Abstract
In the present work, we prepared enormously soluble poly (aniline-co-3-aminophenol) (PA-co-3-AP) copolymers via a simple chemical oxidative method with aniline and 3-aminophenol, and their properties are compared with conventional polyaniline (PA). Concerning the weight percentage (20, 40, 60 and 80%) of 3-aminophenol, the as-prepared polyamines are denoted as PA-co-3-AP20, PA-co-3-AP40, PA-co-3-AP 60 and PA-co-3-AP80, respectively. The structural determination of as-prepared polymers has been explored by X-ray photoelectron spectroscopy (XPS), UV–Vis spectroscopy. The signature property of the conducting polymers is their tunable electrochemical behavior and the copolymers are investigated for their electrochemical activity. In the electrochemical study, the copolymers differ from PA in the single-electron oxidation and reduction, reversibly at various scan rates ranging from 20 to 50 mV. The current density decreases from PA-co-3-AP20 to PA-co-3-AP80 by increasing the loading weight percentage of 3-aminophenol in the polymeric backbone. The present investigation provides further insight into methods to prepare extremely soluble conducting polyanilines for potential electrochemical applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
After the breakthrough and endearing noble prize by the MacDiarmid–Shirakawa–Heeger group, conducting polymers gained a unique status in the realm of electroactive materials for numerous prospective applications. However, the intractable and insoluble nature of acid-doped conducting polyaniline limits its significant applications such as energy storage and energy conversion. Conductive polymers (CPs) including polyaniline (PA), polypyrrole (PPy) and poly(3,4-ethylene dioxythiophene) (PEDOT), etc., have received massive attention in recent years due to their significant applications in diversified applications such as electrochemical sensors, electrochromic, electroluminescence, energy storage systems, and anticorrosive coatings [1,2,3,4,5,6,7,8,9]. Among all the conducting polymers, PA is one kind of conducting polymer [10], and plays a key role in the energy storage [10, 11] and energy conversion devices [12] besides carbonaceous materials and metal-based compounds. Owing to the high flexibility, electrical conductivity, specific capacitance, and excellent environmental stability PA exhibits potential applications in electrochemical supercapacitors [13,14,15]. For PAs, in several cases, being processable and soluble is sometimes more imperative than being highly conductive. The lower solubility and inevitable nature of PA contribute to the most exciting practical applications [16,17,18].
In the past research, enormous progress has been established to improve processability using the following chemical modifications: (i) introducing the N-substituted aniline derivatives [19,20,21,22,23]; (ii) different copolymerization techniques of aniline with appropriately substituted anilines [9, 24,25,26,27,28,29]; (iii) various post-treatments of polyaniline e.g. sulphonation on the polymer matrix [30, 31] and (iv) controlling the molecular weight of the polymer [32]. The aforementioned methods help polyaniline that is soluble in common organic solvents [33]. Among all the above-stated methods, the copolymerization strategy is the most significant and this technique could improve the processability and conductivity [9, 24]. Depending on the type of substitution groups like electron-donating and withdrawing, polyaniline and its copolymers are well properly reported in the literature [24, 34, 35]. Nevertheless, enough reports existed for the structural interpretation of polyanilines. The conventional PA are having the general formula of [(B–NH–B–NH)m (B–N=Q=N)1−m]n, whereas B denotes the benzenoid ring and Q indicates the quinonoid ring of the polymer backbone. In general, the intrinsic oxidation of the aniline-based conducting polymers can be changed from the completely reduced leucoemeraldine base (LEB, m = 1) to the half (50%) oxidized emeraldine base (EB, m = 0.5) to the fully oxidized pernigraniline base (PAB, m = 0). These various intrinsic oxidation structures of PA and copolyanilines have been characterized by formal techniques such as Fourier-transform infrared spectroscopy [36] (FT-IR), ultraviolet–visible spectroscopy [9, 24] (UV–Vis), Raman spectroscopy [37], and electron spin resonance [38] (ESR) and along with electrochemical studies, etc. But, X-ray photoelectronic spectroscopy (XPS) is the advanced and foremost analysis tool extensively used in quantitative analysis of different oxidation states of conducting polyanilines [24].
