Abstract
Preparing blends from fully biodegradable resources is an important alternative in the production of materials less harmful to the environment. Among these materials, thermoplastic cornstarch (TPS) and polycaprolactone (PCL) are an interesting possibility for replacing conventional synthetic materials, and it is desirable that they present relative stability under conditions of processing and use. In the present work, PCL/TPS blends with up to 30% TPS, obtained from cornstarch plasticized with water and glycerin, coconut or babassu oil, were processed in an internal laboratory mixer. Adjusted torque variation rate during the last processing stage is a very sensitive indicator of molar mass alterations with processing time. Rheometry data indicate that TPS obtained with babassu and coconut vegetable oils have a higher molar mass compared to that obtained with glycerin. In addition, the incorporation of TPS promotes thermal degradation in PCL/TPS mixtures, which is more pronounced the higher the TPS content and with TPS-glycerin, except for the PCL/20%TPS blends obtained with glycerin and babassu oil. The mechanical properties of the extruded films decreased with the addition of TPS, this reduction being more evident with the increase in the TPS content and in systems with TPS obtained with babassu oil. In contrast, only in blends containing 10 and 20% TPS of glycerin, there was an increase in elongation at break, being a good feature for flexible films, this property is desirable in certain applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental problems caused by high consumption and improper disposal of conventional polymeric materials led to studies, dissemination and use of environmentally less aggressive materials [1]. One of the solutions to these problems is the replacement of conventional polymers by biodegradable polymers, which are materials that can decompose by microorganisms. Thermoplastic starch (TPS) is a good example of a biodegradable material. Its characteristics are similar to those of synthetic thermoplastics, when a plasticizer (usually glycerol and/or water) is added to its processing [2,3,4,5,6,7,8]. The properties of thermoplastic starch will depend on the origin of the granules and processing conditions, such as temperature, particle size, screw configuration, extruder barrel, material feed rate, the matrix employed, the amount of water in the system, as well as on the type and content of plasticizer [9].
Vegetable oils have been used as plasticizers in the production of TPS [10], in the search for materials less aggressive to the environment. The thermal stability of these oils depends on the amount of saturated fatty acids present in their composition; the higher the more stable. Coconut and babassu oils are examples of vegetable oils that have good thermal stability, having approximately 80% of saturated fatty acids in their composition [11,12,13].
Despite the ease in producing thermoplastic starch (TPS), it still presents low mechanical properties. Strategies used to alleviate this problem include the development of polymer blends [12,13,14,15]. A promising polymer to blend with TPS is polycaprolactone (PCL), as this polymer not only has good thermal and mechanical properties but it is also biodegradable [16,17,18].
Based on the above, here we investigate the effects of glycerin, coconut oil, and babassu oil as plasticizers in the manufacture of thermoplastic starch (TPS), and their incorporation into polycaprolactone (PCL) by analyzing the rheological and mechanical properties of the films developed.
Experimental
Materials
Polycaprolactone (PCL), trade name Capa™ 6500, supplied as granules by Perstorp (Sweden was used as the matrix. In order to obtain the Thermoplastic Starches (TPS) (disperse phase), the following materials were used: Duryea® Maizena (Corn Starch), Glycerin P.A supplied by Nuclear, Babassu Oil supplied by Florestas Brasileiras LTDA, COPRA Extra Virgin Coconut Oil and distilled water.
Methods
Processing of thermoplastic starches (TPS) and PCL/TPS blends
Three thermoplastic corn starches (TPS) were prepared with different plasticizers with the following component ratios:
-
For starches obtained with vegetable oil: 50% corn starch and 50% of a system composed of 30% plasticizer (glycerin (20%) + coconut or babassu oil (10%)) and 20% distilled water.
-
For the starch obtained with glycerin only: 50% corn starch, 30% glycerin and 20% distilled water.
