolysis to obtain nanocellulose materials from softwood pulp, which provided a higher aspect ratio than acid hydrolysis.
TEMPO oxidative method
In some recent research studies, CNC was prepared from banana pseudostems by oxidizing them with 2, 2, 6, 6-Tetramethylpiperidine-1-oxyl radicals (TEMPO) [68]. It was found that TEMPO-oxidized CNC had less and uniform width, as well as a high mass recovery ratio, suggesting that these materials could be used as fillers in polymer matrix. An oxidation reaction with TEMPO involves adding sodium bromide and sodium hypochlorite to water at a higher pH to dissolve the catalytic groups of TEMPO and sodium bromide, and by adding sodium hypochlorite, the cellulose –OH is oxidized to carboxylates [69]. In another approach, the TEMPO method can also be used with neutral or weak acids for the oxidation of sodium hypochlorite and sodium chlorite [70]. In MCC and softwood bleached kraft pulp, CNCs were made by oxidizing the pulp during TEMPO-mediated cycling to obtain uniform diameters (3–4 nm) with good gas barrier properties.[71]. Similarly, the oxidation process followed by the sonication was used to develop CNF in other study and their results show that yield increases as sonication time increases [72]. As cellulose is oxidized with periodate-chlorite, the secondary alcohols of the molecule first undergo oxidation by sodium periodate to aldehyde and then oxidation by sodium chlorite to COOH. In order to do this, the nanofibrils become more ductile [73], and the films incorporated with CNF can behave in a more mechanical way [74].
Water hydrolysis method
It's a great method to use water as the green solvent because it makes less and cleaner waste, corrosion-resistant, and cost effective approach. Subcritical water hydrolysis has been used in limited studies to isolate CNCs [75]. Similarly, the researchers studied that optimizing experimental parameters resulted in a higher yield of CNCs with improved characteristics. After partial hydrolysis of cellulose, 21.9% CNC was obtained, in a rod-like structure and crystallized form, with a similar aspect ratio to conventional CNC, which shows excellent thermal stability. In that study the experiment was conducted to examine the thermal and mechanical properties of packaging film using CNF extracted from canola straw by subcritical water technology [76]. In addition to improving moisture resistance, the film reinforced with CNF also showed improved tensile strength (TS) and reduced water vapor permeability.
The hydrolysis of cellulosic amorphous and semicrystalline areas has also been reported to be facilitated by subcritical water. As it only uses water to hydrolyze, this procedure has tremendous potential, and despite its high energy requirements because of the high pressure reactor, it can be less expensive since many washing processes are not necessary [77]. While, in order to fully understand the process of hydrolysis with subcritical water, more research is needed on various reaction conditions.
Subcritical and supercritical fluids (for polar solvents) have a higher diffusion coefficient and a lower dielectric constant. In both cases, water can more easily break glycosidic bonds by disintegrating cellulose amorphous domains [78]. In addition, hydrothermal processes of hemicellulose removal demonstrate water's ability to hydrolyze polysaccharides [79]. In order to achieve an extensive hydrolysis rate, both H3O+ species and water molecules must be present [80]. While, a lower Kw value in subcritical and supercritical water results in a higher concentration of ionized species [81]. Therefore, they are effective at hydrolysis reactions as a result. It has been observed that water under high pressures and temperatures hydrolyzes lignocellulosic, gasifies biomass, and liquefies cellulose/hemicellulose by several means [77, 82,83,84,85]. The rate of hydrolysis must slow down when the density, ion product, and dielectric constant of water are all extremely lower. Hydrolysis occurs in nature by generating a cellobiose-water intermediate (a transition state) with higher polarity compared to the reactant. One possible mechanism that can be involved known as; hydrolysis occurs when water molecules attack the nucleophilic bond linkage in cellobiose through the nucleophilic attack of water molecules on the glycosidic bonds in cellobiose and other can be hydrolysis occurs when a proton (H+) ion is dissociated from the water molecule and attacks the (1,4)-glycosidic bonds [86].
Acid hydrolysis method
Reinby and coworkers isolated the CNC first time in 1949 by using of H2SO4 [87]. While, a variety of acids were later used, such as phosphoric, maleic, hydrochloric, and bromic acids [88, 89]. Hydrochloric acid and sulfuric acid have been widely used for decades to isolate the CNC using chemical treatments and methods [45, 80, 90]. This method involves de-ionizing water, followed by the addition of sulfuric acid to extracted cellulose. A neutral pH suspension is obtained after filtering, centrifuging, and washing with clean water after a specific reaction time [91]. The optimal conditions of acid hydrolysis have been found by determining reaction time, temperature, and acid concentration in several studies. It has been found that the optimal concentration of hydrochloric acid is 64% (w/v) at a liquor ratio of 1: 8.75 at a specified reaction time and temperature (45 °C, 5 min ultrasonication) [45]. In some studies, it was found that sulfate esters are produced by using sulfuric acid to avoid CNC aggregation in the solution and promote CNC dispersion in water. In addition, due to fewer sulfate groups on their surfaces, sphere-shaped nanoparticles have been examined when sulfuric and hydrochloric acids were combined during hydrolysis of CNC [92]. Structure and dimensional properties of the obtained fibers are greatly influenced by the duration, temperature, and acid concentration of the hydrolysis reaction. Researchers have examined a variety of cellulosic materials for the reaction condition and optimal conditions included: sulfuric acid concentration of 65% (wt), reaction temperature at 20–70 °C, and a hydrolysis duration that varies from 30 min to different range of time limits [93]. Similarly, in other study it has been reported that sulfuric acid hydrolysis resulted in cellulose nanocrystals with diameters of 5 nm and aspect ratios up to 60, can processed from coconut husk fibers [94].
Ammonium persulfate ((NH4)2S2O8) method
In recent years, ammonium persulfate (APS) method has been widely consider to extract the nanocellulose instead of TEMPO-oxidation and acid hydrolysis methods [95]. In order to adopting APS method, it has non-toxic behavior with higher water solubility and considered cost-effective. Moreover, its provides the carboxyl group at the C6 position, therefore, enabling CNC to be generated from cellulose fibers [96]. In addition to removing hemicellulose, pectin, and lignin from biomass materials, the CNC extraction process by the APS method is less harmful for the environment. The APS method relies on dissolving the amorphous parts of cellulosic materials to generate free radicals, hydrogen peroxide, and hydrogen sulfide by oxidizing cellulose fibers [97]. Cellulosic materials have also been dissolvable with ionic liquids, metal solutions, hydrates of molten inorganic salts, alkali/urea solutions, NaOH aqueous solutions, and NaOH/thiourea solutions.[98,99,100,101,102]. Moreover, the cellulose polymer solution obtained by reducing intermolecular hydrogen bonds between the cellulose particles which is the main mechanism of cellulosic material dissolution during these processes. The hydrogen bonding interactions between OH-ions and amino groups supplied by NaOH and urea in NaOH/urea systems are direct, but the hydrogen bonding interactions between urea and cellulosic materials are indirect [103]. It might be that when cellulose particles thaw in a NaOH/urea solution, the NaOH hydrates form new hydrogen bonds in the cellulose molecules (which are relatively stable), and the urea "hydrates" bind the hydrogen bonds to build an inclusion complex that acts as a sheath-like structures [104].
Other isolation methods
Once natural biomass materials are chemically purified, the next process is to convert them into nanoscale particles (CNC or CNF) using a variety of methods, particularly acid hydrolysis [17], and mechanical treatment, in combination to produce nanoparticles with desired characteristics [1, 15, 105]. Amorphous material is removed or decomposed by mechanical methods and chemical treatments [106]. Major preprocessing steps involve removing the matrix material partially and chemically treating the interfibrillar materials to break their hydrogen bonds. Pretreating cellulosic materials in an appropriate manner leads to the availability of hydroxyl groups, a boost in crystallinity and fiber surface area, and the breakdown of hydrogen bonds between fibers, increasing their reactivity [105]. By imparting a charge to the surface of the fibrils, the interfering forces among the fibrils can also be strengthened through oxidation, or by adsorbing polyelectrolytes (such as carboxymethyl cellulose treatment), or by adsorption of charged polyelectrolytes (such as 2,2,6,6-tetramethyl-piperidinyl-1-oxyl radical selective oxidation) [45, 90]. Therefore high-pressure homogenizers [107], cryo crushing [108], microfluidization [109, 110], and high-intensity ultrasonic treatments [111, 112] are some mechanical approaches to converting cellulose fibers into nanofibers.
Types of nanocellulose
Cellulose nanocrystals (CNC)
Cellulose nanocrystals are also named as nano-whiskers [19, 25], demonstrate extended crystalline rod-like forms, and reported to exhibit extensive rigid structure as compared to cellulose nanofibers because of the higher amount of amorphous domains are reduced [8]. Generally, cellulose nanocrystals show a degree of crystallinity within the range of 54 to 88%. Usually, enzymatic treatment has been reported to isolate CNC [113], however, in acid hydrolysis, sulfuric acid is the most commonly used acid for CNC extraction [114]. The most commonly used method involved in cellulose nanocrystals starts with alkaline and bleaching pre-treatments, later on, acid hydrolysis, washing with deionized water, solution centrifuging, repetitive dialysis, and ultra-sonication to produce a suspension for freeze drying or spray drying according to the requirements [90]. Cellulose origin and reaction conditions have been reported the obvious effects on cellulose nanocrystal properties including dimensions, crystallinity index, and morphological characteristics [90]. There are many techniques associated with CNC production, including enzymatic/acid hydrolysis and mechanical treatment or oxidation, which is frequently used to eliminate amorphous parts of cellulosic fiber and obtain the crystalline region cellulose with a particular molecular shape. Therefore, multiple phases are included in the process, such as drying/grinding/dewaxing, purification, delignification (mechanical, chemical, biological, or combined), bleaching, and filtration/washing/drying [115]. Du et al. extracted cellulose, beginning with milled wood taken from the source of fresh Douglas-fir wood chips [116, 117]. The wood chips were pounded into wood flour with a particle diameter of 235 nm and then exposed to a second milling method to shape ball-milled wood with a gear-drive planetary ball mill. The ball-milled wood was then reacted with water and the enzyme Cellic HTec2 to achieve a solid sample (hydrolysis residue) [118]. In a neutral sulfite cooking process, the hydrolysis residue was combined with cooking liquor to produce neutral sulfite cooking residues and lignosulphonate [119]. In the next step, holocellulose is formed by treatment with NaCl and CH3COOH at 70 °C by delignification. After the formation of holocellulose, it was bleached and then reacted with NaOH at 90 °C, followed by filtration, washing, and drying steps. To increase the cellulose purity, repeat the delignification and bleaching procedure as reports mentioned in the literature [120, 121]. After the isolation of cellulose, many methods can be applied to prepare cellulose nanocrystals. The most common way is enzymatic hydrolysis, in which celluloses as endoglucanases and exoglycanases are used [122]. Harsh hydrolysis with concentrated acids such as sulphuric, nitric, formic, and oxalic acid is also used to prepare cellulose nanocrystals. Other commonly used methods for preparing cellulose nanocrystals are subcritical water hydrolysis, oxidation method, mechanical treatment, and combined process (TEMPO oxidation) [123]. Figure 3 shows a scanning electron microscopic view of cellulose nanocrystals and their thermal stability values and lyotropic crystalline behavior.
Cellulose nanocrystals are generally reported as having a width of 3–50 nm and a length is subjected between 50 to 500 nm [127]. Cotton cellulose was evaluated in the form of sulfonated cellulose nanocrystals by Ureña-Benavides et al. [125]. Similarly, in a study Marchessault and co-authors investigated that cellulose nanocrystal aqueous solutions keep liquid crystal properties [128] owing to excellent photonics properties [129]. The results of optical microscope revealed that the concentration beyond 4.5 wt% gives chiral nematic structures to nanomaterials [90, 92]. Cross linking between cellulose nanocrystals and natural or synthetic polymers produces functional nanocomposites. Various reports have been devoted to the techniques and methods used for cellulose nanocrystals composites preparation such as acid hydrolysis, enzymatic hydrolysis and more [130]. Figure 4 shows the sources and extraction process of cellulose nanocrystals.
