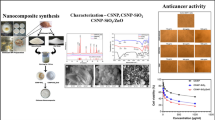
Abstract
Chitosan, the second naturally abundant polysaccharide, has shown promising anticancer activity against many cancer cells. There are various chitosan nanoparticle preparation techniques. This study compared three of these methods, namely, ionotropic gelation, microemulsion, and emulsification solvent diffusion in terms of their product physicochemical and biological properties. To compare different methods, type of chitosan, pH and concentration of chitosan solution were kept constant in all methods. The obtained chitosan nanoparticles were characterized using FTIR, UV–Visible spectroscopy, and SEM. The anticancer activity of the nanoparticles was evaluated by MTT assay in MDA-MB-231 cells at different doses (0.5, 1, 1.5, 2 mg/mL). The morphological alterations of cells were assessed by light inverted microscope. All three methods resulted in nanoparticle formation with the size and zeta potential range of 240–442 nm and + 19.1–34.6 mV, respectively. The ionotropic gelation method yielded smaller nanoparticles with higher zeta potential than those yielded by the microemulsion and emulsification solvent diffusion methods. The cytotoxicity assay showed a dose-dependent effect of nanoparticles. The nanochitosans prepared using the ionotropic gelation, microemulsion, and emulsification solvent diffusion methods showed maximum 77.87%, 63.12%, and 53.17% inhibition against MDA-MB-231 cells, respectively. The results concluded that all obtained nanoparticles have acceptable potency for cytotoxicity against MDA-MB-231 cells with IC50 ranged from 0.89 to 1.67 mg / mL. However, nanoparticles prepared using ionotropic gelation method exhibit the highest anticancer activity. Overall, chitosan nanoparticles obtained using all three methods could serve as anticancer agents and applied in the development of novel antitumor drugs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cancer is a multistep and progressive disease with a low cure rate, usually accompanied by abnormalities in proliferation, metastasis, invasion, and metabolic disorders. A survey in 2020 by the World Health Organization (WHO) estimated about 19.3 million new cancer cases and almost 10.0 million died of the disease worldwide [1]. Age, family history, sunlight and ionizing radiation, some viruses and bacteria including Human papilloma viruses (HPVs), Hepatitis B and hepatitis C viruses, Salmonella typhi, lifestyle, and exposure to some organic and inorganic chemicals such as asbestos, benzene, benzidine, cadmium, nickel, arsenic, radon and vinyl chloride are among the risk factors for cancer development [2]. Although the current approaches for the cancer treatment include chemotherapy, radiotherapy, surgery, and immunotherapy are effective against many types of cancer, but also carry a risk of acute side effects [3]. Therefore, the discovery of new anticancer agents is critical to prevent complications and drug resistance problems caused by common clinical treatment methods.
Over the last few decades, some new anticancer agents have been identified as less toxic and capable of overcoming the resistance induced by the common chemotherapy drugs. Glutamic acid and its derivatives [4], metal ion complexes [5], organic and inorganic nanostructure materials are examples of these new anticancer compounds [6].
Glutamine, a derivative of glutamic acid, plays very important roles in cancer cells. This amino acid is essential for the rapid growth of tumor cells because it acts as a nitrogen donor in the nucleotide and amino acid biosynthesis. It also helps in the uptake of other essential amino acids and maintains the activation of TOR kinase [7]. Therefore, glutamine derivatives are believed to be able to prevent the growth of cancer cells through glutamine antagonism. Ali et al. [8] synthesized glutamic acid derivatives and their Cu (II) and Ru (III) complexes and showed their anticancer activities on a panel of human tumor cell lines.
However, among the new drugs and tools in the field of cancer detection and treatment, nanoparticles (1–100 nm) are one of the most interesting and promising therapeutic approaches. As the size decreases to nanoscale, many special optical, electric, magnetic, and mechanical properties appear, making nanostructure differ from its bulk materials [9]. Nanoparticles generally used in medicine can be divided into three classes: inorganic nanoparticles, organic nanoparticles and composite nanoparticles according to their composition and physical and chemical properties [10]. In the group of inorganic nanoparticles commonly used in medicine, metal and metal oxide nanoparticles are the most suitable candidates for next-generation anticancer treatment due to their unique physical and chemical properties like magnetic and plasmonic properties, making them effective for biomedical applications [11]. In addition, the decrease in the surface-to-volume ratio of metal nanoparticles generally increases their reactivity, leading to the strong interactions with DNA and protein molecules [12]. Many studies in this regard have focused on the efficiency of various metal and metal oxide nanoparticles for the cancer treatment [13]. Hussain et al. [14] were synthesized zinc oxide nanoparticles from aqueous Pandanus odorifer leaf extract, and reported their cytotoxic activity against MCF-7, HepG2 and A-549 cells.