The components mentioned above were mixed manually in a plastic bag until a homogeneous mass was obtained, then they were processed, in a Haake Rheomix 3000 internal mixer operating with "roller" type rotors. The TPS´s thus obtained were ground in a knife mill. The PCL and PCL/TPS blends were processed in the same equipment and subsequently, were ground in a knife mill. The conditions used to manufacture TPS and to process PCL and PCL/TPS blends are shown in Table 1.
Torque-temperature–time relationship analysis
In an internal mixer, on tests performed at constant rotor speed, torque (Z) is directly proportional to melt viscosity (η) in the last stage of processing:
Viscosity is very sensitive to small variations in molar mass and can be used to estimate the effect of processing on polymer degradation. For all known thermoplastics (with very few exceptions) viscosity depends on the weight average molar mass Mw according to the classical "power law 3.4" [19]. For a molten polymer processed at a constant temperature having a pseudoplasticity index n, this relationship is:
For PCL one can assume n = 0.94 [20]. However, torque is also temperature dependent. Consequently, torque drop during the final stages of processing can be attributed to the combined effect of an increase in melt temperature caused by attrition and a decrease in molar mass of the matrix caused by degradation.
The effect of temperature on viscosity—and hence on torque—can be eliminated by adjusting torque to a reference temperature and is given by:
where Z* is the torque adjusted at a reference temperature T* (arbitrary value, but close to the melt temperature T). The adjusted torque is the torque that would be observed if the temperature in the chamber were T*. β is the exponential temperature coefficient of the material which, for PCL, was experimentally determined to be 0.0175 °C−1 [20]. This β value will be used—in a first approximation—for both, the neat PCL polymer and for the PCL/TPS blends.
Once a processing time interval Δt is chosen for the final stage of processing (in the present case the interval between 5 and 10 min of processing), the relative change in adjusted torque at a given temperature T* is considered to be a measure of the degradation rate:
where Z* is the average adjusted torque over the interval Δt. The rate of change of the weight-average molar mass can be estimated by taking into account the dependence of torque on molar mass [21]:
The % change in adjusted torque and the % change in weight average molar mass per minute of processing are obtained by multiplying RZ and RM by 100.
Melt flow index (MFI)
Melt flow index (MFI) measurements were conducted in a DSM MI-3 model Plastometer, according to ASTM D1238 standard, with a 2.16 kg load under 160 °C temperature for PCL and PCL?TPS blends, and at 230 °C for the three thermoplastic starches. To calculate the melt flow index, Eq. 6 is used:
Preparation of PCL and PCL/TPS blend films
PCL and PCL/TPS blends processed in the internal mixer were ground in a knife mill and extruded in a single-screw bench-top extruder model Lab-16 Chill roll from AX PLASTICS fitted with a flat die and standard screw (without mixing elements) to obtain flat films. The operating conditions are shown in Table 2.
Mechanical properties
Mechanical properties were performed on an Emic (DL500) universal testing equipment operating at 50 mm/min, with a load cell of 20 N, following the ASTM D882 standard, specific for mechanical tests on films. The average results of 10 samples were reported as a function of TPS type (oil used as a plasticizer) and content. The thickness of each sample was determined using a micrometer.
Results and discussion
Torque-temperature–time analysis
Figure 1 shows the torque and temperature curves as a function of processing time obtained in the internal mixer during manufacture of the thermoplastic corn starches (TPS) obtained with the different plasticizers. Processing was carried out at 125 °C with rotor speed of 60 rpm for 20 min.
Initially, temperature drops and torque increase as the material is introduced in the mixing chamber. Torque rises as the material is introduced in the chamber and tends to level out as the materials melt. Temperature decreases as cold material is fed into the processing chamber. Torque and temperature continue to increase with time as starch gelatinizes and the granules are hydrated. After about two minutes processing, torque continues to increase due to the loss of water from the system. TPS obtained with glycerin reached a maximum torque after 9–10 min processing. It is believed that maximum gelatinization occurs at this time, when the temperature responsible for this process (123 °C) is reached. Subsequently, this TPS shows a decrease in torque attributed to shear destructuring of the starch granules. However, for the TPS containing vegetable oils, torque continues to increase with time, probably because there was, at the same time, scission and recombination and/or crosslinking of chains, generating an increase in their molar mass. Based on these rheometry results, the TPS obtained with glycerin and that TPS having babassu vegetable oil were chosen to verify their influence on PCL/TPS blends.