Cellulose nanofibrils (CNF)
It’s reported in the open literature that cellulose chains are normally entangled with a higher surface area and CNF is a type of stretched cellulose nanofibrils bundle, also named as cellulose nanofibers and nanofibrillar cellulose [133,134,135]. Unlike cellulose nanocrystals, CNF substantially contains long-chain amorphous regions [136]. Various techniques such as chemical, mechanical, and enzymatic methods have been used to extract cellulose nanofibrils from several types of sources, in which the mechanical treatment method is most extensively used. Generally, CNF extraction by mechanical treatments involved homogenization, cryo crushing, and grinding processes [137,138,139]. Additionally, chemical methods involved alkaline treatment, and biological methodology is applied by using enzymatic treatments [65, 140]. Similar to CNC, the properties of cellulose nanofibrils can be different according to the raw material origin as well as the CNF isolation techniques applied. Thus the properties of cellulose nanofibrils can fluctuate extensively like dimensions, morphological properties, size and shape, as well as fibrillation degree. With the research advancement, a study revealed a quality index to standardize the variety of cellulose nanofibrils [141]. Figure 5 shows the sources and extraction process of cellulose nanofibrils. Generally, cellulose nanofibrils production involves various operations including refining, biological hydrolysis, refining again, and then at the end homogenization [137]. Similarly, TEMPO-mediated oxidation, followed by blending-process [142] or homogenization [143], as well as carboxymethylation, followed by homogenization [144]. So the cellulose nanofibrils extraction technique is an outstanding combination of various processes through which various types of CNF can be attained. Table 3 shows a CNF-based drug delivery system [145], with various modifications agents and drug models. Figure 6 shows mechanical processes for CNF production.
Mechanical processes for CNF production and photographs are adopted through www.niro-soavi.com, www.microfluidicscorp.com, and www.masuko.com
Bacterial nanocellulose (BNC)
The most basic component of the plant cell wall is cellulose which has been considered the most abundant natural polymer on earth [51, 162]. Cellulose is also formed by tunicate, fungi as well as green algae [163,164,165,166]. Similarly, reports revealed that some particular bacteria have been used for cellulose synthesizing called bacterial cellulose [166]. For the first time in the year 1886, bacterial cellulose production through Acetobacter xylinum was defined in a report [167]. Later on, for BNC production various types of bacteria were also used like Agrobacterium, Salmonella, and Rhizobium [168,169,170]. Acetobacter xylinum is reported as an excellent bacterium for the higher yield of bacterial nanocellulose [171] and it’s also named as Komagataeibacter xylinus and Gluconacetobacter xylinum [166]. Generally, BNC fibrils are reported in length as almost 100 nm and width of about 100 nm [163, 172, 173]. As a result, fibrils create an outstanding 3D structure by cross-linking with each other and stabilize by intra- and intermolecular hydrogen bonding [174]. Depending on the fermentation process, bacterial nanocellulose are produced in various shapes like in the form of thin films hydrogel using static culture conditions, and by using agitation culture conditions spheres like hydrogels are produced [175, 176]. BNC is reported to exhibit similar chemical structural properties as plant cellulose [177]. Nevertheless, BNC and plant cellulose are different in cellulose purity as well as morphological and mechanical properties [178, 179]. Bacterial nanocellulose is reported to possess excellent characteristics for example tremendous purity, high surface area and aspect ratio, outstanding 3D ultrafine chemical structure [180], higher water holding capacities, and high porosity values as compared to plant cellulose [181,182,183,184]. Figure 7 shows SEM image of bacterial cellulose and stress–strain curve of cellulose at the various content percentage of bacterial cellulose.
Bacterial nanocelluloses having an elastic modulus of 78 GPa are examined to have excellent water holding properties with a molecular weight of about 8000 Da [185]. Bacterial nanocellulose is promising nanomaterials most widely used in functional materials such as scaffolds [186, 187] optical, and excellent mechanical properties because of higher surface area values and lower density of BNC [188, 189]. Figure 8 shows commonly used bacterial cellulose sources and production techniques and Table 4 shows the properties of various forms of nanocellulose. Bacterial nanocellulose has been used outstandingly in biomedical applications [190] such as tissue engineering, wound dressing [191], and artificial skins [192, 193] due to higher values of physical strength as well as interpenetrating and hydrophilic surface structure of bacterial nanocellulose [190]. Similarly, BNC is used in blood arteries as well as in regenerative medicines [194,195,196].
Characteristics of cellulose biopolymer nanocomposites
Generally, the production and manufacturing design of food packaging materials need various steps and careful consideration to fulfill the desired properties [205]. Multiple characteristics are considered to evaluate the bio-nanocomposite material for specific applications like thermal, mechanical, and rheological properties [206]. In a study, the researchers utilized cellulose nanofibers that were extracted from banana fibers to serve as a reinforcement material for natural rubber (NR) [207]. In this report, by varying the amount of CNF content, the researchers aimed to investigate the impact on the mechanical properties of the resulting composite material. The CNF was extracted from banana fibers through a series of mechanical and chemical treatments, and then introduced into the natural rubber matrix through a mixing process. The composite samples were then subjected to a curing process before testing the mechanical properties. The researchers found that as the CNF content in the composite material increased, there was a corresponding improvement in both the Young's modulus and strength of the material. This is a result of the CNF reinforcing the NR matrix, leading to enhanced load transfer and improved mechanical performance.
In a study conducted by Phomrak and Phisalaphong in 2017 [208], the authors investigated the use of crosslinking agents, specifically Zinc-based compounds and Sulphur, to enhance the interaction between cellulose and rubber. The addition of these agents was found to promote the formation of a 3D network between natural rubber (NR), cellulose nanofibers (CNF), and Zinc metal, leading to improved mechanical properties of the composite material. Furthermore, the authors reported the preparation of a bacterial cellulose-rubber composite (NRBC) using a latex aqueous microdispersion process. This method involves dispersing NR in an aqueous medium and then adding bacterial cellulose to form a composite material. The addition of CNF and crosslinking agents further enhanced the mechanical properties of the NRBC composite. Overall, these findings demonstrate the potential of using crosslinking agents and CNF to improve the interaction between cellulose and rubber, leading to the formation of a stronger 3D network and improved mechanical properties of the composite material.
A typical immiscible system, polylactic acid/Natural rubber blend was prepared by solvent casting and extrusion followed by compression moulding. Cellulose nanocrystals were investigated as compatiblizers for the system. Three different types of fillers were used in this study, one unmodified (CNC), long alkyl chain grafted CNC (C18-g-CNC) and PLA grafted CNC (PLA-g-CNC). The preferential localization of fillers affects the compatibility which was analyzed in the study. Unmodified CNC moved to the PLA phase, thus there was no obvious effect in mechanical properties. Long alkyl chain grafted CNC had affinity for the NR phase and resulted in decrease in Young's modulus. Although the PLA grafted CNC were localized in the PLA phase, the tensile strength could be retained with a slight increase in Young's modulus. Thus PLA grafted CNC having small PLA chains acted as effective nucleating agent [209] reported the reinforcing effect of cellulose nanofibrils and cellulose nanowhiskers on poly ethylene oxide (PEO) matrix.
For the reason, Abdollahi et al. (2013) [210] measure the mechanical properties of neat alginate film as well as reinforcement with nanocellulose material. They verified that the tensile strength (TS) value of the composite films increased from 18.0 to 22.4 MPa with increasing nanoparticles content from 0 to 5 wt%, while the elongation at break (E%) value decreased from 11.5 to 8.2%. The improvement observed in the TS of the nanocomposite by increasing the filler content up to 5% was attributed to the reinforcement effect of homogeneously dispersed high-strength cellulose nanoparticles in the biopolymer matrix. Alves et al. (2015) [211] studied the mechanical properties of starch/gelatine/CNC films, with the increase in gelatine and CNC concentration driving an increase in puncture resistance. This behavior is desirable because a larger resistance to puncture indicates better film resistance, and the resistant film is better suited for applications in the packaging industry.
Applications of nanocellulose
Nanocellulose can be applied in various fields like food packaging, optical materials, aerospace and construction, pharmaceutical, and food additives [212, 213]. Cellulosic materials are considered an essential component used in cosmetics, food, and beverage. Their excellent properties such as hygroscopicity, chemically inactiveness, absence of high sorption, and nontoxicity enable the nanocellulose to be used in various sectors [214,215,216,217]. Nanocellulose possesses excellent unique properties such as creditable mechanical properties, proper strength, cheaper, and lightweight which makes it interesting for wide use. Aerogels prepared by freeze dried CNF are used in various areas like diapers, tampons, hygienic naperies to wound-dressing. Similarly, nanocellulose has been utilized in biotechnology and biomedical applications as an elastic cryo-structured gel [217]. Nevertheless, nanocellulose has several uses like an excellent high scattering substrate for corrosion inhibition, membrane for loudspeakers, computer parts, conductive material reinforcement, and tobacco filter additives. Cellulosic materials are outstandingly capable of holding water, so they are well-matched with the human body.
Nanocellulose has been used in biocompatible coating, design for drug release, scaffold as well as wound dressings. Nanocellulose has been extensively used in biomedical applications because of the very less cytotoxic and excellent biocompatible properties of nanocellulose. In biomedical functional applications, hydrogels and aerogels are reported as tremendous forms of materials dues to higher porosity values, however owning less mechanical stability values. So the aerogels and hydrogels with lower mechanical stability values can have an extensive drawback in specific tissues while cell culturing or in blood vessels. Nanocellulose with cross-linking agents forms an excellent stable structure of aerogels and hydrogels without affecting the porosity values, letting the passage of fundamental nutrients. This review paper will summarize the recent work related to the usage of nanocellulose in food packaging, and biomedical applications such as wound dressing, drug delivery systems, tissue engineering, scaffolds, medical implants, etc.
Nanocellulose in food preservation and packaging
Multifunctional nanocellulose scaffolds have been used in food packaging due to their outstanding characteristics. In nanocomposites, CNF with dimensions between 1 and 100 nm has been used as reinforcement. It is biodegradable, environmentally safe, renewable, cheaper, lighter, stronger, and stiffer than other materials. Transparent CNF-based films have excellent barrier properties and are used for coatings, food packaging, and different other applications. Several industries, including food and preservatives, use thick films to act as oxygen barriers. Polymers such as soy protein, rubber latex, thermosetting resins and starch-based matrixes can benefit from cellulose nanocrystals (CNC) in terms of mechanical properties. A functional food ingredient can be created from these composites as dietary fiber or as a coating for food packaging [218]. Aside from its use as a natural emulsifying and stabilizing ingredient in foods like salad dressing, milk products, ice cream, and bread, while nanocellulose scaffolds are also used as a photo heat resistive material to preserve the foods in terms of packaging [219]. Food industries use BNC for various applications, including texture modifiers, thickeners, gravies, sauces, icings, frosting, additives, deserts, and frozen dairy kinds of stuff as shown Fig. 9. It has been proven that BNC is an excellent hydrocolloid additive that can replace other materials in the food industry [220]. Numerous companies have marketed products with BNC and carboxymethyl combined with sucrose [221]. As well as replacing low-calorie additives and texture modifiers, stabilizers and thickeners, BNC is also used to replace low-calorie additives [222]. Food industries could potentially benefit from BNC's dietetic and technical aspects [223].
The protective applications of nanocellulose for food stuffs [21]
A recent study reported highly stable Pickering emulsions using microfluidization from a variety of oils [224]. The product was more stable to coalescence, but it does show flocculation and creaming under some circumstances. In addition, under the different conditions, CNC-added droplets exhibited greater stability against flocculation. This may be attributed to a decrease in electrostatic repulsion among the particles, which affects or limits their use in a particular food variety. Of course, they also exhibit flocculation and creaming when exposed to highly acidic conditions and strong ionic strength. The indigestible dietary fiber might affect the performance of lipid droplets in the human gut by forming a layer around them. In addition, it could mitigate the effects of lipid spikes in the bloodstream or increase satiety by delaying the digestion of lipids. The indigestible coating, however, can withstand the adverse gastrointestinal conditions caused by the absence of cellulase enzyme, as well as protect the bioactive compounds encapsulated like vitamins, omega-3 fatty acids, curcumin, probiotics, and nutraceuticals, because there is no cellulase enzyme in the human system. While, a deeper understanding of the mechanism of the NC effect requires a detailed investigation of the human model/system.