Among various organic compounds, polymers are of major interest in the preparation of nanoparticles for cancer management. In addition to the size-dependent properties, polymeric nanoparticles provide other advantageous properties such as flexibility and easily modifiable, stability in biological fluids, controlled release of anticancer drugs, biodegradability, biocompatibility, and affinity to cancer-specific Biomarkers [15]. Therefore, a variety of polymeric nanoparticles have been developed over the years to treat cancer and other diseases, and they can be subdivided into two major groups of synthetic and natural polymers [16,17,18]. Although synthetic polymers have easily predictable mechanical features, natural polymers also offer extensive advantages, especially in drug delivery such as the availability of natural resources, nontoxicity, biocompatibly and biodegradably, nonimmunogenicity, and site-specific targeting to particular tissues [19]. Among polymeric-based nanoparticles, chitosan is one of most widely studied. Chitosan, deacetylated chitin, is a natural polysaccharide present in crab and shrimp sells that consists of β-(1, 4)-2-amido-D-glucose linked via (1–4) glycosidic bonds. The amine groups of chitosan influence a large variety of its bioactive features, including mucoadhesion and permeation enhancement. Chitosan nanoparticles are widely applied to deliver anticancer agents and inhibit tumor growth without systemic toxicity [20,21,22]. Anticancer activity of chitosan is associated with induction of apoptosis and cell cycle arrest [23]. To date, different procedures have been developed for the preparation of chitosan nanoparticles such as ionotropic gelation method, microemulsion method, coacervation/precipitation method, solvent evaporation method, etc. [24, 25].
Many studies have shown that the molecular weight and deacetylation degree of chitosan, as well as the concentration and pH of the chitosan solution used in different methods are among the main factors affecting the size, surface charge and morphology of chitosan nanoparticles [26,27,28]. On the other hand, it has been demonstrated that the size, zeta potential, and shape of nanoparticles greatly influence the cellular uptake ratio and result in different biological and anticancer activities [29]. According to the available literature data, these influencing parameters are not constant in different methods used to produce chitosan nanoparticles, leading to the incomparability of these particles in terms of their physicochemical and biological properties. Therefore, in the present study, chitosan nanoparticles were produced using three different methods of the ionotropic gelation, microemulsion, and emulsification solvent diffusion. However, prior to production, type of chitosan (Medium MW, 75–85% deacetylated), pH (5) and concentration of chitosan solution (1 mg/ml) used were kept constant. So far, the characteristics of nanochitosans prepared using the aforementioned methods have not been well established. This study aimed to compare these three methods in terms of their product physicochemical and biological properties in order to find the most appropriate method to prepare chitosan nanoparticles before being used as anticancer agent.
Experimental
Materials
The chemicals which were used in the chitosan nanoparticles preparation step were purchased from Sigma-Aldrich (Saint Louis, MO, USA) and their properties are given in Table 1. The chemicals which were applied in the cytotoxicity assays (Dulbecco’s modified eagle’s medium (DMEM), fetal bovine serum (FBS), 3-(4, 5-dimethyl-thiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), and trypsin–EDTA were provided from Gibco (USA). All of the chemicals were of the highest analytical reagent grade.
Preparation of chitosan nanoparticles
To obtain homogeneous chitosan gel solution, 30 mg of chitosan was dissolved in acetic acid (1% v/v, 30 mL) and stirred for 10 h. Afterward, the size of chitosan nanoparticle was modified using three different methods. The concentrations and the phase volume ratio were optimized to prepare chitosan nanoparticles with good colloidal stability.
In the ionotropic gelation method, 10 mL of STPP solution (1 mg/mL, pH 5) was slowly titrated (at a rate of roughly 1 drop/s) into 30 mL of chitosan solution (1 mg/mL, pH 5). The resulting solution was then centrifuged in 13,000 rpm for 5 min. The supernatant was separated and stored at 4 °C for subsequent analysis [30].