For the first time in literature, the present work discussed the binding energy and electrochemical behavior of polyaniline (PA), poly (3-aminophenol) (P-3-AP), and poly (aniline-co-3-aminophenol) (PA-co-3-AP). The XPS technique determines the chemical composition, chemical bonding of atoms, and the cyclic voltammetry helps to study the oxidation states of the elements and polymers based on their respective binding energies. The foremost characterizations of PA, P-3-AP, and PA-co-3-AP, including electrochemical and XPS studies, were methodically scrutinized as follows.
Experimental section
Chemicals and materials
Aniline, 3-aminophenol, and ammonium persulfate (APS) (99.0 purity) were purchased from Sigma-Aldrich and used as received. Analytical grade hydrochloric acid (HCl), sulfuric acid (H2SO4), and spectroscopic grade dimethyl sulfoxide (DMSO, 99.7%), N-methyl-2-pyrrolidone (NMP, 99.7%), and methanol were used as received from Sigma-Aldrich. Double-distilled water was used in the preparation of aqueous solutions.
Synthesis of PA and P-3-AP homopolymers
The polyaniline (PA) and poly (3-aminophenol) P-3-AP were synthesized as shown in Scheme 1 by chemical oxidation of aniline and 3-aminophenol distinctly with APS in 1 M HCl from our earlier reported work [24, 25]. In a typical reaction, aniline (20 mmol, 1.86 g) was added to 200 mL of 1 M HCl and the mixture was cooled to 5 °C in an ice-cold bath. An ice-cold solution of 20 mmol (4.56 g) ammonium persulphate dissolved in 200 ml of 1 M HCl was then added slowly into the monomer solution with constant stirring under a nitrogen atmosphere at 5 °C. The reaction mixture was further stirred for another 5 h and aged overnight at room temperature. A greenish precipitate of the polymer in emeraldine salt form (PA hydrochloride) was formed. It was filtered and washed with small volumes of 1 M HCl and dried in an air oven for 8 h at 80 °C. The emeraldine base was obtained after the treatment of emeraldine salt with 1 M NH4OH solution and aged for 24 h at room temperature. The separated base was washed with distilled water, filtered, and air-dried. Similarly, Poly (3-aminophenol), P-3-AP was obtained by oxidative polymerization of 3-aminophenol using APS. Finally, the as-prepared polymer procured was black powder.
Synthesis of copolyanilines (PA-co-3-AP)
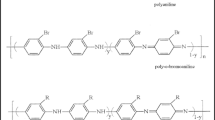
The synthesis of PA-co-3-AP copolymers was processed by the chemical oxidative coupling of the monomers in 1 M HCl using APS. Different copolymers were successfully prepared by varying the amount of 3-aminophenol, namely 20, 40, 60, and 80%. The polyamines with 20, 40, 60, 80% 3-aminophenol will be denoted from here on as PA-co-3-AP20, PA-co-3-AP 40, PA-co-3-AP60, and PA-co-3-AP80, respectively. The synthetic route for homopolymers PA, P-3-AP and PA-co-3-AP copolyanines is presented in Scheme 1 and the 3D repeating units for homopolymers (R=H for PA, R=OH for P-3-AP) are shown in Scheme 2.
Characterization
Cyclic voltammetry
Electrode alteration
Firstly, the working electrode, the glassy carbon electrode (GCE) was polished with 1.0 µm alumina slurry and sonicated in distilled water. The cleaned GCE was subsequently dried using a purified nitrogen stream. Electrode casting solution was prepared in DMF solvent with a concentration of 5 mg/mL. The solution was sonicated for 5 min to have a homogeneous dispersion solution. The modified electrode was prepared by a simple drop-casting method, by pipetting a volume of 10 µL on the GCE and dried at room temperature under vacuum conditions.