Furthermore, functional foods contain new components or higher quantities of existing components, which can promote health and prevent disease [225]. Adding new compounds, however, may adversely affect the properties of food [226]. According to recent reports, NC can be used to reduce fat in formulations. In addition to this study patent results demonstrate that, NC might be used to prepare a salad dressing that is low in calories [227]. NC was combined with vinegar and dried Italian salad in the formulation. This product resembles authentic Italian dressing in both color and texture. As well as being a dietary fiber, NC has other important qualities. In order to support people in meeting their dietary requirements, food scientists are developing functional foods. There are many food products, such as chocolate and hamburger, that can be reduced in calories if they contain NC with a high water content [226, 228]. It is possible to reduce calories in different stuffing by 15–20 weight percent by substituting non-fat additives such as NC for fat [229]. Furthermore, a healthy biscuit cream containing NC as an additive was developed recently [230]. After adding CNC, the cream formulation with sunflower oil and shortening demonstrated the stability of oil related to sensory attributes. Therefore, in order to maintain cream texture properties, it is essential to standardize CNC concentrations.
Similarly, for food packaging, coatings can be used to increase the properties of packaging materials. The coating process is usually based on thin films, which can be applied externally or between two materials and lead to composite structures with excellent characteristics. In recent studies, maltodextrin and NC were combined to extend saffron shelf life. Based on the study results, CNC coating prevented loss of bioactive compounds like crocin in saffron much more efficiently than control samples [231]. In polymer-based packaging materials, NC addition leads to enhance the mechanical and water vapor barrier properties because natural polymers have very little mechanical and water vapor barrier properties. Therefore, alginate-based films were enhanced by the addition of NC. CNCs in packaging films improved their UV barrier properties and oxygen permeability, according to the latest study [232]. On the other hand, it has also been reported that nanoencapsulating nisin in alginate-CNC beads successfully suppresses Listeria monocytogenes growth in ham ready for consumption [233]. In comparison with free nisin, the beads substantially decreased the number of L-monocytogenes during storage. Further, ham's pH and color did not change during storage, nor did its physicochemical properties. In addition, a newly developed bio-nanocomposite was reported with coating method, which primarily based on egg-derived polymers and cellulose nanoparticles and can be used for food packaging [234]. Thus coating is intended to delay food spoilage by delaying dehydration, ripening, and microbial invasion on fresh fruits (such as avocados, bananas, papayas, and strawberries). Due to its edible characteristics, washability, and low-cost nature, it is an effective alternative to commercially available fruit coatings and a solution to the expanding problem of food waste worldwide.
The use of CNC as a food additive is extensive. A recent study evaluated the effect of CNC on retrogradation characteristics, gelatinization, and pasting of usual starch, waxy starch, and sweet potato starch. When gelatinized starch is cooled and stored, retrogradation occurs. Based on viscosity studies, CNC demonstrated that it decreased the setback value of sweet potato starch and raised the peak viscosity of normal maize starch. CNC repressed amylose retrogradation short-term as well as amylopectin retrogradation long-term. Molecular chains of starch can be cross-linked using CNC [235]. CNF derived from discarded brown algae has recently been tested for use as a milk thickener in terms of food additive [236]. As revealed by its rheology behavior, CNF suspensions in water exhibit high viscosity and shear thin ability. As a result of their ability to absorb casein micelles via hydrogen bonds, CNFs showed better thickening properties in milk than other nanofibers. CNF safety and biocompatibility were confirmed by MTT assay. Food producers may use CNF derived from abundant marine bioresources. Therefore, a high aspect ratio bioactive CNF can be produced for food thickening through this study. So consequently, we can say that nanocellulose and its derivatives have great potential in food preservation and packaging.
Nanocellulose in drug delivery system (DDS)
In drug delivery system mostly those materials are preferred to be used which are ideal in sustaining antimicrobial activity, nontoxicity, surface of the skin moisture, speed up the healing process and can be taken out without disrupting to the healed skin. Nanocellulose-based hydrogels accomplish most of the necessities so in recent times they are used in wound dressing applications [237,238,239,240]. An excellent effect of bacterial nanocellulose (BNC) membranes was examined in a report which shows a tremendous recovery of burn wound into nearly 70% closure wound only in 3 weeks as shown in Fig. 10. The bacterial cellulose hydrogel (BCH) was used in a study to treat third-degree burn wounds, results exhibited that BCH assists in growing the fibroblast cells, non-toxic, and cell viability was increased. The burn wound closure activities of BCH were carried out by in-vivo Wistar rats, and the results of that study propose that bacterial cellulose hydrogel can efficiently be used as naturally burn wound dressing materials. Tables 5, 6 and 7 shows nanocellulose based applications in oral drug system [25], nanocellulose-based topical drug delivery system [241] used in biomedical applications, and nanocellulose-based applications in transdermal drug delivery system [242], respectively.
Bacterial cellulose membrane for wound healing showed nearly 70% wound closure in 3 weeks [243]
Cellulose and nanocellulose have been reported to be excellent drug carriers acting as drug excipients in various DDS [263, 264]. The vital benefit of Nanocellulose DDS is sustained drug discharge. Nanocellulose can convert the drug's release through multiple processes, including water retention, film formation, and rheology control [265]. Cellulose nanofibers and cellulose nanocrystals have been significantly used as an excellent safe solution for commonly controlled drugs; they are used in the preparation of several forms of sustainable and controlled DDS such as films, nanoparticles scaffolds, nanocomposites, and micro-particles gels [266]. Different CNF synthesize aerogel serves as a store for oral drug delivery. The CNF aerogels are integrated with beclomethasone dipropionate-coated nanoparticles with amphiphilic hydrophobin proteins. The analysis found that the release of the drug was dependent on the composition and associations between the nanoparticles and the cellulose matrix, so these nanocomposite materials can give new possibilities to monitor the distribution of drugs [267]. For example, cellulose nanofibrils composites loaded with calcium peroxide (CaO2) were proficiently proved as owing high porosity and efficiency in producing H2O2, later on, transformed into O2 with the help of catalase enzyme. The results exhibited that the cell proliferation and wound healing activity of the CNF composites were improved; even the drug release was maintained for 5 days showing the excellent bioactive ability of CNF composites [268]. Similarly, in another paper three model drugs, namely itraconazole, indomethacin, and beclomethasone, were also evaluated using CNF to prepare film matrices with excellent mechanical properties using a filtration process appropriate for heat-sensitive drugs. High drug loading (> 90%) and sustained drug release for more than 3 months were seen in the prepared films, which were accredited to shape a compact fiber network around drug particles as well as to connect the drug to CNF in the molecular form [269]. In a study, porous aerogel by freeze-drying method has been used for oral drug delivery system; the freeze-dried aerogels were later integrated with beclomethasone dipropionate nanoparticles treated with amphiphilic hydrophobic proteins. The results showed that the drug release was dependent on the microstructure and crosslinking between nanoparticles and cellulose-matrix, so the structure of the nanocomposites may offer various potentials for controlled DDS [270]. Additionally, in other article, five model drugs with various structural features (indomethacin, itraconazole, beclomethasone, nafarelin acetate, and lysozyme) have been integrated into CNF hydrogel for film preparation. The research proved that the substance's size-based diffusion has a significant dependency between the drugs and CNF fibers through the films and the pH-dependent electrostatic attachment [271]. Nanocellulose hydrogels are applied in various biomedical applications like drug delivery system, cell therapy, and tissue engineering. Depending on the source origin, nanocellulose delivers the desired properties from the biomedical point of view. Cellulose nanofibers have been extracted from an abundant plant source and CNF hydrogel was prepared to achieve the desired functionalities in a recent study [272]. Results evaluated that CNF hydrogel exhibited no cytotoxicity, created a 3D environment for cells, and prompted spheroid formation of HepaRG and HepG2 cells. Similarly, cellulose nanofibers hydrogel from a novel plant was produced and used for human pluripotent stem cells. The pluripotency of human pluripotent stem cells cultured in CNF hydrogels was sustained even for 26 days [273].
CNC was also used to start preparing dynamic extended-release drug delivery systems, such as the modified surface properties of CNC bound to cetyltrimethylammonium bromide, which improved hydrophobic binding drugs etoposide, paclitaxel, and docetaxel, extending the release of these drugs for 2 days [271]. Cellulose nanofibers in a study [274] have been extracted using the spray drying method and utilized as drug carriers using 6 model drugs such as verapamil hydrochloride, ibuprofen, metoprolol tartrate, atenolol, indomethacin, and nadolol. Investigations of this study reveal that the dimensions of the prepared nanoparticles were nearly 5 μm in size and the drug release was quick for the first 10 to 14 days, and a continuous drug releasing behavior was noted for 2 months. Cellulose nanofibers can maintain the drug release because of the nano-fiber structural network which assures the certain drug release. In various studies for drug delivery systems, i.e., tablets and nanoparticles, CNF and CNC in the preparation of rapid drug delivery systems were incorporated. CNF has the potential to prepare fast delivery systems for drugs. Results revealed that the speediest drug release was shown by tablets prepared by direct compression using CNF, which could be attributed to CNF's faster dissolving time than Avicel PH102 [275].
In a similar study, results acknowledged the cellulose nanocrystals efficiency in increasing the mechanical properties and adjusting the drug release in theophylline-loaded alginate microspheres because of the excellent properties of nanocellulose to limit the movement of alginate restraints [276]. Additionally, nanocellulose and sodium alginate were prepared in the occurrence of calcium ions exhibited efficient improvement in ibuprofen release [277]. Meanwhile, it’s reported that microcrystalline cellulose's adsorptive properties tend to result in incomplete drug release from the tablets. Akhlaghi et al. revealed the execution of CNC in the preparation of fast-release nanoparticles, where the researchers grafted CNC with chitosan oligosaccharide into carboxylic groups through the oxidation of the primary alcohol groups on CNC, which reacted to chitosan oligosaccharide with the available amino groups. For the preparation of procaine hydrochloride-loaded nanoparticles, the grafted CNC/chitosan oligosaccharide was used to release the drug at pH 8 within 1 h [278].
Nanocellulose in nano-generator, piezo-electric, biosensing and bioimaging
Biosensors are considered outstanding materials in recent technology due to their excellent properties like simple, cheap, and very appropriate for multiple areas of application as shown in Fig. 11. Due to the boom in technology, measurements are taken with different approaches to use the biosensors and electronic devices in medical applications as a diagnosing materials. From the last few years, scientists are trying to use environment friendly, sustainable, and biodegradable materials in biosensors and electronic devices to replace the plastic/glass-based substrates in bio-sensing equipment’s like actuators, electrochromic instruments, electrodes, and sensors. That’s why instead of plastic materials, recently nanopaper-based biosensors and electronic devices are highly recommended to be used in sensor technology. The utilization of nanopapers in electronic devices and sensors is advantageous over common papers because of owing excellent characteristics like higher mechanical strength and outstanding stability values in various conditions.
Schematic illustration of nanocellulose usage in biosensing and bioimaging [279]
Research has proposed multiple binary systems involving polymers (particularly polypyrrole (PPy) or polyaniline (PANI)) and nanocellulose. To initiate polymerization, BC was dissolved in a concoction of protonic acid (hydrochloric) and polar solvent, then added to an oxidant agent and conductive monomers. When acid hydrolysis was used to dope the PPy particles, formed a nanohybrid of PPy-CNs on the cellulosic layer. A desirable electrical conductivity and a reasonable degree of flexibility were demonstrated by the attained structure. An NC-PPy nanocomposite exhibiting a BC ratio of 1:10 and a core-sheath structure was described to exhibit a conductivity value as high as 77 (S cm−1). CN-based electrodes' charge capacity is significantly influenced by the type of nanocellulose and PPy pore size distribution. While a coating composed of PPy and CN substrate has weaker electrostatic forces than a substrate composed of pure CN, which has a higher number of NH groups. For making CN-carbon composites, coating methods are generally more effective than polymer-based ones. However, the weak carbon bonds between the layers may prevent an increase in conductivity beyond a certain thickness [280]. So in support to these conductive CN composites used in to build nanogenerators and piezoelectric devices.