In the microemulsion method, sodium chloride (2 mg) were dissolved in 20 mL of chitosan solution (1 mg/mL, pH 5) and stirred for 3 h. Then, this chitosan gel was added dropwise to the stirred olive oil and polysorbate 80 at 4 °C. Next, 5 mL of acetone was added, and the formed mixture was stirred for an additional hour. The particles were then solidified by glutaraldehyde cross-linking agent saturated with toluene and recovered by centrifugation and finally dried at 50 °C for 24 h [31].
In the emulsification solvent diffusion method, an oil-in-water emulsion was formed by injecting methylene chloride/acetone (3:1) organic solvent into aqueous chitosan solution (1 mg/ mL, pH 5) containing lecithin as a stabilizing agent. The emulsion was then stirred to evaporate methylene chloride/acetone at room temperature. The obtained chitosan nanoparticles by centrifugation (21,000 rpm, 30 min) were then washed with distilled water and dried at 30 °C [32].
The nanochitosans prepared by three different methods were characterized for particle size, electrophoretic mobility, and morphology.
Particle size and zeta potential analysis
Particle size distribution (PDI) and size averages were evaluated using a particle size analyzer (Horiba scientific SZ100, USA) equipped with a 90° scattering angle. Zeta potential was measured after sonication of the samples, and then subjecting to dynamic light scattering (DLS).
Fourier transform infrared (FTIR) analysis
The formation of chitosan nanoparticles by the three methods was confirmed using FTIR spectrometry (Bruker Tensor 27 IR spectrophotometer) between 400 and 4000 cm−1 at room temperature.
UV–visible spectral analysis of chitosan nanoparticles
The absorbance of prepared chitosan nanoparticles was scanned using a UV–visible spectrophotometer (PerkinElmer, lambda 25, USA) at all wavelengths between 200 and 600 nm.
Morphological evaluation
The morphological structure of developed chitosan nanoparticles was assessed using high resolution scanning electron microscope (Tescan MiRa II LMU) at an acceleration voltage 15 kV. The samples were coated with a thin layer of gold.
In vitro cytotoxicity studies
MDA-MB-231 cells were purchased from the Pasteur Institute of Iran and maintained in DMEM medium supplemented with 10% FBS, penicillin and streptomycin (100 IU/100 μg). The cells were incubated at 37 °C and 5% CO2 in a humidified atmosphere. The efficacy and the inhibitory concentration (IC50) of the prepared chitosan nanoparticles were determined using the MTT assay. Briefly, MDA-MB-231 cells were seeded in a 96-well plate at a density of 1 × 104 per well for 24 h. The cells were subsequently exposed to 0.5–2 mg / mL of chitosan nanoparticles and incubated for an additional 24 h. Next, the culture medium was replaced with 100 μL of the MTT solution (0.5 mg / mL), and the plate was incubated at 37 °C for an additional 4 h. Afterward, 100 μL of DMSO was added to each well to dissolve the formazan crystals, and the optical density was read at 570 nm (BioTek, USA). The percentage of cell viability was calculated as followed:
Viability % = 100 × OD of treated cells/ OD of control cells.
Statistical analyses
Results were analyzed using an ANOVA, followed by Student’s t-test with GraphPad Prism. Values were expressed as the mean with the standard deviation. The reported p values were considered statistically significant when p < 0.05.
Result and discussion
Preparation and characterization of chitosan nanoparticles
In our experiments, chitosan nanoparticles were first synthesized using three different methods. The following describes each of the methods used to prepare the nanoparticle library:
To date, different approaches have been developed to produce chitosan nanoparticles, but the most common approach is ionotropic gelation method since it is simple, cost-effective, and does not require organic solvents, but the nanoparticles obtained using this method shows poor mechanical strength in acidic medium. In this method as shown in Fig. 1, chitosan polymer is dissolved in an acetic acid solution and then mixed with the STPP aqueous solution under intensive stirring. Then, electrostatic cross-linking occurs between the positively charged amino groups (− NH3+) of chitosan and the negatively charged phosphate groups in the STPP, leading to nanoparticle production with a size range of 200–1000 nm [30, 33].
The microemulsion technique is used to prepare polymeric nanoparticles with a narrow size distribution. This technique uses a lipophilic surfactant such as polysorbate 80 in an organic solvent such as acetone to produce reverse micelles [31]. Bovine serum albumin-loaded chitosan nanoparticles (80–180 nm) were produced using this approach [34]. An illustration of this strategy is shown in Fig. 2.
