Abstract
In this paper, a cyclotetrasiloxane modified with epoxy resin was synthesized via hydrosilylation with 1, 3, 5, 7-tetramethylcyclotetrasiloxane (D4H) and 4-vinyl-1-cyclohexane 1, 2-epoxide (VCHO). The structure of tetramethylcyclotetrasiloxane modified with cyclohexane epoxide (D4H–VCHO) was characterized by Fourier transform infrared (FT-IR), 1H nuclear magnetic resonance (1H-NMR) and gel permeation chromatography (GPC). The effects of feeding methods, feeding ratios, catalyst dosages, reaction temperature and reaction time on the products were investigated. After 4-h reaction with 8 ppm catalyst at 90 °C, D4H–VCHO was obtained with an epoxy value of 0.521 and a refractive index of 1.4920. Then, with 4-methylhexahydrophthalic anhydride as a curing agent and tetrabutylammonium bromide as a catalyst, the obtained D4H–VCHO was cured at 90 °C for 1 h and then 120 °C for another hour. The cured products showed high light transmittance, strong thermal stability, suitable hardness and low water absorption. The glass transition temperature of cured materials was 151.6 °C, indicating the improvement of flexibility. In addition, the introduction of epoxy groups greatly enhanced the adhesion of silicone resins to substrates. The results demonstrated that the epoxy-modified silicone materials could be applied as LED packaging materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The new generation of solid-state cold light source, light-emitting diode (LED), has advantages of low power consumption, environmental friendliness and long working life, compared to traditional light sources [1]. Nowadays, LEDs are widely used in many fields, such as ordinary light source, automotive headlights, indicator lights and backlights. LEDs are mainly composed of substrates, chips, gold wires, packaging materials and lead frames.
The dominant packaging materials for LED are mainly epoxy resins and silicone materials. Traditional epoxy packaging materials perform well in terms of mechanical, electrical and adhesive properties [2, 3], but they have the large internal stress and poor UV/thermal resistance in cured solids. Silicone materials play a significant role in LED encapsulation due to high thermal stability, strong thermal shocking resistance and low moisture absorption [4,5,6,7], but they have the poor adhesion to the metal or polymer substrates. Thus, epoxy-modified silicon materials, with the advantages of both silicon and epoxy resins [8,9,10], have attracted wide attention in recent years.
Epoxy-modified silicone resins have been synthesized by hydrosilylation [11,12,13], hydrolytic condensation [14, 15] and so forth. Accordingly, epoxy groups have been introduced to the molecular structure of siloxane. In addition, a cured epoxy-modified silicone material can also be prepared by mixing epoxy resin with silane coupling agents [16, 17]. However, it is difficult to gain homogeneous, well-performing and transparent materials through simply physical blending [18]. Therefore, researchers preferred to synthesize epoxy-modified polysiloxane through hydrosilylation reaction or hydrolysis condensation, other than physical mixing [19,20,21,22,23].
Murias et al. [24] performed hydrosilylation reaction between 1, 1, 3, 3-tetramethyldisiloxane and allyl glycidyl ether. In order to obtain enough epoxy groups and large molecular weight, modified siloxane was mixed with other epoxy resins before curing. The content of siloxane in resins was reduced; thus, resulting in poor thermal stability of cured materials. Romo-Uribe et al. [25] prepared poly(dimethylsiloxane) diglycidyl ether terminated to replace the above-modified siloxane. To increase the content of epoxy groups, other epoxy resins were added to the poly(dimethylsiloxane) diglycidyl ether. Accordingly, the content of siloxane decreased. The mass loss of the cured products at 287.9 °C reached 5%. To prepare long-chain polysiloxanes with a high epoxy value, Feng et al. [26] prepared a PDMS-block-DGEBA copolymer with DGEBA, hydroxyl-terminated PDMS and silane coupling agent. The PDMS modified DGEBA systems had higher impact strength and lower weight-loss rate than the pristine DGEBA system. Yang et al. [27] synthesized an epoxy-modified polysiloxane with methyl hydrodiethoxy silane and 4-vinyl-1-cyclohexane 1, 2 -epoxide (VCHO) as raw materials via hydrosilylation. Then, an epoxy-modified silicone oil was obtained through hydrolysis condensation reaction between monomer and diethoxydimethylsilane. Finally, it was cured with methylhexahydrophthalic anhydride (MHHPA) and catalyzed by tetrabutylammonium (DTAB). The cured product possessed high thermal stability, high light transmittance and high hardness. However, the process was too complex for industrial applications.