The working principle of nanogenerators is based on piezoelectric or triboelectric effects, which convert external mechanical tension into electricity. There are also pyroelectric nanogenerators, which produce electricity by varying temperature. A piezoelectric effect occurs when the charge distribution of a material changes as a result of mechanical deformation. In many electro-active applications, such as microelectromechanical systems, actuators, robotics, and sensors, CNs exhibit piezoelectric properties due to their asymmetric crystal arrangement [281]. Taking advantage of this distinctive property of nanocellulose, the CNF-poly (dimethylsiloxane) (CNF-PDMS) aerogel was developed into a nanogenerator. An aerogel coated with PDMS was layered between two PDMS films followed by aluminum foils in that study. As a result of the piezoelectric signal in the layered structure, 19 light-emitting diodes were turned on and the capacitor charged to 3.7 V [282]. Similarly, as shown in Fig. 12, using bacterial cellulose, a biotriboelectric nanogenerator was developed in which a transparent and gel-state BC was generated using a Cu current collector to create a self-powering system. Based on results, a light input force (16.8 N) generated with 8.1 C m−2 and 4.8 mW m−2 of accumulative charge and peak power, respectively [283].
Schematic illustration of; a preparation of bacterial nanocellulose, and b usage of BC-triboelectric nanogenerators to harvest triboelectric energy [283]
Using CNF in a perfluorosulfonic acid-based copolymer, recent studies have described an IPMC composed of an ionic polymer-metal composite. Through an oxidation–reduction reaction, CNF could increase platinum (Pt) plating surface area. It was discovered that the hybridized structures exhibited better mechanical characteristics, as well as electro responsive properties, with the potential to be used as artificial muscles and actuators in the future [284]. Further, the sensors were constructed by using electronic beam evaporating CN film between electrodes on a 125 mm thick PET substrate as shown in Fig. 13.
Schematic illustration of; a CNF film that stands upright and bends, b, c double layer of PET and Cu sandwiched between a layer of CNF shown in the side view of a sensor assembly, d sensor assembling, e, f CNF film under room temperature conditions for ferroelectric hysteresis based on voltage curves 40–50 V m−1 and 5–15 V m−1 electric fields [285]
In comparison to the piezoelectric effect of metal oxide as a reference material, Csoka et al. demonstrated that ultra-thin CNC films with a high shear piezoelectric constant of 2.1 V−1 have an acceptable piezoelectric effect [286]. It has been demonstrated that BC films can play a significant role as smart electro-active actuators by controlling crystallinity and chemical properties [287]. Similarly, the piezoelectric polymer polyvinylidene fluoride (PVDF) has been compared with a self-standing film of CNF [285]. As well as boosting piezoelectric response, microcrystalline chitosan can also reduce the brittleness of CNF films through its incorporation with CNF [288]. A nanocomposites composed of poly(vinylidene fluoride) and cellulose nanocrystals (PVDF-CNC) has recently been developed to improve piezoelectricity in PVDF devices [289]. In addition to ferroelectricity, CNF films have also been investigated as materials that can reverse their polarization when exposed to external electric fields. As shown in Fig. 13e–f a ferroelectric test on CNF films was conducted to determine the ferroelectric hysteresis of the films. At low electric fields (Fig. 13e), the film's capacitance value 5–15 V μm−1 shows a linear trend. This indicates that CNF film does not show ferroelectric hysteresis at these low electric fields. On the other hand, (Fig. 13f), at higher voltages (40–50 V m−1), a nonlinear polarization trend is observed, suggesting ferroelectric hysteresis may be present. The porous CNF demonstrated its ability to resist high electric fields during this characterization testing [285].
Similarly, nanocellulose has been used in optical sensors by fabricating the silver nano-particles with bacterial cellulose in a nanocomposite form using the citrate method. This study reveals that the silver nanoparticles and bacterial cellulose nanocomposite were exploited to detect the 2,2-dithiodipyridine and amino acid acting as a substrate for Raman scattering [290]. Silver nanoparticles and bacterial cellulose nanocomposites have also been used in Raman scattering for detecting the carbamazepine and atrazine [291]. Similarly, an eco-friendly, sustainable and biocompatible material cellulose nanofibers have been used in pH sensors, which are advantageous due to recyclable materials and demonstrate a very stable performance. These can be used for 1 month at various temperatures and for various pH with an excellent visual-ability to illustrate different colors for different pH 1–14 [292]. Due to the excellent sensing properties of these bio-composites based sensors, can be used to detect pH of biological fluids (blood, urine, etc.), and this pH sensor based on biocomposite could potentially serve as an alternative tool for diagnosing alcoholics, monitoring health, or even tracking the progression of certain illnesses. Similarly, nano-paper of bacterial cellulose has been used in developing the nanocomposites to design the biosensors for exhibiting plasmonic and photoluminescent characteristics which can be used for various applications [293]. This paper confirmed the properties of the optical sensor in a cheap, transparent, tunable, reusable, lightweight, and perhaps for wearable applications. Moreover, to develop the optical sensors, it is also important to measure the transmission haze, particularly in printed electronics and photovoltaics. The optical property occurs when light passing through the material deviates by more than 2.5 degrees from its path. The combination of high transparency and superb light scattering can greatly enhance the performance of material. CNF nanopaper has been shown to have excellent light scattering coefficient and high transparency, making it suitable for such systems [294]. It is the nanoscale dimension and extensive internal bonds of CNCs that give them their superior optical properties, in comparison with dense plastic films [126].
Similarly, in another article, nanocellulose surface characteristics, its performance, and design issues to develop biosensor with different approaches were discussed [292]. For this purpose, CNCs were extracted from cotton cellulose and used in detecting human neutrophil elastase (HNE) by covalently attaching HNE tripeptide substrate with glycine esterified cellulose nanocrystals. Authors described outstanding results for colorimetric detection of HNE that involves very low amounts (few milligrams) of peptide-CNC to create a visually obvious response at HNE levels. Similarly, transparent and tunable BC nanopapers have been described for the in-situ generation of silver nanoparticles [295]. In this technique, the reduction of silver ions and BC nanopaper is done by using hydroxyl groups of cellulose nanofibers (performing as a reducing agent to generate bio-nanocomposite). The major advantages of silver nanoparticles and BC nanopapers nanocomposites were obvious because these nanocomposites preparation was done without any external reducing or cross-linking agent. Similarly, this study also reveals the excellent chemical detecting properties as well as determine the amount of cyanide ions and 2-mercaptobenzothiazole in various water samples. On the other hand, a novel study has also been revealed in which supramolecular functionalized nanocellulose with β-cyclodextrin was characterized for the first time, having easy and simple conjugation through amination [296]. Hence, nanocellulose is a potential biopolymer with many applications in electronic and biomedical diagnostics, such as piezoelectric sensors, actuators, energy generators and biosensor. In addition, their surface-modification as a nanomaterials proved as an outstanding platform because of their easy design according to the suggested applications.
Future prospect and recommendations
Nanocellulose has emerged as a next-generation nanomaterial due to its customizable surface chemistry, high mechanical strength, and surface characteristics. The development of economical and environmentally friendly nanocellulose based materials will likely reduce the need for petrochemical based products. Nanocellulose can be used as a biopolymer to manufacture glucose molecules in large quantities without harming the body. As a result, biocompatibility, renewability, hydrophilicity, and biodegradability are the primary viewpoints for its biomedical uses. The current review article offers advanced data on recent developments in the sustainable manufacture of various nanocellulose kinds, including BNC, CNC, and CNF, from various sources and manufacturing pathways.
Appropriate resources, pretreatment, and physicochemical conditions are essential to induce desired characteristics in nanocellulose-based bio-inks. Researchers have developed various methodologies to optimize procedure parameters and functionalities to broaden their application using different biomaterials, including chondrocytes, mesenchymal stem cells, fibroblast alginate, hyaluronic acid, and gelatin. Nanocellulose can effectively be utilized to produce the 3D bioprinting of nanocellulose-based functional hydrogels. The advantages of shear thinning, and gelling characteristics are attained by CNF/alginate at low concentrations, as demonstrated by various works based on bio-ink synthesis through CNFs, alginates, and chondrocyte cells [297]. Further research should investigate the impact of shear-induced CNC alignment on cell viability during 3D bioprinting, as well as the effects of high concentration-induced osmolarity.
In general, nanocelluloses have demonstrated effectiveness and versatility as biomaterials suitable for various biomedical applications, either in their original form or after incorporating bioactive components. These applications encompass a wide range, including wound healing, drug delivery, biosensing, bioimaging, and tissue engineering. However, certain challenges need to be addressed before their use in biomedical applications. First of all, it is essential to thoroughly evaluate the biosafety of nanocellulose and its derived biomaterials, by practicing the established standard methods and clinically accepted protocols such as the ISO 10993 standards [298]. Secondly, there is a need for advanced characterization techniques to understand the real-time interactions between biomolecules and the surface of nanocellulose, as well as the interactions between nanocellulose and cells or tissues [299, 300]. Finally, the life cycle assessment and biodegradation profiles of nanocellulose-based biomaterials, particularly those designed for implantation and long-term use in the body, should be evaluated and precisely controlled [301, 302]. The properties required for nanocellulose vary across different biomedical applications, from surface chemistry to micro/nano-structure.
Conclusion
In the recent time, eco-friendly and biodegradable materials are replacing petroleum-based products in the early stages of scientific research. So, this review article exhibits that nanocellulose is likely to replace conventional plastic materials at the industrial level in near future. Nano-cellulosic materials are sustainable, biocompatible, cheap, nontoxic, and biodegradable polymers. They have a lot of marvelous characteristics and thus can be incorporated with a variety of materials. The various types of nanocellulose, dimensions, and applications in different fields have been described in this literature. However, we primarily focus on research and development in the areas of cellulose nanocrystals, cellulose nanofibers, and bacterial nanocellulose. A wide range of application prospects were discussed, including food packaging, drug delivery, piezoelectric sensors, actuators, energy generators, biosensing, and bioimaging, as well as biomedical applications to maximize the scope of nanocellulose. There is a great deal of challenge in choosing the right nanocellulose for a particular application. Therefore, studies suggest that in contrast with all other types of nanocellulose, bacterial nanocellulose is the best choice when it comes to drug delivery system. Similarly, cellulose nanofibers are ideal for large-scale applications, including food packaging, composites, and strength additives. On the other hand, in polymer composites, biosensors, piezoelectric sensors, actuators, energy generators and bioimaging applications, the cellulose nanocrystals, and cellulose nanofibers are suitable for stabilizing interfaces, reinforcing, and modifying rheology. In order to maximize the production and commercialization of nanocellulose, various scientists and companies conducted numerous research studies. Essentially, nanocellulose has multiple applications that can help and resolve a number of society's problems, but due to its goal oriented sustainable properties, cellulosic materials production can still be expensive, thus further studies are still important to make possible and wide range of optimized production.