For preparation of chitosan nanoparticles by emulsion solvent diffusion method (Fig. 3), an organic phase such as methylene chloride and acetone was added to an aqueous solution containing chitosan and a stabilizer such as lecithin under stirring. Thus an O/W emulsion is formed. Next, methylene chloride evaporates at room temperature and acetone transfers to the aqueous phase, decreasing chitosan solubility and thus, chitosan nanoparticles are produced upon polymer precipitation. Although the emulsion solvent diffusion method leads to better particle size control, strong cross-linking agents are commonly used in this technique and the total deletion of the residual cross-linking agents can be challenging [32].
Determination of particle size, PDI, zeta potential and morphology
The mean size, PDI, and zeta potential of nanoparticles prepared by the three methods are given in Table 2.
The mean size of chitosan samples was found to be between 200 and 450 nm. Although the mean size of the samples is within the expected limits, chitosan nanoparticles synthesized using ionotropic gelation method have a significantly lower size compared to chitosan nanoparticles prepared using O/W emulsion solvent diffusion and microemulsion methods. In addition, the nanoparticles which were prepared using O/W emulsion solvent diffusion method, have a relatively higher PDI compared to the nanoparticles prepared using ionotropic gelation method, while their zeta potential was lower than the nanoparticles prepared with microemulsion and ionotropic gelation methods. Surface potential was positive for all the particles due to the presence of positively charged amine groups in the chitosan structure.
Zeta potential values of these nanoparticles are shown in Table 2, were significantly different, decreasing from + 35 mv to + 12 mv. In detail, mean zeta potentials of the obtained chitosan nanoparticles were + 34.6 mv for the ionotropic gelation method, + 19.1 for the microemulsion method, and + 12.2 mv for the emulsion solvent diffusion method. The higher zeta potential in a particular range implies that the nanoparticles are stable in the formulation, suggesting the prevention of aggregation. The mean size and zeta potential of the chitosan nanoparticles were consistent with Rebbouh-Nouiouat et al. [35] and Hasanzadeh Kafshgari et al. [36] studies.
Size distribution is also an important parameter for evaluating quality of the nanoparticles. As reported in Table 2, PDI values were determined as 0.26, 0.97, and 0.54 for the chitosan nanoparticles prepared by the ionotropic gelation, O/W emulsion solvent diffusion method, and microemulsion methods. PDI values should be between 0 and 1.0. PDI value in homogeneous dispersion is less, whereas heterogeneous dispersions have PDI values greater than 0.3.
The morphology of the synthesized chitosan nanoparticles was assessed by SEM. The SEM micrographs of chitosan nanoparticles are represented in Fig. 4. The chitosan nanoparticles obtained from ionotropic gelation method had smooth surfaces and spherical topography and less and more narrow size distributions, whereas the particles prepared by using the microemulsion method and emulsification solvent diffusion method showed irregular shape and pattern with some agglomerations.
Spectroscopic characterization
UV–visible spectroscopy
The recorded UV–visible spectral of chitosan and chitosan nanoparticles prepared using the microemulsion, the O/W emulsion solvent diffusion, and the ionotropic gelation methods are shown in Fig. 5. The UV–vis absorption of chitosan revealed an absorption band at 218 nm. But in the case of chitosan nanoparticles, the absorption bands were appeared at around 230 to 245 nm, which corresponds to the π- π* transition of the nanoparticles [37].
FTIR analysis
The characteristic spectrum of chitosan as shown in Fig. 6a indicated N–H and O–H stretching (3430–3420 cm−1), C-H symmetric and asymmetric stretch at 2951 cm−1and 2881 cm−1, C=O stretching of amide I at 1655 cm−1, N–H stretch at 1561 cm−1, CH2 stretch at 1429 cm−1, C–O stretch at 1072 cm−1, and C–O–C stretch at 1155 cm−1 and 895 cm−1 [38]. The chitosan nanoparticles produced using the three methods exhibited similar characteristic spectra as that of chitosan with several redshifts, indicating an increase in the hydrogen bonds or the interaction between amine groups of chitosan with aldehyde groups of glutaraldehyde (Fig. 6b–d).