Therefore, it is necessary to prepare long-chain polymethylsiloxane with a high content of epoxy. In the study, the cyclotetrasiloxane modified with epoxy resin was prepared through the hydrosilylation reaction between 1, 3, 5, 7-tetramethyolcyclotetrasiloxane (D4H) and 4-vinyl-1-cyclohexane-1, 2-epoxide (VCHO). Then, the optimum hydrosilylation conditions were further investigated. At last, the prepared cyclotetrasiloxane was cured with methylhexahydrophthalic anhydride. The cured products showed high light transmittance (> 90% at 450 nm) and strong thermal reliability. The epoxy-modified silicone materials could be applied as encapsulants for LED.
Experimental
Raw materials
4-Vinylcyclohexane 1, 2- epoxide (VCHO) and 4-methylhexahydrophthalic anhydride (MHHPA) were provided by Chembridge International Co., Ltd., Taipei, China. 1, 3, 5, 7-Tetramethylcyclosiloxane (D4H) was supplied by Shenzhen Superior Changhao Technology Co., Ltd., Guangdong, China. Tetrabutylammonium bromide (DTAB) was purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd., Shanghai, China. Chloroplatinic acid was obtained from Guangdong Kejunchi Technology Co., Ltd., Guangdong, China. The 3535 surface-mounted devices (SMD) LED lead frames were purchased from Shenzhen Wenliang Electronics Co., Ltd., Guangdong, China.
Synthesis of D4H–VCHO
A certain amount of D4H was dropped into 4-vinyl-1-cyclohexene 1, 2-epoxide (VCHO) within 30 min at 85 °C. Subsequently, the reactants were continuously stirred for 6 h under the catalyst of chloroplatinic acid. Finally, the product performed vacuum distillation and the unreacted VCHO was removed. A viscous, transparent and colorless cyclotetrasiloxane modified with epoxy resin (D4H–VCHO) was synthesized. The synthesis route is shown in Fig. 1.
Preparation of cured D4H–VCHO with anhydride
The synthesized D4H–VCHO was cured with anhydride and catalyzed with DTAB. The curing reaction was carried out at 90 °C for 1 h and then 120 °C for another hour. Finally, the product cured with D4H–VCHO was successfully prepared.
Analysis and measurement
FT-IR
A NICOLET ANTARI spectrometer (Thermo Fisher Scientific Co., Ltd., America) was used to record the FT-IR spectra. The test was performed in the range from 4000 to 500 cm−1. Liquid samples were filmed on the surface of KBr pellets.
1H-NMR
The 1H-NMR spectra were recorded by using an AVANCE III HD 600 Spectrometer (Bruker, Germany) with chloroform as solvent.
Gel permeation chromatography (GPC)
The molecular weight and the distribution of the sample were determined by using Agilent 1000 gel permeation chromatography (Agilent, America). The chloroform was used as solvent, while polystyrene was used as standard.
Thermogravimetric analysis (TGA)
The thermal stability of the epoxy-modified silicone materials was measured through thermogravimetric analysis with a STA-449C thermogravimetric analyzer (Netzsch Group, Germany) at a heating rate of 10 °C/min from 40 to 800 °C in a nitrogen atmosphere.
Dynamic thermomechanical analysis (DMA)
The glass transition temperature of epoxy-modified silicone materials was measured through dynamic thermomechanical analysis with a DMA Q800 thermogravimetric analyzer (TA Instruments, America) at a heating rate of 5 °C/min from 25 to 200 °C. The sample size was 40 \(\times \hspace{0.17em}\)12 \(\times \hspace{0.17em}\)3 mm3.
Measurements of optical properties
2WA-J abbe refractometer (Shanghai CSOIF Co., Ltd., China) was used to measure the refractive index. The light transmittance of cured materials was measured in the range from 300 to 800 nm with a Hitachi U-3010 (Hitachi, Japan) spectrophotometer. The sample was a disc with a diameter of 30 mm and a thickness of 2 mm.
Measurement of the mechanical properties of cured products
The hardness of the prepared encapsulation materials was measured with a Yuanling LX-A Shore A durometer (Shanghai Yuanling Instrument Co., Ltd., Yuanling, China) according to ISO 7619: 1986.