References
Sacui IA et al (2014) Comparison of the properties of cellulose nanocrystals and cellulose nanofibrils isolated from bacteria, tunicate, and wood processed using acid, enzymatic, mechanical, and oxidative methods. ACS Appl Mater Interfaces 6(9):6127–6138
Plackett D et al (2014) A review of nanocellulose as a novel vehicle for drug delivery. Nord Pulp Pap Res J 29(1):105–118
Du H et al (2019) Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohyd Polym 209:130–144
Jahan MS et al (2011) Jute as raw material for the preparation of microcrystalline cellulose. Cellulose 18(2):451–459
Liu R, Yu H, Huang Y (2005) Structure and morphology of cellulose in wheat straw. Cellulose 12(1):25–34
Kondo T (1997) The assignment of IR absorption bands due to free hydroxyl groups in cellulose. Cellulose 4(4):281–292
Liu Y et al (2020) Modified ammonium persulfate oxidations for efficient preparation of carboxylated cellulose nanocrystals. Carbohyd Polym 229:115572
John MJ, Thomas S (2008) Biofibres and biocomposites. Carbohydr Polym 71(3):343–364
Azizi S et al (2013) Preparation, characterization, and antimicrobial activities of ZnO nanoparticles/cellulose nanocrystal nanocomposites. BioResources 8(2):1841–1851
Oun AA, Shankar S, Rhim J-W (2020) Multifunctional nanocellulose/metal and metal oxide nanoparticle hybrid nanomaterials. Crit Rev Food Sci Nutr 60(3):435–460
Kaushik M, Moores A (2016) Nanocelluloses as versatile supports for metal nanoparticles and their applications in catalysis. Green Chem 18(3):622–637
Shankar S, Oun AA, Rhim J-W (2018) Preparation of antimicrobial hybrid nano-materials using regenerated cellulose and metallic nanoparticles. Int J Biol Macromol 107:17–27
Pathania D et al (2016) Preparation of a novel chitosan-g-poly (acrylamide)/Zn nanocomposite hydrogel and its applications for controlled drug delivery of ofloxacin. Int J Biol Macromol 84:340–348
Spagnoli P, Santos SC, Caetano A (2017) A contribution toward the adaptation and validation of the entrepreneurial self-efficacy scale in Italy and Portugal. J Career Assess 25(4):670–687
Trache D et al (2016) Microcrystalline cellulose: Isolation, characterization and bio-composites application—a review. Int J Biol Macromol 93:789–804
Shaghaleh H, Xu X, Wang S (2018) Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers, and cellulose derivatives. RSC Adv 8(2):825–842
Mbakop S, Nthunya LN, Onyango MS (2021) Recent advances in the synthesis of nanocellulose functionalized–hybrid membranes and application in water quality improvement. Processes 9(4):611
Muqeet M et al (2020) Insight into cellulose-based-nanomaterials-a pursuit of environmental remedies. Int J Biol Macromol 163:1480–1486
Hitam C, Jalil A (2022) Recent advances on nanocellulose biomaterials for environmental health photoremediation: an overview. Environ Res 204:111964
Soares da Silva FAG et al (2022) Development of a layered bacterial nanocellulose-PHBV composite for food packaging. J Sci Food Agric 103:1077–1087
Perumal AB et al (2022) Nanocellulose: recent trends and applications in the food industry. Food Hydrocolloids 127:107484
Trache D, Thakur VK (2020) Nanocellulose and nanocarbons based hybrid materials: synthesis, characterization and applications. Nanomaterials 10:1800
Omran AAB et al (2021) Micro-and nanocellulose in polymer composite materials: a review. Polymers 13(2):231
Huang S et al (2020) Recent developments and prospective food-related applications of cellulose nanocrystals: a review. Cellulose 27(6):2991–3011
Hasan N et al (2020) Recent advances of nanocellulose in drug delivery systems. J Pharm Investig 50(6):553–572
Wang F et al (2022) Self-healable nanocellulose composite hydrogels combining multiple dynamic bonds for drug delivery. Int J Biol Macromol 203:143–152
Wang M et al (2013) Grafting of carboxybetaine brush onto cellulose membranes via surface-initiated ARGET-ATRP for improving blood compatibility. Colloids Surf B 103:52–58
Carrillo CA, Laine J, Rojas OJ (2014) Microemulsion systems for fiber deconstruction into cellulose nanofibrils. ACS Appl Mater Interfaces 6(24):22622–22627
Abo-Elseoud WS et al (2018) Chitosan nanoparticles/cellulose nanocrystals nanocomposites as a carrier system for the controlled release of repaglinide. Int J Biol Macromol 111:604–613
Shamsuddin IM et al (2017) Bioplastics as better alternative to petroplastics and their role in national sustainability: a review. Adv Biosci Bioeng 5(4):63
Chen YJ (2014) Bioplastics and their role in achieving global sustainability. J Chem Pharm Res 6(1):226–231
Arikan E, Ozsoy H (2015) A review: investigation of bioplastics. J Civ Eng Arch 9:188–192
Jabeen N, Majid I, Nayik GA (2015) Bioplastics and food packaging: a review. Cogent Food Agric 1(1):1117749
Ilyas R, et al (2016) Nanocrystalline cellulose reinforced starch-based nanocomposite: a review. In: 5th Postgraduate seminar on natural fiber composites. Universiti Putra Malaysia Serdang, Selangor
Reddy RL, Reddy VS, Gupta GA (2013) Study of bio-plastics as green and sustainable alternative to plastics. Int J Emerg Technol Adv Eng 3(5):76–81
Sidek IS et al (2019) Current development on bioplastics and its future prospects: an introductory review. INWASCON Technol Mag 1:03–08
Andrady AL, Neal MA (2009) Applications and societal benefits of plastics. Philos Trans R Soc B Biol Sci 364(1526):1977–1984
Thomas P et al (2020) Comprehensive review on nanocellulose: recent developments, challenges and future prospects. J Mech Behav Biomed Mater 110:103884
Chirayil CJ, Mathew L, Thomas S (2014) Review of recent research in nano cellulose preparation from different lignocellulosic fibers. Rev Adv Mater Sci 37:20–28
Faruk O et al (2012) Biocomposites reinforced with natural fibers: 2000–2010. Prog Polym Sci 37(11):1552–1596
Rajinipriya M et al (2018) Importance of agricultural and industrial waste in the field of nanocellulose and recent industrial developments of wood based nanocellulose: a review. ACS Sustain Chem Eng 6(3):2807–2828
Liu Y et al (2017) Efficient cleavage of lignin–carbohydrate complexes and ultrafast extraction of lignin oligomers from wood biomass by microwave-assisted treatment with deep eutectic solvent. Chemsuschem 10(8):1692–1700
Lee H, Hamid SBA, Zain S (2014) Conversion of lignocellulosic biomass to nanocellulose: structure and chemical process. Sci World J 2014:1–20
Reid MS, Villalobos M, Cranston ED (2017) Benchmarking cellulose nanocrystals: from the laboratory to industrial production. Langmuir 33(7):1583–1598
Moon RJ et al (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev 40(7):3941–3994
Trache D (2018) Nanocellulose as a promising sustainable material for biomedical applications. AIMS Mater Sci 5:201–205
do Nascimento DM et al (2016) A comprehensive approach for obtaining cellulose nanocrystal from coconut fiber. Part I: proposition of technological pathways. Ind Crops Prod 93:66–75
Brown RM, Montezinos D (1976) Cellulose microfibrils: visualization of biosynthetic and orienting complexes in association with the plasma membrane. Proc Natl Acad Sci 73(1):143–147
Ljungberg N et al (2005) New nanocomposite materials reinforced with cellulose whiskers in atactic polypropylene: effect of surface and dispersion characteristics. Biomacromol 6(5):2732–2739
McNamara JT, Morgan JL, Zimmer J (2015) A molecular description of cellulose biosynthesis. Annu Rev Biochem 84:895–921
Klemm D et al (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed 44(22):3358–3393
Kobayashi S, Sakamoto J, Kimura S (2001) In vitro synthesis of cellulose and related polysaccharides. Prog Polym Sci 26(9):1525–1560
Zhu JY, Sabo R, Luo X (2011) Integrated production of nano-fibrillated cellulose and cellulosic biofuel (ethanol) by enzymatic fractionation of wood fibers. Green Chem 13(5):1339–1344
Stanisławska A (2016) Bacterial nanocellulose as a microbiological derived nanomaterial. Adv Mater Sci 16(4):45–57
Farooq A et al (2020) Cellulose from sources to nanocellulose and an overview of synthesis and properties of nanocellulose/zinc oxide nanocomposite materials. Int J Biol Macromol 154:1050–1073
Kenned JJ, Sankaranarayanasamy K, Kumar CS (2021) Chemical, biological, and nanoclay treatments for natural plant fiber-reinforced polymer composites: a review. Polym Polym Compos 29(7):1011–1038
Kabir M et al (2012) Chemical treatments on plant-based natural fibre reinforced polymer composites: an overview. Compos B Eng 43(7):2883–2892
Belgacem MN, Gandini A (2005) The surface modification of cellulose fibres for use as reinforcing elements in composite materials. Compos Interfaces 12(1–2):41–75
Bhatnagar A, Sain M (2005) Processing of cellulose nanofiber-reinforced composites. J Reinf Plast Compos 24(12):1259–1268
Noremylia M, Hassan MZ, Ismail Z (2022) Recent advancement in isolation, processing, characterization and applications of emerging nanocellulose: a review. Int J Biol Macromol 206:954–976
Onkarappa H et al (2020) Hevea brasiliensis mediated synthesis of nanocellulose: effect of preparation methods on morphology and properties. Int J Biol Macromol 160:1021–1028
Vitz J et al (2009) Extended dissolution studies of cellulose in imidazolium based ionic liquids. Green Chem 11(3):417–424
Wahlström R, Suurnäkki A (2015) Enzymatic hydrolysis of lignocellulosic polysaccharides in the presence of ionic liquids. Green Chem 17(2):694–714
Phanthong P et al (2018) Nanocellulose: extraction and application. Carbon Resour Convers 1(1):32–43
Janardhnan S, Sain MM (2006) Isolation of cellulose microfibrils—an enzymatic approach. BioResources 1(2):176–188
Henriksson M et al (2007) An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur Polym J 43(8):3434–3441
López-Rubio A et al (2007) Enhanced film forming and film properties of amylopectin using micro-fibrillated cellulose. Carbohydr Polym 68(4):718–727
Faradilla RF et al (2017) Characteristics of a free-standing film from banana pseudostem nanocellulose generated from TEMPO-mediated oxidation. Carbohydr Polym 174:1156–1163
Isogai A, Saito T, Fukuzumi H (2011) TEMPO-oxidized cellulose nanofibers. Nanoscale 3(1):71–85
Nechyporchuk O, Belgacem MN, Bras J (2016) Production of cellulose nanofibrils: a review of recent advances. Ind Crops Prod 93:2–25
Zhou Y et al (2018) Acid-free preparation of cellulose nanocrystals by TEMPO oxidation and subsequent cavitation. Biomacromol 19(2):633–639
Qingqing L, Renneckar S (2011) Supramolecular structure characterization of molecularly thin cellulose ı nanoparticles. Biomacromol 12(3):650–659
Larsson PA, Berglund LA, Wågberg L (2014) Highly ductile fibres and sheets by core-shell structuring of the cellulose nanofibrils. Cellulose 21(1):323–333
Liimatainen H et al (2013) High-strength nanocellulose–talc hybrid barrier films. ACS Appl Mater Interfaces 5(24):13412–13418
Novo LP et al (2016) A study of the production of cellulose nanocrystals through subcritical water hydrolysis. Ind Crops Prod 93:88–95
Zhao Y, Huerta RR, Saldaña MD (2019) Use of subcritical water technology to develop cassava starch/chitosan/gallic acid bioactive films reinforced with cellulose nanofibers from canola straw. J Supercrit Fluids 148:55–65
Novo LP et al (2015) Subcritical water: a method for green production of cellulose nanocrystals. ACS Sustain Chem Eng 3(11):2839–2846
Adschiri T et al (2011) Green materials synthesis with supercritical water. Green Chem 13(6):1380–1390
Vallejos ME et al (2012) Low liquid–solid ratio (LSR) hot water pretreatment of sugarcane bagasse. Green Chem 14(7):1982–1989