Figure 2 shows the torque and temperature versus time curves, obtained in the same equipment (Haake Rheomix 3000 internal mixer operating with “roller” rotors), for the PCL and for the PCL/TPS blends with the TPS described above.
As expected, as the materials are introduced in the mixing chamber, temperature drops and torque increases. When the systems start melting, torque decreases toward a plateau (steady state), and the temperature increases, surpasses the wall temperature and also tends to stabilized. This plateau, which is reached when the polymer is substantially molten, is reached during the first 5 min of processing. It was possible to observe that with the increase of the PCL/TPS-GLYCERIN content, the torque value is lower than that of PCL. On the other hand, with increasing PCL/TPS-BABASSU content, the torque value is higher than that of PCL.
The interval between 5 and 10 min of processing was chosen to analyze the torque-temperature–time relationship (Fig. 3), according to the procedure described previously, using the set chamber wall temperature as the reference temperature T* = 125 °C, and \(\beta\) = 0.0175 °C−1 [20].
The data show that the adjusted torque linearly depends on processing time, stabilizes with time, and results in straight torque-time lines with a nearly zero slope, depending on the composition of the mixture. It is verified that the adjusted torque values (a measurement that depends solely on the molar mass) are greater and nearly independent of processing for the PCL curve than for the PCL/TPS blends, which is taken as an indication of the greater thermal stability of PCL over the PCL/TPS blends. The blend containing 30% TPS with babassu oil, however, displayed adjusted torque values equivalent to that of neat PCL.
Table 3 and Fig. 4 show the variation in adjusted torque (RZ) and the variation in weight average molar mass (RM) per minute of processing in the final processing stages (5–10 min).
It is observed that the values of the variation of adjusted torque as a function of time (RZ), which is a measurement of the rate of degradation, as well as the variation of the weight average molar mass (RM) for the blends, are higher than those of PCL and that these changes tend to increase with TPS content. This behavior is taken as an indication that the incorporation of TPS to PCL increased its degradation. In general PCL/20%TPS blends obtained with glycerin and babassu oil, displayed relatively lower values for RZ and RM, which seems to indicate that to be an optimal TPS concentration for PCL/TPS blends, as degradation of these systems was minimal.
Melt flow index (MFI)
As described previously, three thermoplastic starches (TPS) were obtained during processing in an internal mixer operating with roller rotors, at 60 rpm, 125 °C for 20 min, and the materials obtained in this test were used for this analysis. The test was conducted at 230 °C, as the TPS did not flow at 160 °C, especially the TPS containing vegetable oils. It is believed this behavior might be due to the higher molar mass acquired in obtaining these materials in the internal mixer. When the test was carried out at a temperature of 230 °C, all TPS´s flowed quickly, generating melt flow index values for the thermoplastic starches obtained with the vegetable oils a little higher than the thermoplastic starch obtained with glycerin. It is believed this behavior can be associated to the chain breakage of these higher molar mass TPS´s obtained leading to their degradation, and therefore higher melt flow indices.
Figure 5 and Table 4 show the Melt Flow Index of the three thermoplastic starches (TPS) manufactured.
The results of the determination of the PCL and the PCL/TPS blends melt flow indices are shown in Fig. 6 and Table 5.