Anticancer evaluation of chitosan nanoparticles
The cytotoxicity of chitosan nanoparticles obtained using three different methods was determined on MDA-MB-231 cells by MTT assay. To test the potential cytotoxicity of chitosan nanoparticles, MDA-MB-231 cells were treated with test samples at various concentrations (0.5, 1, 1.5, 2 mg/mL). As shown in Fig. 7, all samples tested induced dose-dependent cytotoxic effects on MDA-MB-231 cells. Chitosan nanoparticles formed by the ionotropic gelation method at concentrations of 0.5, 1, 1.5, and 2 mg/mL significantly decreased the cell viability to 64.53%, 49.22%, 35.64%, and 22.13% of control group, respectively. However, under the corresponding conditions, chitosan nanoparticles produced by O/W emulsion solvent diffusion method at the same concentrations decreased the cell viability to 81.39%, 70.42%, 59.52%, and 46.83%. Moreover, the cytotoxicity of chitosan nanoparticles prepared by microemulsion method was lower than chitosan nanoparticles obtained by ionotropic gelation method and higher than chitosan nanoparticles obtained by O/W emulsion solvent diffusion method. They at concentrations of 0.5, 1, 1.5, and 2 mg/mL decreased the cell viability to 72.51%, 56.79%, 47.12%, and 36.88% of control group, respectively. The IC50 values of chitosan nanoparticles obtained by ionotropic gelation, microemulsion, and O/W emulsion solvent diffusion methods on MDA-MB-231 cells were 0.89 mg/mL, 1.24 mg/mL and 1.67 mg/mL, respectively.
In fact, chitosan nanoparticles produced by ionotropic gelation method, which had the smallest size and the largest zeta potential compared to the nanoparticles prepared by the other two methods, showed the highest cytotoxicity cell inhibition effect among all the groups tested.
The success of nanotherapeutics depends on effective cellular uptake of nanoparticles, which strongly rely on the nanoparticles’ size. Nanoparticles generally enter cells through the endocytosis process. Smaller nanoparticles could interact more effectively with cellular and subcellular compartments due to their higher surface/volume ratios and penetrate intracellular locations such as nucleus and mitochondria, making them more toxic [39]. In accordance with our results, some previous studies have demonstrated that nanoparticles with smaller sizes are more cytotoxic compared to larger nanoparticles. For example, Ko et al. [40] indicated that smaller gold nanoparticles (30–50 nm) have higher internalization efficiency to human adipose-derived stem cells compared to larger gold nanoparticles with sizes of 75 and 100 nm. Surface charge is another determining factor in cellular uptake and toxicity potential of nanoparticles [41]. It has been reported that tumor cells have a net negative charge. Therefore, it is considered that positively charged nanoparticles, in contrast to anionic and neutral nanoparticles, adhere easily to the cell membrane and increase the membrane-engulfing process due to electrostatic adhesion-mediated targeting [42]. Li and Malmstadt (2013) [43] revealed that the strong electrostatic interaction between cationic polystyrene nanoparticles and the phosphate groups of the membrane results in increasing nanoparticle–membrane binding and membrane surface tension which in turn, assists in the formation of pores. In this regard, we hypothesize that more potent anticancer activity of chitosan nanoparticles obtained using ionotropic gelation procedure compared to the nanoparticles produced using two other techniques is the result of an increased capacity to penetrate into cancer cells. Of course, it is well known that in addition to the size and surface charge, the cytotoxicity of nanoparticles depends on many other factors [44]. Therefore the exact cause for the marked differences in the cytotoxicity among these three different-sized and surface charged chitosan nanoparticles warrants further study.
Morphological changes
The morphological changes in MDA-MB-231cells exposed to various concentrations of chitosan nanoparticles obtained using ionotropic gelation, microemulsion, and emulsion solvent diffusion methods are shown in Fig. 8. Alterations in the morphology of MDA-MB-231cells were observed under phase contrast inverted microscope. Results showed that MDA-MB-231 cells exposed to 1 and 2 mg/mL concentrations of all samples for 24 h reduced the cell density. Most of the cells at 2 mg/mL of the nanoparticles lost their typical morphology and appeared smaller in size, shrunken, and rounded. The chitosan nanoparticles might enhance cell detachment by interacting with intercellular junctions. In addition, the extensive morphological changes (plasma membrane blebbing and vacuolation) observed in the MDA-MB-231 cells treated with 2 mg/mL of the nanoparticles, indicate an autophagic mechanism of cell death [45].