Xiang Q et al (2003) Heterogeneous aspects of acid hydrolysis of α-cellulose. In: Davison BH, Lee JW, Finkelstein M, McMillan JD (eds) Biotechnology for fuels and chemicals. Springer, pp 505–514
Bandura AV, Lvov SN (2006) The ionization constant of water over wide ranges of temperature and density. J Phys Chem Ref Data 35(1):15–30
Cantero DA et al (2015) Simultaneous and selective recovery of cellulose and hemicellulose fractions from wheat bran by supercritical water hydrolysis. Green Chem 17(1):610–618
Louw J et al (2014) Thermodynamic modelling of supercritical water gasification: Investigating the effect of biomass composition to aid in the selection of appropriate feedstock material. Bioresour Technol 174:11–23
Cantero DA et al (2015) Energetic approach of biomass hydrolysis in supercritical water. Bioresour Technol 179:136–143
Meillisa A, Woo H-C, Chun B-S (2015) Production of monosaccharides and bio-active compounds derived from marine polysaccharides using subcritical water hydrolysis. Food Chem 171:70–77
Sasaki M et al (2002) Kinetics and mechanism of cellobiose hydrolysis and retro-aldol condensation in subcritical and supercritical water. Ind Eng Chem Res 41(26):6642–6649
Ranby B (1949) Aqueous colloidal solutions of cellulose micelles. Munksgaard International Publishers Ltd., Copenhagen, pp 649–650
Liu Y et al (2014) A novel approach for the preparation of nanocrystalline cellulose by using phosphotungstic acid. Carbohydr Polym 110:415–422
Lu P, Hsieh Y-L (2012) Preparation and characterization of cellulose nanocrystals from rice straw. Carbohydr Polym 87(1):564–573
Habibi Y, Lucia LA, Rojas OJ (2010) Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem Rev 110(6):3479–3500
Kargarzadeh H et al (2012) Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 19(3):855–866
Revol J-F et al (1992) Helicoidal self-ordering of cellulose microfibrils in aqueous suspension. Int J Biol Macromol 14(3):170–172
Dong XM, Revol J-F, Gray DG (1998) Effect of microcrystallite preparation conditions on the formation of colloid crystals of cellulose. Cellulose 5(1):19–32
Rosa M et al (2010) Cellulose nanowhiskers from coconut husk fibers: Effect of preparation conditions on their thermal and morphological behavior. Carbohydr Polym 81(1):83–92
Leung AC et al (2011) Characteristics and properties of carboxylated cellulose nanocrystals prepared from a novel one-step procedure. Small 7(3):302–305
Zhang K et al (2016) Extraction and comparison of carboxylated cellulose nanocrystals from bleached sugarcane bagasse pulp using two different oxidation methods. Carbohydr Polym 138:237–243
Mascheroni E et al (2016) Comparison of cellulose nanocrystals obtained by sulfuric acid hydrolysis and ammonium persulfate, to be used as coating on flexible food-packaging materials. Cellulose 23(1):779–793
Isogai A, Atalla R (1998) Dissolution of cellulose in aqueous NaOH solutions. Cellulose 5(4):309–319
Li Y et al (2014) High yield preparation method of thermally stable cellulose nanofibers. BioResources 9(2):1986–1997
Qu P et al (2010) Nanocomposites of poly (lactic acid) reinforced with cellulose nanofibrils. BioResources 5(3):1811–1823
Wang S, Lu A, Zhang L (2016) Recent advances in regenerated cellulose materials. Prog Polym Sci 53:169–206
Yang Y-J et al (2014) Cellulose dissolution in aqueous lithium bromide solutions. Cellulose 21(3):1175–1181
Jiang F, Hsieh Y-L (2014) Synthesis of cellulose nanofibril bound silver nanoprism for surface enhanced Raman scattering. Biomacromol 15(10):3608–3616
Cai J et al (2008) Dynamic self-assembly induced rapid dissolution of cellulose at low temperatures. Macromolecules 41(23):9345–9351
Yu H-Y et al (2013) Comparison of the reinforcing effects for cellulose nanocrystals obtained by sulfuric and hydrochloric acid hydrolysis on the mechanical and thermal properties of bacterial polyester. Compos Sci Technol 87:22–28
Shahi N et al (2021) Biopolymers fractionation and synthesis of nanocellulose/silica nanoparticles from agricultural byproducts. ACS Sustain Chem Eng 9(18):6284–6295
Dufresne A (2010) Processing of polymer nanocomposites reinforced with polysaccharide nanocrystals. Molecules 15(6):4111–4128
Chakraborty A, Sain M, Kortschot M (2005) Cellulose microfibrils: a novel method of preparation using high shear refining and cryocrushing. Holzforschung 59:102–107
Lee S-Y et al (2009) Preparation of cellulose nanofibrils by high-pressure homogenizer and cellulose-based composite films. J Ind Eng Chem 15(1):50–55
Ferrer A et al (2012) Valorization of residual Empty Palm Fruit Bunch Fibers (EPFBF) by microfluidization: production of nanofibrillated cellulose and EPFBF nanopaper. Bioresour Technol 125:249–255
Frone AN et al (2011) Preparation and characterization of PVA composites with cellulose nanofibers obtained by ultrasonication. BioResources 6(1):487–512
Johnson RK et al (2009) A new bio-based nanocomposite: fibrillated TEMPO-oxidized celluloses in hydroxypropylcellulose matrix. Cellulose 16(2):227–238
Filson PB, Dawson-Andoh BE, Schwegler-Berry D (2009) Enzymatic-mediated production of cellulose nanocrystals from recycled pulp. Green Chem 11(11):1808–1814
Habibi Y (2014) Key advances in the chemical modification of nanocelluloses. Chem Soc Rev 43(5):1519–1542
Dufresne A (2017) Nanocellulose: from nature to high performance tailored materials. Walter de Gruyter GmbH & Co KG
Bharimalla AK et al (2015) Energy efficient manufacturing of nanocellulose by chemo-and bio-mechanical processes: a review. World J Nano Sci Eng 5(04):204
Du L et al (2017) A co-production of sugars, lignosulfonates, cellulose, and cellulose nanocrystals from ball-milled woods. Bioresour Technol 238:254–262
Niu F et al (2017) The characteristic and dispersion stability of nanocellulose produced by mixed acid hydrolysis and ultrasonic assistance. Carbohydr Polym 165:197–204
Zhao J et al (2015) Polyethylenimine-grafted cellulose nanofibril aerogels as versatile vehicles for drug delivery. ACS Appl Mater Interfaces 7(4):2607–2615
Li N et al (2018) Rod-like cellulose nanocrystal/cis-aconityl-doxorubicin prodrug: a fluorescence-visible drug delivery system with enhanced cellular uptake and intracellular drug controlled release. Mater Sci Eng C 91:179–189
Lee H et al (2018) Improved thermal stability of cellulose nanofibrils using low-concentration alkaline pretreatment. Carbohydr Polym 181:506–513
Ganguly K et al (2020) Stimuli-responsive self-assembly of cellulose nanocrystals (CNCs): Structures, functions, and biomedical applications. Int J Biol Macromol 155:456–469
Mariano M, El Kissi N, Dufresne A (2014) Cellulose nanocrystals and related nanocomposites: review of some properties and challenges. J Polym Sci Part B Polym Phys 52(12):791–806
Espino E et al (2014) Isolation and characterization of cellulose nanocrystals from industrial by-products of Agave tequilana and barley. Ind Crops Prod 62:552–559
Ureña-Benavides EE et al (2011) Rheology and phase behavior of lyotropic cellulose nanocrystal suspensions. Macromolecules 44(22):8990–8998
Tayeb AH et al (2018) Cellulose nanomaterials—binding properties and applications: a review. Molecules 23(10):2684
Malucelli LC et al (2017) Preparation, properties and future perspectives of nanocrystals from agro-industrial residues: a review of recent research. Rev Environ Sci Bio/Technol 16(1):131–145
Marchessault R, Morehead F, Walter N (1959) Liquid crystal systems from fibrillar polysaccharides. Nature 184(4686):632–633
Parker RM et al (2018) The self-assembly of cellulose nanocrystals: hierarchical design of visual appearance. Adv Mater 30(19):1704477
Dufresne A (2013) Nanocellulose: a new ageless bionanomaterial. Mater Today 16(6):220–227
Mugaanire IT, Wang H, Sun J (2019) Fibrous microcrystalline cellulose from Ficus natalensis barkcloth. Eur J Wood Wood Prod 77(3):483–486
Wang H et al (2020) Extraction of cellulose nanocrystals using a recyclable deep eutectic solvent. Cellulose 27(3):1301–1314
Ahola S, Österberg M, Laine J (2008) Cellulose nanofibrils—adsorption with poly (amideamine) epichlorohydrin studied by QCM-D and application as a paper strength additive. Cellulose 15(2):303–314
Abe K, Iwamoto S, Yano H (2007) Obtaining cellulose nanofibers with a uniform width of 15 nm from wood. Biomacromol 8(10):3276–3278
Henriksson M et al (2008) Cellulose nanopaper structures of high toughness. Biomacromol 9(6):1579–1585
Li P et al (2017) Cellulose nanofibrils from nonderivatizing urea-based deep eutectic solvent pretreatments. ACS Appl Mater Interfaces 9(3):2846–2855
Pääkkö M et al (2007) Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromol 8(6):1934–1941
Alemdar A, Sain M (2008) Isolation and characterization of nanofibers from agricultural residues—wheat straw and soy hulls. Biores Technol 99(6):1664–1671
Hassan ML et al (2012) Nanofibers from bagasse and rice straw: process optimization and properties. Wood Sci Technol 46(1–3):193–205
Tonoli G et al (2012) Cellulose micro/nanofibres from Eucalyptus kraft pulp: preparation and properties. Carbohydr Polym 89(1):80–88
Desmaisons J et al (2017) A new quality index for benchmarking of different cellulose nanofibrils. Carbohydr Polym 174:318–329
Saito T, Isogai A (2006) Introduction of aldehyde groups on surfaces of native cellulose fibers by TEMPO-mediated oxidation. Colloids Surf A 289(1–3):219–225
Besbes I, Vilar MR, Boufi S (2011) Nanofibrillated cellulose from alfa, eucalyptus and pine fibres: preparation, characteristics and reinforcing potential. Carbohydr Polym 86(3):1198–1206
Aulin C et al (2010) Self-organized films from cellulose I nanofibrils using the layer-by-layer technique. Biomacromol 11(4):872–882
Löbmann K et al (2017) Cellulose nanopaper and nanofoam for patient-tailored drug delivery. Adv Mater Interfaces 4(9):1600655
Farooq A et al (2019) Structure and properties of high quality natural cellulose nano fibrils from a novel material Ficus natalensis barkcloth. J Ind Text 51:664–680
Wei S, Ching YC, Chuah CH (2020) Synthesis of chitosan aerogels as promising carriers for drug delivery: a review. Carbohydr Polym 231:115744
Halib N et al (2017) Potential applications of nanocellulose-containing materials in the biomedical field. Materials 10(8):977
Salimi S et al (2019) Production of nanocellulose and its applications in drug delivery: a critical review. ACS Sustain Chem Eng 7(19):15800–15827
Akhlaghi SP et al (2014) Comparative release studies of two cationic model drugs from different cellulose nanocrystal derivatives. Eur J Pharm Biopharm 88(1):207–215
Miraftab R, Huining X (2019) Feasibility and potential of graphene and its hybrids with cellulose as drug carriers: a commentary. J Bioresour Bioprod 4(4):200–201
Gopinath V et al (2018) A review of natural polysaccharides for drug delivery applications: special focus on cellulose, starch and glycogen. Biomed Pharmacother 107:96–108
Khan MI et al (2019) Chitosan-based polymer matrix for pharmaceutical excipients and drug delivery. Curr Med Chem 26(14):2502–2513
Bernkop-Schnürch A, Dünnhaupt S (2012) Chitosan-based drug delivery systems. Eur J Pharm Biopharm 81(3):463–469
Siepmann J, Peppas NA (2012) Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Rev 64:163–174
Liu L et al (2003) Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials 24(19):3333–3343
Sriamornsak P (2011) Application of pectin in oral drug delivery. Expert Opin Drug Deliv 8(8):1009–1023
Evdokimova OL et al (2018) Hybrid drug delivery patches based on spherical cellulose nanocrystals and colloid titania—synthesis and antibacterial properties. Nanomaterials 8(4):228