The data show the melt flow index of PCL to be lower than the blends, that is, its viscosity is higher than that of blends, a fact proven by toque rheometry as the torque on PCL was higher than that observed for the blends. The melt flow index of both TPS´s under investigation is higher than that of PCL. If the individual values for PCL and TPS were used in the rule of mixtures, a lower melt flow rate than the one obtained experimentally would be expected for all blends with increasing TPS content as values ranging from 2.63 to 2.95 and from 2.68 to 3.11 would be expected for PCL/TPS-gycerin PCL/TPS-babassu, respectively. The higher experimentally determined melt flow index values for the blends seems to indicate some sort of degradation.
It is also worth mentioning that the blends with TPS of glycerin had slightly higher melt index values than those obtained with TPS of babassu oil, this consequence was expected, since in the rheometric analysis the final torque of the blends containing TPS-glycerin was lower than the blends containing TPS-babassu, indicating a more pronounced degradation in the systems with TPS-glycerin. It is believed that during the processing of materials in the internal mixer to obtain the blends, the two TPS analyzed may have degraded at different rates and that this has affected their melt flow behavior.
Mechanical properties
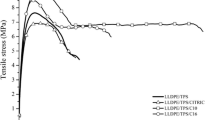
The tensile modulus of elasticity, tensile strength and elongation at break were determined according to ASTM D 882 standards. The portions of the stress–strain curve where the tensile modulus, the yield strength, the maximum strength and elongation at break were determined are shown in the example in Fig. 7 for PCL. The other samples followed the same procedure.
a Example of analysis of a stress–strain graph (black curve), in this case of PCL. The modulus obtained by linear regression (blue line) was E = 165.04 MPa. The maximum stress (red dot) is σmax = 10.10 MPa and the maximum strain (green dot) is εmax = 582.94%. The repeated upward and downward trend on the stress–strain curve is due to decreased cross-sectional area (necking). b Magnification of the elastic region of the stress–strain graph, in which the yield strength (blue dot) is σe = 7.16 MPa (color figure online)
Table 6 and Fig. 8 present the tensile properties for all investigated systems: elastic modulus E, maximum tensile strength σmax, elongation at break εmax, and yield strength σe. Mean values and standard deviations are reported.
The introduction of thermoplastic starches (TPS) to PCL led to decreases in modulus, tensile and yield strength, and elongation at break. This decrease was more significant at higher TPS contents and particularly for the systems containing TPS obtained with babassu oil. Despite this, the incorporation of 10 and 20% of glycerin TPS promoted an increase in elongation at break, a very positive characteristic, since more flexible films are desirable for certain packaging applications. These results can be explained based on the lower properties of TPS compared to PCL and thus, the incorporation of TPS to PCL led, in general, to reduced values of mechanical properties. Based on rheometry data, however, one would expect the properties of PCL/TPS-babassu blends to be higher than those of PCL/TPS-glycerin blends. It is possible that this discrepancy can be attributed to better TPS-glycerin dispersion and/or interaction with the matrix, but further analyzes must be conducted in order to determine the reason for the observed behavior.
Conclusion
Rheometry data obtained during processing indicate that there was gelatinization and destructuring of granules in thermoplastic cornstarch obtained with glycerin, and that thermoplastic starches obtained with vegetable oils had a higher molar mass compared to that obtained with glycerin. The addition of different starches to PCL increases the degradation of the matrix, and this degradation, in general, is more pronounced the higher the TPS content and for the blends containing TPS-glycerin, except for the PCL/20%TPS blends obtained with glycerin and babassu oil, the degradation it is less pronounced. Furthermore, there is a decrease in the mechanical properties of PCL with the incorporation of thermoplastic starches. This reduction is more significant with the increase in the TPS content, and in systems with TPS obtained with babassu oil. Only in blends containing 10 and 20% TPS of glycerin, there was an increase in elongation at break, this is a good feature for flexible films as, in certain packaging applications, this property is desirable.