Electrochemical tests
All electrochemical tests were performed on a laboratory potentiostat (Gamry 3000, USA) with a glassy carbon electrode (GCE, 3 mm diameter, Bioanalytical systems, USA) used as a working electrode. Ag/AgCl/3 M KCl electrode and Pt electrode (Bioanalytical systems, USA) were applied in a three-electrode cell system as reference and counter electrodes, respectively. Cyclic voltammetric measurements were acquired under Gamry instruments framework software, and data acquired were evaluated using Echem Analyst and OriginPro 8. Cyclic voltammetry test was conducted in 0.1 M H2SO4 acid solution as the electrolyte under N2 atmosphere, in the potential range from − 0.4 to 0.8 V at a scan rate of 100 mVs−1. Scan rate influence was also studied under similar conditions except for, scan rates varied at 10, 20, 30, 40, and 50 mVs−1 in the potential range between − 0.2 to 0.7 V.
XPS Studies
The XPS signals on the powdered sample were recorded using AXIS ULTRA DLD (kratos analytical Ltd, UK) equipped with an Al Ka X-ray source. The spectra were acquired in the constant analyzer energy mode with a pass energy of 160 eV, 10 kV, and 10 mA emission current for the survey. The individual scans were performed with a pass energy of 10 eV, 15 kV, and 15 mA emission current. The vision manager 2 software was used for digital acquisition and data processing. Spectral calibration was determined using the automated calibration routine and the internal C1s standard. In 100 ml of DMSO solvent, the solubility of PA and PA-co-3-AP derivatives in the powder form is to be determined by dissolving 10 mg of the as-synthesized polymer and allowed to disperse homogeneously. The dispersion is aged for a few hours at the room temperature of 25 °C, and filtered by sintered glass crucible (porosity 2 microns).
Structural studies
UV–Visible absorption spectra of the polymer samples in NMP solvent were recorded at room temperature on a thermo spectronic genesys2 research grade spectrophotometer.
Morphological studies
Scanning electron micrographs were recorded using a JEOL-6700 field emission microscope to study the morphology of the polymers.
Results and discussion
UV–vis absorption study
UV–Vis electronic absorption spectroscopy is used to determine the extent of conjugation and the sorts of transitions involved in electrically conducting polymers [29]. Figure 1 shows the UV–Vis absorption spectra of PA, P-3-AP, and PA-co-3-AP80 copolymers salts in NMP solvent is observed in the range of 250–1000 nm. The as-synthesized polymers show two characteristic absorption bands at ~ 320–350 and ~ 600–620 nm [24, 39]. The band at lower wavelength is ascribed to the π–π* transition of the π conjugated systems and the higher wavelength band is consigned to the n–π* benzenoid to quinonoidexcitonic transition [24, 40,41,42,43,44,45]. Furthermore, the band at ~ 470 nm is signifying the polaron–π* transition [29]. A wide range of absorption at visible region ~ 600–900 nm is assigned to the π–polaron transitions, which is endorsed to the emeraldine salt form of PA, P-3-AP, and PA-co-3-AP copolyanines [42, 43]. These observations confirm the obtained PA, P-3-AP, and PA-co-3-AP copolymers exist in the conducting emeraldine salt form (ES).