Conclusion
In the present study, chitosan nanoparticles were produced using three different methods (ionotropic gelation, microemulsion and emulsification solvent diffusion methods), and compared for the first time, in terms of their physicochemical and biological properties. The obtained nanoparticles were characterized by various techniques, including FTIR, SEM and UV–vis spectroscopy. The focus of this study was to prepare nanoparticles with anticancer activity. Hence, the anticancer activity of the prepared chitosan nanoparticles was evaluated in MDA-MB-231cells by MTT assay and phase-contrast microscopy. All the chitosan nanoparticles showed good cytotoxic activity against MDA-MB-231 cells but the best results were of the nanoparticles obtained using ionotropic gelation method with an average size of 253 nm. In fact, an inverse relationship was observed between the chitosan nanoparticles’ size and the cytotoxic activity, while a direct relationship between the nanoparticles’ surface charge and the anticancer activity was noticed. Taken together, the results of this study establish that the preparation of chitosan nanoparticles through any of the methods mentioned above leads to the formation of nanoparticles with anticancer activity. These nanoparticles could be considered as an effective anticancer agent for the treatment of human breast cancer but further investigations are needed.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Arem H, Loftfield E (2018) Cancer epidemiology: a survey of modifiable risk factors for prevention and survivorship. Am J Lifestyl Med 12(3):200–210. https://doi.org/10.1177/1559827617700600
Chan H-K, Ismail S (2014) Side effects of chemotherapy among cancer patients in a Malaysian general hospital: experiences, perceptions and informational needs from clinical pharmacists. Asian Pac J Cancer Prev 15(13):5305–5309. https://doi.org/10.7314/apjcp.2014.15.13.5305
Ali I, Wani WA, Haque A, Saleem K (2013) Glutamic acid and its derivatives: candidates for rational design of anticancer drugs. Future Med Chem 5(8):961–978. https://doi.org/10.4155/fmc.13.62
Saleem K, Wani WA, Haque A, Lone MN, Hsieh M-F, Jairajpuri MA, Ali I (2013) Synthesis, DNA binding, hemolysis assays and anticancer studies of copper(II), nickel(II) and iron(III) complexes of a pyrazoline-based ligand. Future Med Chem 5(2):135–146. https://doi.org/10.4155/fmc.12.201
Grigore ME (2017) Organic and inorganic nano-systems used in cancer treatment. J Med Res Health Educ https://www.imedpub.com/medical-research-and-health-education/
Dutta S, Ray S (2013) Glutamic acid as anticancer agent: An overview. Saudi Pharm J 21(4):337–343. https://doi.org/10.1016/j.jsps.2012.12.007
Ali I, Wani WA, Saleem K, Wesselinova D (2013) Syntheses, DNA binding and anticancer profiles of L-glutamic acid ligand and c(II) and ruthenium(III) complexes. Med Chem 9:11–21. https://doi.org/10.2174/157340613804488297
Yu Z, Gao L, Chen K, Zhang W, Zhang Q, Li Q, Hu K (2021) Nanoparticles: A new approach to upgrade cancer diagnosis and treatment. Nanoscale Res Lett. https://doi.org/10.1186/s11671-021-03489-z
Joudeh N, Linke D (2022) Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists. J Nanobiotechnol. https://doi.org/10.1186/s12951-022-01477-8
Vinardell MP, Mitjans M (2015) Antitumor activities of metal oxide nanoparticles. Nanomaterials 5(2):1004–1021. https://doi.org/10.3390/nano5021004
Abarca-Cabrera L, Fraga-García P, Berensmeier S (2021) Bio-nano interactions: binding proteins, polysaccharides, lipids and nucleic acids onto magnetic nanoparticles. Biomater Res 25:1–18. https://doi.org/10.1186/s40824-021-00212-y
Xu J-J, Zhang W-C, Guo Y-W, Chen X-Y, Zhang Y-N (2022) Metal nanoparticles as a promising technology in targeted cancer treatment. Drug Deliv 29:664–678. https://doi.org/10.1080/10717544.2022.2039804