Galkina O et al (2015) Antibacterial and photochemical properties of cellulose nanofiber–titania nanocomposites loaded with two different types of antibiotic medicines. J Mater Chem B 3(35):7125–7134
Liu L, Fishman ML, Hicks KB (2007) Pectin in controlled drug delivery—a review. Cellulose 14(1):15–24
Galkina O et al (2015) Cellulose nanofiber–titania nanocomposites as potential drug delivery systems for dermal applications. J Mater Chem B 3(8):1688–1698
Huang C et al (2019) Production of dissolving grade pulp from tobacco stalk through SO2-ethanol-water fractionation, alkaline extraction, and bleaching processes. BioResources 14(3):5544–5558
Iguchi M, Yamanaka S, Budhiono A (2000) Bacterial cellulose—a masterpiece of nature’s arts. J Mater Sci 35(2):261–270
Sirajunnisa AR, Surendhiran D (2016) Algae—a quintessential and positive resource of bioethanol production: a comprehensive review. Renew Sustain Energy Rev 66:248–267
Li D-C, Papageorgiou AC (2019) Cellulases from thermophilic fungi: recent insights and biotechnological potential. In: Fungi in extreme environments: ecological role and biotechnological significance, pp 395–417
Römling U, Galperin MY (2015) Bacterial cellulose biosynthesis: diversity of operons, subunits, products, and functions. Trends Microbiol 23(9):545–557
Brown AJ (1886) XLIII.—on an acetic ferment which forms cellulose. J Chem Soc Trans 49:432–439
Brown RM Jr (1996) The biosynthesis of cellulose. J Macromol Sci Part A Pure Appl Chem 33(10):1345–1373
Augimeri RV, Varley AJ, Strap JL (2015) Establishing a role for bacterial cellulose in environmental interactions: lessons learned from diverse biofilm-producing Proteobacteria. Front Microbiol 6:1282
Rangaswamy B, Vanitha K, Hungund BS (2015) Microbial cellulose production from bacteria isolated from rotten fruit. Int J Polym Sci 2015:1–8
Wang J, Tavakoli J, Tang Y (2019) Bacterial cellulose production, properties and applications with different culture methods—a review. Carbohydr Polym 219:63–76
Bäckdahl H et al (2008) Engineering microporosity in bacterial cellulose scaffolds. J Tissue Eng Regen Med 2(6):320–330
Gatenholm P, Klemm D (2010) Bacterial nanocellulose as a renewable material for biomedical applications. MRS Bull 35(3):208–213
Portela R et al (2019) Bacterial cellulose: a versatile biopolymer for wound dressing applications. Microb Biotechnol 12(4):586–610
Ramana K, Tomar A, Singh L (2000) Effect of various carbon and nitrogen sources on cellulose synthesis by Acetobacter xylinum. World J Microbiol Biotechnol 16(3):245–248
Cirillo G et al (2013) Incorporation of carbon nanotubes into a gelatin–catechin conjugate: innovative approach for the preparation of anticancer materials. Int J Pharm 446(1–2):176–182
Lin W et al (2020) Insight into understanding the performance of deep eutectic solvent pretreatment on improving enzymatic digestibility of bamboo residues. Bioresour Technol 306:123163
Brown RM, Willison J, Richardson CL (1976) Cellulose biosynthesis in Acetobacter xylinum: visualization of the site of synthesis and direct measurement of the in vivo process. Proc Natl Acad Sci 73(12):4565–4569
Skiba EA et al (2020) A technology for pilot production of bacterial cellulose from oat hulls. Chem Eng J 383:123128
Chiozzini GC et al (2021) Bacterial nanocellulose membrane as bolus in radiotherapy:" proof of concept". Cellulose 28(2):607–613
Hsieh Y-C et al (2008) An estimation of the Young’s modulus of bacterial cellulose filaments. Cellulose 15(4):507–513
Nakagaito A, Iwamoto S, Yano H (2005) Bacterial cellulose: the ultimate nano-scalar cellulose morphology for the production of high-strength composites. Appl Phys A 80(1):93–97
Trovatti E et al (2010) Novel bacterial cellulose–acrylic resin nanocomposites. Compos Sci Technol 70(7):1148–1153
Zhang HY, et al (2011) Development and characteristic of bacterial cellulose for antimicrobial wound dressing. In: Advanced materials research. Trans Tech Publ.
Klemm D, et al (2006) Nanocelluloses as innovative polymers in research and application. In: Polysaccharides II, pp 49–96
Khan MR, Liao S, Farooq A, Naeem MA, Wasim M, Wei Q (2023) Regeneration and modification of cellulose acetate from cigarette waste: Biomedical potential by encapsulation of tetracycline hydrochloride. Int J Biologic Macromol 250:126266
Olsson RT et al (2010) Making flexible magnetic aerogels and stiff magnetic nanopaper using cellulose nanofibrils as templates. Nat Nanotechnol 5(8):584–588
Wang L et al (2014) Carbon aerogels from bacterial nanocellulose as anodes for lithium ion batteries. RSC Adv 4(34):17549–17554
Marestoni LD, Barud HDS, Gomes RJ, Catarino RPF, Hata NNY, Ressutte JB, Spinosa WA (2021) Commercial and potential applications of bacterial cellulose in Brazil: ten years review. Polímeros 30:e2020047
Wiegand C et al (2015) Antimicrobial functionalization of bacterial nanocellulose by loading with polihexanide and povidone-iodine. J Mater Sci Mater Med 26(10):1–14
Celes FS et al (2022) A pilot and open trial to evaluate topical bacterial cellulose bio-curatives in the treatment of cutaneous leishmaniasis caused by L. braziliensis. Acta Trop 225:106192
Wan W et al (2006) Bacterial cellulose and its nanocomposites for biomedical applications. ACS Publications
Wang J, Zhu Y, Du J (2011) Bacterial cellulose: a natural nanomaterial for biomedical applications. J Mech Med Biol 11(02):285–306
Rajwade J, Paknikar K, Kumbhar J (2015) Applications of bacterial cellulose and its composites in biomedicine. Appl Microbiol Biotechnol 99(6):2491–2511
Grande CJ et al (2009) Nanocomposites of bacterial cellulose/hydroxyapatite for biomedical applications. Acta Biomater 5(5):1605–1615
Ullah H et al (2016) Advances in biomedical and pharmaceutical applications of functional bacterial cellulose-based nanocomposites. Carbohydr Polym 150:330–352
Li G et al (2017) An environmentally benign approach to achieving vectorial alignment and high microporosity in bacterial cellulose/chitosan scaffolds. RSC Adv 7(23):13678–13688
Luo H et al (2018) Preparation of oriented bacterial cellulose nanofibers by flowing medium-assisted biosynthesis and influence of flowing velocity. J Polym Eng 38(3):299–305
Torres FG, Commeaux S, Troncoso OP (2012) Biocompatibility of bacterial cellulose based biomaterials. J Funct Biomater 3(4):864–878
Campano C et al (2016) Enhancement of the fermentation process and properties of bacterial cellulose: a review. Cellulose 23(1):57–91
Wei B, Yang G, Hong F (2011) Preparation and evaluation of a kind of bacterial cellulose dry films with antibacterial properties. Carbohydr Polym 84(1):533–538
Chen L et al (2016) Highly thermal-stable and functional cellulose nanocrystals and nanofibrils produced using fully recyclable organic acids. Green Chem 18(13):3835–3843
Chen Y et al (2017) An efficient method for cellulose nanofibrils length shearing via environmentally friendly mixed cellulase pretreatment. J Nanomater 2017:1–12
Alkhatib Y et al (2017) Controlled extended octenidine release from a bacterial nanocellulose/Poloxamer hybrid system. Eur J Pharm Biopharm 112:164–176
Weber C et al (2002) Production and applications of biobased packaging materials for the food industry. Food Addit Contam 19(S1):172–177
De Azeredo HM (2009) Nanocomposites for food packaging applications. Food Res Int 42(9):1240–1253
Abraham E et al (2013) Physicomechanical properties of nanocomposites based on cellulose nanofibre and natural rubber latex. Cellulose 20:417–427
Phomrak S, Phisalaphong M (2017) Reinforcement of natural rubber with bacterial cellulose via a latex aqueous microdispersion process. J Nanomater 2017:1–9
Bitinis N et al (2013) Poly (lactic acid)/natural rubber/cellulose nanocrystal bionanocomposites part I. Processing and morphology. Carbohydr Polym 96(2):611–620
Abdollahi M et al (2013) Comparing physico-mechanical and thermal properties of alginate nanocomposite films reinforced with organic and/or inorganic nanofillers. Food Hydrocolloids 32(2):416–424
Alves J et al (2015) Effect of cellulose nanocrystals and gelatin in corn starch plasticized films. Carbohydr Polym 115:215–222
Cui S et al (2016) Green preparation and characterization of size-controlled nanocrystalline cellulose via ultrasonic-assisted enzymatic hydrolysis. Ind Crops Prod 83:346–352
Dufresne A (2019) Nanocellulose processing properties and potential applications. Curr For Rep 5(2):76–89
Charreau H, Cavallo E, Foresti ML (2020) Patents involving nanocellulose: analysis of their evolution since 2010. Carbohydr Polym 237:116039
Mardirossian M et al (2017) D-BMAP18 antimicrobial peptide is active in vitro, resists to pulmonary proteases but loses its activity in a murine model of Pseudomonas aeruginosa lung infection. Front Chem 5:40
Trache D, Thakur VK, Boukherroub R (2020) Cellulose nanocrystals/graphene hybrids—a promising new class of materials for advanced applications. Nanomaterials 10(8):1523
Xue Y, Mou Z, Xiao H (2017) Nanocellulose as a sustainable biomass material: structure, properties, present status and future prospects in biomedical applications. Nanoscale 9(39):14758–14781
Mu R et al (2019) Recent trends and applications of cellulose nanocrystals in food industry. Trends Food Sci Technol 93:136–144
Zanchetta G, Rocchi E, Piazza L (2017) Seeing is believing: coupling between liquid crystalline ordering and rheological behavior in cellulose nanocrystals suspensions. Chem Eng Trans 57:1933–1938
da Gama FMP, Dourado F (2018) Bacterial nanocellulose: what future? Bioimpacts 8(1):1
Khan T, Park JK, Kwon J-H (2007) Functional biopolymers produced by biochemical technology considering applications in food engineering. Korean J Chem Eng 24(5):816–826
Nascimento DM et al (2018) Nanocellulose nanocomposite hydrogels: technological and environmental issues. Green Chem 20(11):2428–2448
Azeredo HM et al (2019) Bacterial cellulose as a raw material for food and food packaging applications. Front Sustain Food Syst 3:7
Bai L et al (2019) Oil-in-water Pickering emulsions via microfluidization with cellulose nanocrystals: 1. Formation and stability. Food Hydrocolloids 96:699–708
Chi K, Catchmark JM (2018) Sustainable development of polysaccharide polyelectrolyte complexes as eco-friendly barrier materials for packaging applications. In: Green polymer chemistry: new products, processes, and applications. ACS Publications, pp 109–123
Robin F, Schuchmann HP, Palzer S (2012) Dietary fiber in extruded cereals: limitations and opportunities. Trends Food Sci Technol 28(1):23–32
Turbak AF, Snyder FW, Sandberg KR (1983) Microfibrillated cellulose, a new cellulose product: properties, uses, and commercial potential. In: Journal of Applied Polymer Science: Applied Polymer Symposium
Ström G, Öhgren C, Ankerfors M (2013) Nanocellulose as an additive in foodstuff. Innventia Rep 403:1–25
Kleinschmidt D, et al (1988) US 4.774. 095. Washington, DC: US Patent and Trademark Office
Asghari M, Karimi Zarchi AA, Taheri RA (2021) Preparation and characterization nanocrystalline cellulose as a food additive to produce healthy biscuit cream. Starch-Stärke 73(3–4):2000033
Jafari SM et al (2018) The influence of nanocellulose coating on saffron quality during storage. Carbohydr Polym 181:536–542
Mugwagwa LR, Chimphango AF (2020) Enhancing the functional properties of acetylated hemicellulose films for active food packaging using acetylated nanocellulose reinforcement and polycaprolactone coating. Food Packag Shelf Life 24:100481