XPS study
X-ray photoelectronic spectroscopy (XPS) is widely used to investigate the chemical composition and elemental states, especially in conducting polymers and composite materials. Figure 2a–c shows the XPS survey spectra of PA, P-3-AP, and PA-co-3-AP80 copolymer and it confirms that the presence of anticipated elements in all the samples. Figure 3a denotes the N 1s deconvoluted spectra of PA and which includes three characteristic peaks of C–N (389.9), neutral or quinonoid imine (C=N) 400.5, and quaternary or protonated quinonoid imine (C=NH+) 402.3 eV. The deconvoluted peaks of P-3-AP, at 397.1, 398.2 and 400.2 eV in Fig. 3b, and in Fig. 3b, for PA-co-3-AP80 copolymer, at 397.2, 398.3 and 400.1 eV are perceived. The deconvoluted data found for N 1s of the three polymers are well agreed with the literature [24, 44]. As shown in Fig. 4a–c, the C 1s deconvoluted spectra of PA, P-3-AP, and PA-co-3-AP80 polymers are resolved into four distinctive peaks at ~ 283.3, 284.5, 285.6, and 286.2 eV and which endorses the C–C, C=N, C=NH+ and carbonyl (C=O) functional groups, respectively. The C 1s first peak is observed at ~ 283.3 eV, which is ascribed to the neutral C–C bonds present in the polymer backbone. The second integral peak centered at ~ 284.5 eV which is assigned to the neutral quinonoid imine (C=N) bond, which confirms the presence of the quinonoid ring in the polymer backbone. The third peak is observed at ~ 285.6 eV, which can be assigned to the protonated quinonoid imine (C=NH+) and which reveals the formation of polyaniline emeraldine salt (ES). The characteristic peak of the carbonyl (C=O) functional groups (i.e. carbon connected with oxygen) due to the realization of BQ (p–benzoquinone) and HQ (hydroquinone quinol) as degradation products in PA, P-3-AP, and PA-co-3-AP80 copolymer is perceived at utmost binding energy (~ 288 eV). In this study, the O 1s spectra were not recorded for further investigations because it is highly subjective to the moisture and other oxide impurities which are very common in the XPS technique [24].
Electrochemical performance
The electrochemical study is a remarkable property of conducting polyanilines to scrutinize the application of PA as electrode material in numerous electrochemical applications. In the present study, the electrochemical tests for PA, P-3-AP, and PA-co-3-AP copolymers are performed in 0.1 M H2SO4 electrolyte under inert (N2) atmosphere using GCE electrode in a three-electrode system within the potential window of − 0.4 to 0.8 V at various scan rates of 20–50 mVs−1. In Fig. 5a, the PA shows two oxidation forward peaks at 0.269 and 0.517 V and two reduction peaks at 0.114 and 0.431 V reversibly. The oxidation peaks correspond to the transformation of leucoemeraldine base to emeraldine base (EB) and EB to PA (emeraldine base to complete oxidation state of pernigraniline state) [45]. While in Fig. 5b, d, P-3-AP and PA-co-3-AP copolymers exhibit only one single electron oxidation at ~ 0.152 V, and on reversal, the scan shows a single reduction at around 0.075 V. The current density of PA is higher when compared to the P-3-AP and which is almost 5 times greater than the copolymers. It is due to the distorted planarity of the phenyl rings while increasing the hydroxyl (–OH) groups on the polymer backbone, which results in deprived electrical conductivity and electrochemical activity. Eventually, the current density of the copolymers PA-co-3-AP20 to PA-co-3-AP80 gradually decreases with the higher weight percent of 3-aminophenol.
Solubility test
A Buchner-type filter funnel, a 100-mL round-bottom flask, and an electronic weighing balance are used to determine the solubility of the polymer powder. There are only two weighings and no undissolved polymer transfer in this simple technique. In this, method [46], the as-prepared polymer, is weighed directly into a clean, dry, filter crucible. The polymer is then dried in the filter crucible for one hour at 70 °C in an oven. The crucible is then appropriately placed in the round-bottom flask, together with a specified volume of DMSO solvent (100 mL). The left out polymer in the filter crucible is then dried at 70 °C in an oven for one hr and then reweighed. The mass of the polymer dissolved in a given volume of solvent is referred to as weight loss at a temperature of 25 ± 2 °C.