The tensile strength of the samples was carried out on a CMT 4303 universal electronic material testing machine (Shenzhen Sans Instruments Co., Ltd., China) according to GB/T 1040-92.
Water absorption
The water absorption ratios of the cured cyclotetrasiloxane modified with epoxy resin were measured after immersing it in distilled water at room temperature for 24 h according to the standard of ASTM D570-98(2005). Subsequently, the samples were taken out and free water on sample surfaces was absorbed with filter paper. The water absorption ratios of cured samples were calculated. The water absorption ratios can be calculated as
where m1 and m2 are, respectively, the weight of a sample before and after the water absorption test.
Red dye penetration test
The adhesive properties of packaging materials were evaluated by the red dye penetration test. In this test, 3535 SMD LED lead frames were encapsulated by the cured materials and the lead frames were immersed into boiling red ink for 6 h. Subsequently, whether red ink was permeated into the LED frames was observed. If the penetration phenomenon was not observed, the packaging materials had strong adhesion to the LED substrate.
Results and discussion
Characterization of D4H–VCHO
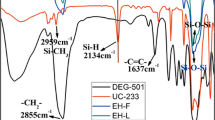
Figure 2 shows the FT-IR spectra of D4H and D4H–VCHO. After the hydrosilylation reaction, the characteristic peaks of D4H–VCHO were different from those of D4H. The absorption peaks in the range of 990–1120 cm−1 were ascribed to Si–O–Si peaks. The characteristic bands at 2965 cm−1 and 1260 cm−1 were assigned to the stretching vibration of C–H from CH3 and the bending vibration of Si–CH3. Moreover, the Si–O–Si peaks and Si–CH3 peaks of D4H–VCHO were stronger than those of D4H, demonstrating the introduction of D4H. In addition, the Si–H peaks at 2160 cm−1 were weakened greatly in D4H–VCHO and the epoxy groups at 870 cm−1 appeared in D4H–VCHO, indicating the successful addition reaction between D4H and VCHO (Figs. 3, 4).
The structures of D4H and D4H–VCHO were analyzed by 1H-NMR. The signals of solvent chloroform were determined at 7.26 ppm. The chemical shifts between 0 and 0.3 ppm were assigned to the hydrogen on Si-CH3. The Si-CH2-CH band at 3.6 ppm appeared in D4H–VCHO, whereas the Si–H band at 4.9 ppm obviously decreased, illustrating the occurrence of addition reaction. What’s more, the chemical shifts of epoxy groups appeared in 3.1 ppm. The absorption peaks from 0.5 to 2.16 ppm were ascribed to the proton peaks and multiplets of cyclohexyl.
The molecular weight of D4H–VCHO was analyzed by GPC. The number average molecular weight of D4H–VCHO was 585, while the weight average molecular weight was 605. The popydispersity index (PDI) was as low as 1.034, revealing the homogeneity of products. Based on the above analyzed, the epoxy-modified polysiloxane was successfully synthesized.
Optimization of feeding method
The color and appearance of the synthesized product were closely related to the feeding methods in the reaction process. This paper investigated three feeding methods, including Method 1 (disposable batch feeding), Method 2 (adding D4H into VCHO in a dropwise manner) and Method 3 (adding VCHO into D4H in a dropwise manner). The reaction phenomena and the appearance of products of different methods are summarized in Table 1. In Method 1, as the reactants were mixed, a large quantity of heat was released within a short time. Therefore, heat induced the ring-opening and cross-linking of epoxy groups. In Method 3, the concentration of hydrogen was much greater than that of C=C, thus resulting in the agglomeration of platinum to form Pt–Pt colloids and Pt–Si colloids. Thus, the D4H–VCHO turned yellowish [28]. In Method 2, excessive C=C formed the Pt–C colloids, which caused less discoloration [29]. Therefore, adding D4H into VCHO in a dropwise manner was the optimal feeding method.
Optimization of feeding ratio
In hydrosilylation reaction, the molar ratio of Si–H to C=C significantly affected the conversion rate and epoxy value. A large epoxy value of D4H–VCHO was beneficial to the formation of stable curing materials. Thus, the relationship between the molar ratio of Si–H to C=C and the properties of D4H–VCHO was investigated (Table 1). When n(Si–H):n(C=C) was 1:0.8, insufficient vinyl groups caused a large amount of Si–H residues and a lower epoxy content in final products [30]. Then, increasing the amount of vinyl groups could obviously increase the epoxy content in D4H–VCHO. Once the molar ratio of n(Si–H):n(C=C) was increased above the ratio of 1:1.2, improving the vinyl groups had little effect on the increase in the epoxy content. Too much VCHO would result in the waste of raw materials and adversely affect the performance of cured products. Therefore, the best molar ratio of n(Si–H):n(C=C) was 1:1.2.