Hussain A, Oves M, Alajmi MF, Hussain I, Amir S, Ahmed J, Rehman MT, El-Seed HR, Ali I (2019) Biogenesis of ZnO nanoparticles using Pandanus odorifer leaf extract: anticancer and antimicrobial activities. RSC Adv 9:15357–15369. https://doi.org/10.1039/C9RA01659G
Bigaj-Józe MJ, Grześkowiak BF (2022) Polymeric nanoparticles wrapped in biological membranes for targeted anticancer treatment. Eur Polym J 176:111427. https://doi.org/10.1016/j.eurpolymj.2022.111427
Jana S, Sen KK, Gandhi A (2016) Alginate based nanocarriers for drug delivery applications. Curr Pharm Des 22:3399–3410
Masood F (2016) Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater Sci Eng C 60:569–578. https://doi.org/10.1016/j.msec.2015.11.067
Acharya S, Sahoo SK (2011) PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv Drug Deliv Rev 63(3):170–183. https://doi.org/10.1016/j.addr.2010.10.008
Wong KH, Aiping LuA, Chen X, Yang Z (2020) Natural ingredient-based polymeric nanoparticles for cancer treatment. Molecules 25(16):3620. https://doi.org/10.3390/molecules25163620
Ding J, Guo Y (2022) Recent advances in chitosan and its derivatives in cancer treatment. Front Pharmacol l 13:888740. https://doi.org/10.3389/fphar.2022.888740
Zivarpour P, Hallajzadeh J, Asemi Z, Sadoughi F, Sharifi M (2021) Chitosan as possible inhibitory agents and delivery systems in leukemia. Cancer Cel Int 21(1):544. https://doi.org/10.1186/s12935-021-02243-w
Key J, Park K (2017) Multicomponent, tumor-homing chitosan nanoparticles for cancer imaging and therapy. Int J Mol Sci 18(3):594. https://doi.org/10.3390/ijms18030594
Wimardhani YS, Suniarti DF, Freisleben HJ, Wanandi SI, Siregar NC, Ikeda MA (2014) Chitosan exerts anticancer activity through induction of apoptosis and cell cycle arrest in oral cancer cells. J Oral Sci 56:119–126. https://doi.org/10.2334/josnusd.56.119
Divya K, Jisha MS (2018) Chitosan nanoparticles preparation and applications. Environ Chem Lette 16:101–112. https://doi.org/10.1007/s10311-017-0670-y
Rizeq BR, Younes NN, Rasool K, Nasrallah GK (2019) Synthesis, bioapplications, and toxicity evaluation of chitosan-based nanoparticles. Int J Mol Sci 20(22):5776. https://doi.org/10.3390/ijms20225776
Babiia O, Wanga Z, Liu G, Martinez EC, Littel-van den Hurk SD, Chen L (2020) Low molecular weight chitosan nanoparticles for CpG oligodeoxynucleotides delivery: impact of molecular weight, degree of deacetylation, and mannosylation on intracellular uptake and cytokine induction. Int J Biol Macromol 159:46–56. https://doi.org/10.1016/j.ijbiomac.2020.05.048
Antonioua J, Liua F, Majeeda H, Qi J, Yokoyama W, Zhong F (2015) Physicochemical and morphological properties of size-controlled chitosan–tripolyphosphate nanoparticles. Colloids Surf A: Physicochem Eng Asp 465:137–146. https://doi.org/10.1016/j.colsurfa.2014.10.040
Ngan LTK, Wang S-L, Hiep DM, Luong PM, Vui NT, Dinh TM, Dzung NA (2014) Preparation of chitosan nanoparticles by spray drying, and their antibacterial activity. Res Chem Intermed 40:2165–2175
Sadat SMA, Jahan ST, Haddadi A (2016) Effects of size and surface charge of polymeric nanoparticles on in vitro and in vivo applications. J Biomater Nanobiotechnol 7:91–108. https://doi.org/10.4236/jbnb.2016.72011
Hou Z, Zhan C, Jiang Q, Hu Q, Li L, Chang D, Yang X, Wang Y, Li Y, Ye S, Xie L, Yi Y, Zhang Q (2011) Both FA- and mPEG-conjugated chitosan nanoparticles for targeted cellular uptake and enhanced tumor tissue distribution. Nanoscale Res Lett 6(1):563. https://doi.org/10.1186/1556-276X-6-563
Khanmohammadi M, Elmizadeh H, Ghasemi K (2015) Investigation of size and morphology of chitosan nanoparticles used in drug delivery system employing chemometric technique. Iran J Pharm Res 14(3):665–675. https://doi.org/10.22037/IJPR.2015.1761
Abdelkader H, Hussain SA, Abdullah N, SuryaniKmaruddin S (2018) Review on micro-encapsulation with chitosan for pharmaceuticals applications. MOJ Curr Res Rev 1(2):77–84. https://doi.org/10.15406/mojcrr.2018.01.00013