In electrochemical study, solubility plays a key role in conducting polyanilines and their potential electrochemical applications. The solubility of PA, P-3-AP and the PA-co-3-P dissolved separately in DMSO solvent, and the results are depicted in Table 1. The obtained data show the solubility of PA is lower than that of P-3-AP and PA-co-3-AP. Owing to the higher reactivity of –OH (hydrogen bonding occurs, which enhances the polarity of the molecule) group, the solubility of P-3-AP, and PA-co-3-AP copolymers was improved. As the weight percentage of 3-aminophenol (20–80%) in the polymer chain is increased, the solubility of copolymers tends to increase, with the greatest solubility of 69.84 × 10–2 to 78.70 × 10–2 (W/V percent), (g/dL)). However, the interesting property noticed here is that the copolymer PA-co-3-AP shows lower solubility than our earlier reported 2-amino phenol copolymers in our previous work [24, 25]. Due to presence of a –OH (hydrogen bonding) group on the polymer backbone, 2-amino phenol copolymers have a higher solubility than 3-amino phenol copolymers. This gives the copolymer a more polar character [24, 26]. The 3D structure of the copolymer (PA-co-3-AP) is shown in Scheme 2. The structure explains the hydrogen bonding in the molecules.
Morphological studies
The field emission scanning electron microscopy (FE-SEM) is a vital tool to investigate the morphology of the conducting polymers. The surface morphology of the PA, P-3-AP, and the PA-co-3-AP copolymers were examined using the magnification of 80 k and depicted in Fig. 6. As can be realized from Fig. 6a, b both PA and P-3-AP polymer own distinct and dissimilar morphology of the sample in the size range of 0.2–1 µm. The PA has a regular granular morphology, whereas P-3-AP polymer has an aggregated globular structure. But, the PA-co-3-AP 20 polymer has both mixed granular and globular morphology Fig. 6c, while PA-co-3-AP 80 polymer has aggregated globular morphology Fig. 6d. These globules and aggregation formation could have occurred due to the formation of the hydrogen bonding between the monomer units and the rapid stirring environmental condition which furnished the doped polymer’s alteration into globular structures.
Conclusions
Herein, we synthesized the highly soluble (PA-quinonoid-3-AP) copolymers by the chemical oxidative polymerization method. The homopolymer P-3-AP procures better solubility than the conventional PA and copolymers. The solubility of homopolymer P-3-AP is ten-fold higher than the simple PA. The solubility of the copolymers (PA-co-3-AP) increases progressively as the weight percent of meta-hydroxy aniline monomers is increased in the polymer chain. Cyclic voltammograms suggest that the current density of PA is higher than the current density of the homopolymer and which is almost five-fold higher. This is due to the presence of distorted planarity of the entire polymer backbone, which results in poor electrical conductivity and electrochemical activity. However, the current density in the copolymers decreases gradually as the weight percent of 3-aminophenol in the polymer backbones is increased. The present study provides better insight into the method that prepares extremely soluble conducting polyanilines which will offer potential electrochemical applications including corrosion protective coatings and energy storage applications etc.
References
Bhati VS, Nigam ANA, Sharma CS, Kumar M (2019) PAN/(PAN-b-PMMA) derived nanoporous carbon nanofibers loaded on ZnO nanostructures for hydrogen detection. Sens Actuators B Chem 299:126980