Huq T et al (2014) Microencapsulation of nisin in alginate-cellulose nanocrystal (CNC) microbeads for prolonged efficacy against Listeria monocytogenes. Cellulose 21(6):4309–4321
Jung S et al (2020) Multifunctional bio-nanocomposite coatings for perishable fruits. Adv Mater 32(26):1908291
Nasution H, Afandy Y, Al-fath MT (2018) Effect of cellulose nanocrystals (CNC) addition and citric acid as co-plasticizer on physical properties of sago starch biocomposite. In: AIP conference proceedings. AIP Publishing LLC
Gao H et al (2018) Fabrication of cellulose nanofibers from waste brown algae and their potential application as milk thickeners. Food Hydrocolloids 79:473–481
Carpenter MA, Geletkanycz MA, Sanders WG (2004) Upper echelons research revisited: antecedents, elements, and consequences of top management team composition. J Manag 30(6):749–778
Liu J et al (2016) Hemicellulose-reinforced nanocellulose hydrogels for wound healing application. Cellulose 23(5):3129–3143
Liu H et al (2018) Self-healing and injectable polysaccharide hydrogels with tunable mechanical properties. Cellulose 25(1):559–571
Huang W et al (2018) On-demand dissolvable self-healing hydrogel based on carboxymethyl chitosan and cellulose nanocrystal for deep partial thickness burn wound healing. ACS Appl Mater Interfaces 10(48):41076–41088
Silva NH et al (2020) Topical drug delivery systems based on bacterial nanocellulose: Accelerated stability testing. Int J Mol Sci 21(4):1262
Prausnitz MR, Langer R (2008) Transdermal drug delivery. Nat Biotechnol 26(11):1261–1268
Rebouillat S, Ortega-Requena S (2015) Potential applications of milk fractions and valorization of dairy by-products: a review of the state-of-the-art available data, outlining the innovation potential from a bigger data standpoint. J Biomater Nanobiotechnol 6(03):176
Yu H et al (2013) Facile extraction of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acid hydrolysis under hydrothermal conditions. J Mater Chem A 1(12):3938–3944
Mauricio MR et al (2015) Synthesis of a microhydrogel composite from cellulose nanowhiskers and starch for drug delivery. Carbohydr Polym 115:715–722
Svagan AJ et al (2016) Solid cellulose nanofiber based foams—towards facile design of sustained drug delivery systems. J Control Release 244:74–82
Rao KM, Kumar A, Han SS (2017) Poly (acrylamidoglycolic acid) nanocomposite hydrogels reinforced with cellulose nanocrystals for pH-sensitive controlled release of diclofenac sodium. Polym Test 64:175–182
Badshah M et al (2017) Preparation, characterization and in-vitro evaluation of bacterial cellulose matrices for oral drug delivery. Cellulose 24(11):5041–5052
Barbosa AM et al (2016) Cellulose nanocrystal membranes as excipients for drug delivery systems. Materials 9(12):1002
Yang J et al (2011) Biotemplated preparation of CdS nanoparticles/bacterial cellulose hybrid nanofibers for photocatalysis application. J Hazard Mater 189(1–2):377–383
Gupta A et al (2020) Synthesis of silver nanoparticles using curcumin-cyclodextrins loaded into bacterial cellulose-based hydrogels for wound dressing applications. Biomacromol 21(5):1802–1811
Eichhorn SJ (2011) Cellulose nanowhiskers: promising materials for advanced applications. Soft Matter 7(2):303–315
Lazarini SC et al (2016) Characterization of bilayer bacterial cellulose membranes with different fiber densities: a promising system for controlled release of the antibiotic ceftriaxone. Cellulose 23(1):737–748
Plappert SF et al (2019) Anisotropic nanocellulose gel–membranes for drug delivery: Tailoring structure and interface by sequential periodate–chlorite oxidation. Carbohydr Polym 226:115306
Merindol R et al (2020) Assembly of anisotropic nanocellulose films stronger than the original tree. ACS Nano 14(12):16525–16534
Sarkar G et al (2017) Cellulose nanofibrils/chitosan based transdermal drug delivery vehicle for controlled release of ketorolac tromethamine. New J Chem 41(24):15312–15319
Orasugh JT et al (2018) Jute cellulose nano-fibrils/hydroxypropylmethylcellulose nanocomposite: a novel material with potential for application in packaging and transdermal drug delivery system. Ind Crops Prod 112:633–643
Abbas M et al (2019) (Bio) polymer/ZnO nanocomposites for packaging applications: a review of gas barrier and mechanical properties. Nanomaterials 9(10):1494
Verri T, Maffia M, Storelli C (1992) H+/glycyl-glycine cotransport in eel intestinal brush-border membrane vesicles: studies with the pH-sensitive dye Acridine orange. Biochim Biophys Acta (BBA) Biomembr 1110(1):123–126
Saïdi L et al (2017) Poly (N-methacryloyl glycine)/nanocellulose composites as pH-sensitive systems for controlled release of diclofenac. Carbohydr Polym 169:357–365
Medhi P et al (2017) Lidocaine-loaded fish scale-nanocellulose biopolymer composite microneedles. AAPS PharmSciTech 18(5):1488–1494
Müller D et al (2011) Chemical in situ polymerization of polypyrrole on bacterial cellulose nanofibers. Synth Metals 161(1–2):106–111
Fakes MG et al (2009) Enhancement of oral bioavailability of an HIV-attachment inhibitor by nanosizing and amorphous formulation approaches. Int J Pharm 370(1–2):167–174
Onofrei M, Filimon A (2016) Cellulose-based hydrogels: designing concepts, properties, and perspectives for biomedical and environmental applications. Polym Sci Res Adv Pract Appl Educ A, 108–120
Kamel S et al (2008) Pharmaceutical significance of cellulose: a review. Express Polym Lett 2(11):758–778
Di Natale C, De Gregorio V, Lagreca E, Mauro F, Corrado B, Vecchione R, Netti PA (2022) Engineered bacterial cellulose nanostructured matrix for incubation and release of drug-loaded oil in water nanoemulsion. Front Bioeng Biotechnol 10:851893
Taheri A, Mohammadi M (2015) The use of cellulose nanocrystals for potential application in topical delivery of hydroquinone. Chem Biol Drug Des 86(1):102–106
Chang C-W, Wang M-J (2013) Preparation of microfibrillated cellulose composites for sustained release of H2O2 or O2 for biomedical applications. ACS Sustain Chem Eng 1(9):1129–1134
Kolakovic R et al (2012) Nanofibrillar cellulose films for controlled drug delivery. Eur J Pharm Biopharm 82(2):308–315
Ruiz-Palomero C et al (2017) Photoluminescent sensing hydrogel platform based on the combination of nanocellulose and S, N-codoped graphene quantum dots. Sens Actuators B Chem 245:946–953
Kolakovic R et al (2013) Evaluation of drug interactions with nanofibrillar cellulose. Eur J Pharm Biopharm 85(3):1238–1244
Bhattacharya M et al (2012) Nanofibrillar cellulose hydrogel promotes three-dimensional liver cell culture. J Control Release 164(3):291–298
Lou Y-R et al (2014) The use of nanofibrillar cellulose hydrogel as a flexible three-dimensional model to culture human pluripotent stem cells. Stem Cells Dev 23(4):380–392
Kolakovic R et al (2012) Spray-dried nanofibrillar cellulose microparticles for sustained drug release. Int J Pharm 430(1–2):47–55
Kolakovic R et al (2011) Spray-dried cellulose nanofibers as novel tablet excipient. AAPS PharmSciTech 12(4):1366–1373
Lin N et al (2011) Effect of polysaccharide nanocrystals on structure, properties, and drug release kinetics of alginate-based microspheres. Colloids Surf B 85(2):270–279
Supramaniam J et al (2018) Magnetic nanocellulose alginate hydrogel beads as potential drug delivery system. Int J Biol Macromol 118:640–648
Akhlaghi SP, Berry RC, Tam KC (2013) Surface modification of cellulose nanocrystal with chitosan oligosaccharide for drug delivery applications. Cellulose 20(4):1747–1764
Golmohammadi H et al (2017) Nanocellulose in sensing and biosensing. Chem Mater 29(13):5426–5446
Du X et al (2017) Nanocellulose-based conductive materials and their emerging applications in energy devices-a review. Nano Energy 35:299–320
Kim J-H et al (2015) Review of nanocellulose for sustainable future materials. Int J Precis Eng Manuf Green Technol 2(2):197–213
Zheng Q et al (2016) High-performance flexible piezoelectric nanogenerators consisting of porous cellulose nanofibril (CNF)/poly (dimethylsiloxane)(PDMS) aerogel films. Nano Energy 26:504–512
Kim H-J et al (2017) Bacterial nano-cellulose triboelectric nanogenerator. Nano Energy 33:130–137
Noonan C et al (2019) Structure-property relationships in hybrid cellulose nanofibrils/Nafion-based ionic polymer-metal composites. Materials 12(8):1269
Rajala S et al (2016) Cellulose nanofibril film as a piezoelectric sensor material. ACS Appl Mater Interfaces 8(24):15607–15614
Csoka L et al (2012) Piezoelectric effect of cellulose nanocrystals thin films. ACS Macro Lett 1(7):867–870
Jeon J-H et al (2010) Bacterial cellulose actuator with electrically driven bending deformation in hydrated condition. Sens Actuators B Chem 146(1):307–313
Hänninen A et al (2018) Nanocellulose and chitosan based films as low cost, green piezoelectric materials. Carbohydr Polym 202:418–424
Fashandi H et al (2016) Morphological changes towards enhancing piezoelectric properties of PVDF electrical generators using cellulose nanocrystals. Cellulose 23(6):3625–3637
Marques PA et al (2008) Silver-bacterial cellulosic sponges as active SERS substrates. J Raman Spectrosc 39(4):439–443
Wei H, Vikesland PJ (2015) pH-triggered molecular alignment for reproducible SERS detection via an AuNP/nanocellulose platform. Sci Rep 5(1):1–10
Devarayan K, Kim B-S (2015) Reversible and universal pH sensing cellulose nanofibers for health monitor. Sens Actuators B Chem 209:281–286
Morales-Narváez E et al (2015) Nanopaper as an optical sensing platform. ACS Nano 9(7):7296–7305
Hu L, et al. Transparent and conductive paper from nanocellulose
Pourreza N et al (2015) Green in-situ synthesized silver nanoparticles embedded in bacterial cellulose nanopaper as a bionanocomposite plasmonic sensor. Biosens Bioelectron 74:353–359
Ruiz-Palomero C, Soriano ML, Valcárcel M (2015) β-Cyclodextrin decorated nanocellulose: a smart approach towards the selective fluorimetric determination of danofloxacin in milk samples. Analyst 140(10):3431–3438
Heggset EB et al (2019) Viscoelastic properties of nanocellulose based inks for 3D printing and mechanical properties of CNF/alginate biocomposite gels. Cellulose 26:581–595
Alexandrescu L et al (2013) Cytotoxicity tests of cellulose nanofibril-based structures. Cellulose 20:1765–1775
Balea A et al (2021) Nanocellulose characterization challenges. BioResources 16(2):4382
Lin N, Dufresne A (2014) Nanocellulose in biomedicine: current status and future prospect. Eur Polym J 59:302–325
Endes C et al (2016) A critical review of the current knowledge regarding the biological impact of nanocellulose. J Nanobiotechnol 14(1):1–14
Isogai A (2021) Emerging nanocellulose technologies: recent developments. Adv Mater 33(28):2000630
Acknowledgements
This review work was funded by the National first-class discipline program of Light Industry Technology and Engineering: LITE2018-21, Jiangsu, China. Moreover, this review paper acknowledges the training of young academic scholars on nanocellulose materials by Key Laboratory of Eco Textiles, Jiangnan University's Wuxi, Jiangsu, China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors don’t have any type of conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khan, M.R., Wasim, M., Farooq, A. et al. A review study on derivation of nanocellulose to its functional properties and applications in drug delivery system, food packaging, and biosensing devices. Polym. Bull. 81, 9519–9568 (2024). https://doi.org/10.1007/s00289-024-05190-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-024-05190-4