Fan W, Yan W, Xu Z, Ni H (2012) Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf B Biointerfaces 90:21–27. https://doi.org/10.1016/j.colsurfb.2011.09.042
Hasanzadeh Kafshgari M, Khorram M, Mansouri M, Samimi A, Osfouri S (2012) Preparation of alginate and chitosan nanoparticles using a new reverse micellar system. Iran Polym J 21(2):99–107. https://doi.org/10.1007/s13726-011-0010-1
Grenha A (2012) Chitosan nanoparticles: A survey of preparation methods. J Drug Target 20(4):291–300. https://doi.org/10.3109/1061186X.2011.654121
Rebbouh-Nouiouat F, Marie France ME, Laraba-Djebari F (2020) Chitosan nanoparticles as a delivery platform for neurotoxin II from Androctonus australis hector scorpion venom: Assessment of toxicity and immunogenicity. Acta Trop 205(4):105353. https://doi.org/10.1016/j.actatropica.2020.105353
Thamilarasan V, Sethuraman V, Gopinath K, Balalakshmi C, Govindarajan M, Mothana RAA, Siddiqui NA, Khaled JM, Benelli K (2018) Single step fabrication of chitosan nanocrystals using penaeus semisulcatus: potential as new insecticides, antimicrobials and plant growth. J Clust Sci 29:375–384. https://doi.org/10.1007/s10876-018-1342-1
Song C, Yu H, Zhang M, Yang Y, Zhang G (2013) Physicochemical properties and antioxidant activity of chitosan from the blowfly Chrysomya megacephala larvae. Int J Biol Macromol 60:347–354. https://doi.org/10.1016/j.ijbiomac.2013.05.039
Huang K, Ma H, Liu J, Huo S, Kumar A, Wei T, Zhang X, Jin S, Gan Y, Wang PC, He S, Zhang X, Liang X-J (2012) Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano 6:4483–4493. https://doi.org/10.1021/nn301282m
Ko W-K, Heo DN, Moon H-J, Lee SJ, Bae MS, Lee JB, Sun I-C, Jeon HB, Park HK, Kwon IK (2015) The effect of gold nanoparticle size on osteogenic differentiation of adipose-derived stem cells. J Colloid Interface Sci 438:68–76. https://doi.org/10.1016/j.jcis.2014.08.058
Honary S, Zahir F (2013) Effect of zeta potential on the properties of nano-drug delivery systems. Trop J Pharm Res 12:265–273. https://doi.org/10.4314/tjpr.v12i2.20
Jeon S, Clavadetscher J, Lee DK, Chankeshwara SV, Bradley M, Cho WS (2018) Surface charge-dependent cellular up-take of polystyrene nanoparticles. Nanomaterials 8:1028. https://doi.org/10.3390/nano8121028
Li S, Malmstadt N (2013) Deformation and poration of lipid bilayer membranes by cationic nanoparticles. Soft Matter 9(20):4969–4976. https://doi.org/10.1039/C3SM27578G
SohrabiKashani A, Packirisamy M (2021) Cancer-nano-interaction: from cellular uptake to mechanobiological responses. Int J Mol Sci 22:9587. https://doi.org/10.3390/ijms22179587
Mizushima N (2018) A brief history of autophagy from cell biology to physiology and disease. Nat Cell Biol 20:521–527. https://doi.org/10.1038/s41556-018-0092-5
Acknowledgements
This work was supported by the Iranian Research Organization for Science and Technology [grant number 4404]
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by FsH and MH. The first draft of the manuscript was written by FsH and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that there is no conflict of interest.
Ethical approval
We the undersigned declare that this manuscript is original, has not been published before and is not currently being considered for publication elsewhere. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We understand that the corresponding author is the sole contact for the editorial process. She is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs signed by all authors as follows: Forouh Sadat Hassani, Mahnaz Hadizadeh, Davood Zare, and Saeedeh Mazinani.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hassani, F.s., Hadizadeh, M., Zare, D. et al. Comparison of different methods for preparation of nanochitosan as anticancer agent. Polym. Bull. 81, 827–842 (2024). https://doi.org/10.1007/s00289-023-04739-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-04739-z