Al-Hussaini AS, Eltabie KR, Hassan MER (2016) One-pot modern fabrication and characterization of TiO2@terpoly(aniline, anthranilic acid and o-phenylenediamine) core-shell nanocomposites via polycondensation. Polymer 101:328–337
Khaled MO, Mohamed ERH, Al-Hussaini AS (2019) Novel Fe2O3@PANI-o-PDA core-shell nanocomposites for photocatalytic degradation of aromatic dyes. J Polym Res 199
Al-Hussaini AS, El-Bana WE, El-Ghamaz NA (2020) New semiconducting core-shell nanocomposites. Compos nterfaces 27:385–399
Al-Hussaini AS (2016) New polymeric based materials: terpoly(aniline, diphenyl amine, and o-anthranilic acid)/kaolinite composites. Polym Adv Technol 27:1604–1608
Al-Hussaini AS (2016) Inexpensive fabrication and characterization of crystalline poly(o-anthranilic acid-co-o-phenylenediamine) emeraldine base/bentonite nanocomposites. Polym Plast Technol Eng 55:1386–1392
Al-Hussaini AS, Mohamedein AM, Hassan MER (2021) Towards appraising influence of new economical polymeric core-shell nanocomposite. J Inorg Organomet Polym Mater 31:1491–1502
Al-Hussaini AS, Eltabie KR, Hassan MER (2018) Fabrication of core–shell nanocomposites with enhanced photocatalytic efficacy. Polym Int 67:1419–1428
Apparao T, Arukula R, Narayan R, Rao CRK, Raju KVSN (2015) Energy storage and surface protection properties of dianiline co-polymers. RSC Adv 5:106523–106535
Al-Hussaini AS, Eltabie KR, Hassan MER (2020) Synthesis of smart core-shell nanocomposites with enhanced photocatalytic efficacy. Polym Plast Technol Eng 59:1956–1966
Li T, Wang X, Liu P, Yang B, Diao S, Gao Y (2019) Synthesis of feather fan-like PANI electrodes for supercapacitors. Synth Metals 258:1161
Ravi A, Vinothkannan M, Kim AR, Yoo DJ (2019) Cumulative effect of bimetallic alloy, conductive polymer and graphene toward electrooxidation of methanol: an efficient anode catalyst for direct methanol fuel cells. J Alloys Compd 771:477–488
Belanger D, Ren XM, Davey J, Uribe F, Gottesfeld S (2000) Characterization and long-term performance of polyaniline-based electrochemical capacitors. J Electrochem Soc 147:2923
Li D, Huang JX, Kaner R (2009) B, Polyaniline nanofibers: a unique polymer nanostructure for versatile applications. Acc Chem Res 42:135
Fusalba F, Gouerec P, Villers D, Belanger D (2001) Electrochemical characterization of polyaniline in nonaqueous electrolyte and its evaluation as electrode material for electrochemical supercapacitors. J Electrochem Soc 148:A1
Skotheim TA (1986) Handbook of conducting polymers, vol I and II. Marcel Dekker, New York
Trivedi DC, Nalwa HS (1997) Handbook of organic conductive molecules and polymers, vol 2. Chichester/Wiley, England
Al-Hussaini AS (2021) Eco-friendly synthesis and antimicrobial performance of new heteropolymer composites. J Polym Environ 29:1717–1726
Manohar SK, MacDiarmid AG, Cromack KR, Gindey JM, Enstein AJ (1989) N-substituted derivatives of polyaniline. Synth Met 29:E349-356
Watanabe A, Mori K, Iwabuchi Y, Iwasaki Y, Nakamura Y, Ito O (1989) Electrochemical polymerization of aniline and N-alkylanilines. Macromolecules 22:3521–3525
Chevalier JW, Bergeron J-Y, Dao LH (1992) Synthesis, characterization, and properties of poly (N-alkylanilines). Macromolecules 25:3325
Ito A, Oto K-I, Tanaka K, Yamabe T, Yoshizawa K (1995) n-Alkyl group-substituted poly (m-aniline) s: syntheses and magnetic properties. Macromolecules 28:5618
Chan HSO, Ho PKH, Ng SC, Tan BTG, Tan KLJ (1995) A new water-soluble, self-doping conducting polyaniline from poly (o-aminobenzylphosphonic acid) and its sodium salts: synthesis and characterization. Am Chem Soc 117:8517
Waware US, Arukula R, Hamouda AMS et al (2020) Electrochemical and X-ray photoelectron spectroscopic investigations of conductive polymers. Ionics 26:831–838