References
Machado T AM (2016) Obtaining fibers from polycaprolactone/poly (n-isopropylacrylamide) blends using the electrospinning technique. 2016. 84f. Course Conclusion Paper (Degree in Chemistry), Federal Technological University of Paraná-UTFPR, Londrina-Paraná
Averous F, Bouquillon N (2004) Biocomposites based on plasticized starch: thermal and mechanical behaviors. Carbohyd Polym 56(2):111–122
Stepto RFT (2003) The processing of starch as a thermoplastic. Macromolecular Symposia, vol 201, pp 203–212
Curvelo AAS, Carvalho AJF, Agnelli JAM (2001) Thermoplastic starch-cellulosic fibers composites: preliminary results. Carbohyd Polym 45:183–188
Shogren RL, Fanta GF, Doane WM (1993) Development of starch based plastics—a reexamination of selected polymer systems in historical perspective. Starch 45(8):276–280
Doane WM, Swanson C, Fanta G (1992) Emerging polymeric materials based on starch. In: Rowell RM, Schietz TP, Narayan R (eds) Emerging technologies for materials and chemicals from biomass. American Chemical Society, Washington, p 469
Avérous L (2004) Biodegradable multiphase systems based on plasticized starch: a review. J Macromol Sci Polym C24:231–274
Ma XF, Yu JG, Ma YB (2005) Urea and formamide as a mixed plasticizer for thermoplastic wheat flour. Carbohidrate Polym 60:111–116
Corradini E, Lotti C, Medeiros ES, Carvalho AJF, Curvelo AAS, Mattoso LHC (2005) Comparative study of corn-derived thermoplastic starches with different amylose contents. Polymers 15(4):268–273
Hagenmaier RD, Shaw PE (1992) Gas pertmeability of fruit coating waxes. J Am Soc Hortic Sci 1:105–109
Moretto E, Fett R (1998) Technology of vegetable oils and fats in the food industry. São Paulo, Varela
Reda SY, Carneiro PIB (2007) Oils and fats: applications and implications. Anal Mag 27:60–67
Andersen PJ, Ong S, Christensen BJ, Hodson SK (2001) Compositions and methods for manufacturing starch-based compositions. USA: Patent n. 6, pp 168–857
Lawton JW, Shogren RL, Tiefenbacher KF (1999) Effect of batter solids and starch type on the structure of baked starch foams. Cereal Chem 76(5):682–687
Soykeabkaew N, Supaphol P, Rujiravanit R (2004) Preparation and characterization of jute and flax reinforced starch-based composite foams. Carbohydrate Polym 58(1):53–63
Hayashi T (1994) Biodegradable polymers for biomedical uses. Progress Polym Sci 19:663–702
Woodruff MA, Hutmacher DW (2010) The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog Polym Sci 35:1217–1256
Labet M, Thielemans W (2009) Synthesis of polycaprolactone: a review. Chem Soc Rev 38:3484–3504
Dealy JM, Larson RG (2006) Structure and rheology of molten polymers. Hanser, Munich & Cincinnati
Reul LTA, Pereira CAB, Sousa FM, Santos RM, Carvalho LH, Canedo EC (2019) Polycaprolactone/babassu compounds: rheological, thermal, and morphological characteristics. Polym Compos 40:E540–E549
Canedo EL, Wellen RMR, Almeida YMB (2016) Polymer Crystallization—Data Treatment and Macrokinetic Modeling. ANP PRH-28/DEQ/UFPE, Recife
Acknowledgements
The authors are grateful for the financial support of the Coordination for the Improvement of Higher Education Personnel (CAPES/Brazil) and to the Scientic and Technological Council (CNPq) for the fellowships of Carvalho, Vinhas and Almeida.
Funding
The funding was provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, 88882.455375/2019-01,Vithoria Alexandre Duarte Marinho.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marinho, V.A.D., Sousa, F.M., Vinhas, G.M. et al. Influence of the content of corn thermoplastic starches (TPS) prepared with different plasticizers on the rheological and mechanical properties of PCL/TPS blends. Polym. Bull. 80, 7627–7640 (2023). https://doi.org/10.1007/s00289-022-04416-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04416-7