Optimization of catalyst dosage
Catalyst dosages directly influenced the conversion rate of addition reaction. Under the same reaction conditions and different catalyst dosages, the changes in conversion rate were analyzed. When catalyst dosage was lower than 5 ppm, the fewer effective collisions between chloroplatinic acid and reactants resulted in insufficient catalyst efficiency and slower reaction rate [31]. Increasing catalyst dosage below 8 ppm improved the conversion rate significantly. However, when the catalyst dosage increased above 8 ppm, the increase in the conversion rate was not significant. Too much catalyst easily caused a lot of heat and the ring-opening reaction of epoxy groups. In addition, excessive chloroplatinic acid led to the precipitation of platinum colloids and the darker color of products. Thus, the dosage of chloroplatinic was 8 ppm.
Optimization of reaction time
The hydrosilylation reaction could not be completed within a short time. However, the long reaction time led to further condensation of polysiloxane, and the instability and even gelation of final products [32]. The conversion rate was improved with the increase in reaction time (Fig. 5). When the reaction time increased above 6 h, the rate remained unchanged. In addition, the increase in reaction time led to the darker color in D4H–VCHO. Consequently, the optimal reaction time should be 6 h.
Optimization of reaction temperature
At low temperature, the platinum catalytic activity is quite low [33], but the high temperature induces side reactions and damages the homogeneity of products [34]. Figure 6 shows the conversion rate of hydrosilylation reaction at different temperatures. When the temperature rose from 60 to 90 °C, the conversion rate significantly increased. When the temperature rose above 90 °C, the conversion rate was seldom changed. When the reaction temperature was higher than 110 °C, the product became a yellowish liquid with high viscosity. This phenomenon could be explained by the ring-opening reaction of epoxy groups at a high temperature. Therefore, the reaction temperature was chosen as 90 °C.
The optimal reaction conditions were determined as follows: adding D4H into VCHO in a dropwise manner, the mole ratio of n(Si–H) to n(C=C) (1:1.2), 8 ppm catalyst, reaction time of 6 h and reaction temperature of 90 °C.
Performance of cured D4H–VCHO
D4H–VCHO was synthesized under the optimal conditions. Then, D4H–VCHO was cured to prepare the epoxy-modified methyl silicone materials. The cured products showed the refractive index of 1.4920. The hardness of cured materials was 91.3 shore A and that tensile strength was 3.646 MPa, meeting the requirement of packaging materials for mechanical properties. What’s more, the water absorption of cured materials was 0.08%, indicating remarkable waterproof performance.
The high transparency of encapsulants can reduce light loss and improve the brightness of LED devices. The light transmittance of D4H–VCHO before the aging test was maintained above 90% in the visible range from 400 to 800 nm (Fig. 7) and met the optical requirements of LEDs.
LED devices are inevitably affected by external heat and UV. It is important for packaging materials to maintain high light transmittance in the external environment. Thus, the thermal aging test of the cured D4H–VCHO was carried out at 200 °C for 120 h and the aging test was performed with 365 nm UV for 120 h, respectively. However, the light transmittance of D4H–VCHO was still maintained above 80% in the range from 450 to 800 nm after thermal aging or UV aging, indicating the high resistance to thermal and UV.
When the LEDs are under working conditions, the heat generated from chips is transferring through encapsulants to the outer environment. The packaging materials are required to be stable at a working temperature above 120 °C [35]. A thermogravimetric analysis was carried out to evaluate the thermal reliability of cured D4H–VCHO. Figure 8 shows the TGA of cured D4H–VCHO from 40 to 800 °C. The cured D4H–VCHO showed no mass loss below 200 °C, indicating the complete curing of silicone materials. The initial decomposition temperature was about 236 °C, and 5% mass loss was reached at 330 °C. The high thermal reliability could be attributed to the stability of siloxane.
The glass transition temperature represents the lowest temperature of molecular chain motion, which is directly related to the flexibility of materials. Due to introduction of Si–O–Si, the flexibility of cured materials was improved and the glass transition temperature was 151.6 °C (Fig. 9).