Waware US, Rashid M, Hamouda AMS (2019) Poly (aniline-co-3-aminophenol): enhanced crystallinity and solubility. Appl Phys A 125:846
Parthiban A, Le Guen A, Yansheng Y, Hoffmann U, Klapper M, Müllen K (1997) Amino-functionalized poly (arylene ether ketone)s. Macromolecules 30:2238–2243
Wei Y, Harihara R, Patel SA (1990) Chemical and electrochemical copolymerization of aniline with alkyl ring-substituted anilines. Macromolecules 23:758
Pandey SS, Annapoorni S, Malhotra BD (1993) Synthesis and characterization of poly (aniline-co-o-anisidine). A processable conducting copolymer. Macromolecules 26:3190
Nguyen MT, Diaz AF (1995) Water-soluble poly (aniline-co-o-anthranilic acid) copolymers. Macromolecules 28:3411
Yue J, Wang ZH, Cromack KR, Epstein AJ, MacDiarmid AG (1991) Effect of sulfonic acid group on polyaniline backbone. J Am Chem Sot 113:2665
Chen S-A, Hwang G-W (1995) Water-soluble self-acid-doped conducting polyaniline: structure and properties. J Am Chem Sot 117:10055
Cameron RE, Clement SK (1991) US Patent 5008041
Macinnes D, Funt BL (1988) Poly-o-methoxyaniline: a new soluble conducting polymer. Synth Metals 25:235–242
Al-Hussaini AS, Elias AM, Abd El-Ghaffar MA (2017) New poly (aniline-co-o-phenylenediamine)/kaolinite microcomposites for water decontamination. J Polym Environ 25:35–45
Al-Hussaini AS (2018) Novel benzidine and o-phenylenediamine copolymer–matrix microcomposites. J Inorg Organomet Polym Mater 28:871–879
Shacklette LW, Wolf JF, Gould S, Baughman RH (1988) Structure and properties of polyaniline as modeled by single-crystal oligomers. J Chem Phys 88:3955
Cao Y, Li S, Xue Z, Guo D (1986) Spectroscopic and electrical characterization of some aniline oligomers and polyaniline. Synth Met 16:305–315
Masanori K, Akira K, Kazuo S (1986) EPR studies of the charging process of polyaniline electrodes. Chem Lett 15:147–150
Yang Z, Wang X, Yang Y, Liao Y, Wei Y, Xie X (2010) Synthesis of electroactive Tetraaniline−PEO−Tetraaniline triblock copolymer and its self-assembled vesicle with acidity response. Langmuir 26:9386–9392
Manohar SK, MacDiarmid AG (1991) Polyaniline: pernigranile, an isolable intermediate in teh conventional chemical synthesis of emeraldine. Synth Met 41:711–714
Jing X, Wang Y, Wu D, Qiang J (2007) Sonochemical synthesis of polyaniline nanofibers. Ultrason Sonochem 14:75–80
Feng X, Shi Y, Jin S (2015) Three-dimensional microporous polypyrrole/polysulfone composite film electrode for supercapacitance performance. Appl Surf Sci 353:788–792
Tan L, Cao L, Yang M, Wang G, Sun D (2011) Formation of dual-responsive polystyrene/polyaniline microspheres with sea urchin-like and core-shell morphologies. Polymer 52:4770–4776
Al-Hussaini AS, Abdullah MM, Mohamed ERH (2021) Highest degradation of aromatic dyes by new MgO@ PANI-o-PDA core–shell nanocomposites. Polym Bull. https://doi.org/10.1007/s00289-021-03682-1
Albuquerque JE, Mattoso L, Faria R, Masters J, MacDiarmid A (2004) Highest degradation of aromatic dyes by new MgO@ PANI-o-PDA core–shell nanocomposites. Synth Met 146(1):1–10
Harle HD, Ingram JA, Leber PA, Hess KR, Yoder CH (2003) A simple method for determination of solubility in the first-year laboratory. J Chem Edu 80:560
Acknowledgements
We gratefully acknowledge the Qatar University, Doha, for providing the necessary research funding. We also acknowledge the instrumentation facilities provided at CLU and CAM of Qatar University, Doha.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Waware, U.S., Arukula, R., Hamouda, A.M.S. et al. Cyclic voltammetry and XPS studies of poly (aniline-co-3-aminophenol). Polym. Bull. 80, 3897–3910 (2023). https://doi.org/10.1007/s00289-022-04213-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04213-2