Red dye penetration tests were conducted to investigate the adhesion and sealing conditions between the encapsulated resin and lead frames. D4H–VCHO was used as the encapsulating material to encapsulate LEDs. The packaged 3535 SMD LED lead frames were immersed in boiling red ink for 6 h. No red ink was penetrated into the chips after 6-h boiling (SMD encapsulated with D4H–VCHO, Fig. 9), indicating that the modified materials and lead frame were in high adhesion and sealing conditions (Fig. 10).
Conclusions
The epoxy-modified methyl polysiloxane D4H–VCHO was synthesized via hydrosilylation reaction between 4-vinyl-1-cyclohexane-1, 2-epoxide (VCHO) and 1, 3, 5, 7-tetramethyltetracyclosiloxane. The characterization results indicated that epoxy groups were successfully introduced into polysiloxane. The optimal conditions were as follows, adding D4H into VCHO in a dropwise manner, n(Si–H):n(C=C) of 1:1.2, 8 ppm catalyst and 6-h reaction at 90 °C.
After curing, the epoxy-modified methyl silicone materials showed great light transmittance, suitable hardness and low water absorption ratios. Due to the high bond energy of Si–O–Si and the adhesion of epoxy groups, D4H–VCHO possessed the high stability and better adhesion performance. Therefore, the D4H–VCHO can be used as LED encapsulants.
References
Feng QB, Zhu R, Tang G, Zhang JJ, Zhu Y (2013) Thermal analysis of heat sink employed on tunnel lighting lamps. Appl Mech Mater 303:2719–2723
Huang JC, Chu YP, Wei M, Deanin RD (2004) Comparison of epoxy resins for applications in light-emitting diodes. Adv Polym Technol 23:298–306. https://doi.org/10.1002/adv.20018
Zhang X, Yin H, Cheng X (2008) Preparation of needle shaped nano-copper by microwave-assisted water system and study on its application of enhanced epoxy resin coating electrical conductivity. Appl Surf Sci 254:5757–5759. https://doi.org/10.1016/j.apsusc.2008.03.078
Zhan XB, Zhang JY, Wang XL (2012) Progress on silicone packaging materials for power LED. Procedia Eng 27:687–692. https://doi.org/10.1016/j.proeng.2011.12.506
Pan Z, Sun R, Zhu S, Kang Y (2018) The synthesis, characterization and properties of silicone adhesion promoters for addition-cure silicone rubber. J Adhes Sci Technol 32:1517–1530. https://doi.org/10.1080/01694243.2018.1428059
Kim JS, Yang SC, Kwak SY, Choi Y (2012) High performance encapsulant for light-emitting diodes (LEDs) by a sol–gel derived hydrogen siloxane hybrid. J Mater Chem 22:7954–7960. https://doi.org/10.1039/c2jm16907j
Kim JS, Yang SC, Bae BS (2012) Thermally stable transparent sol−gel based siloxane hybrid material with high refractive index for light emitting diode (LED) encapsulation. Chem Mater 22:3549–3555. https://doi.org/10.1021/cm100903b
Reusmann G (2002) New epoxy-siloxane hybrid binder for high performance coatings. Macromol Symp 187:235–242. https://doi.org/10.1002/1521-3900(200209)187:1
Wang WJ, Perng LH, Hsiue GH, Chang FC (2000) Characterization and properties of new silicone-containing epoxy resin. Polymer 41:6113–6122. https://doi.org/10.1016/S0032-3861(99)00790-9
Chen Z, Kim BJ, Stafslien S (2008) UV-curable, oxetane-toughened epoxy-siloxane coatings for marine fouling-release coating applications. Polym Int 57:879–886. https://doi.org/10.1002/pi.2422
Crivello JV, Bi D (1993) Regioselective hydrosilations. II. The synthesis of silicon–hydrogen functional compounds. J Polym Sci Part A Polym Chem 31:2729–2737. https://doi.org/10.1002/pola.1993.080311108
Crivello JV, Lee JL (1990) The synthesis, characterization, and photoinitiated cationic polymerization of silicon-containing epoxy resins. J Polym Sci Part A Polym Chem 28:479–503. https://doi.org/10.1002/pola.1990.080280303
Ho TH, Wang CS (2001) Modification of epoxy resin with siloxane containing phenol aralkyl epoxy resin for electronic encapsulation, application. Eur Polym J 37:267–274. https://doi.org/10.1016/S0014-3057(00)00115-4
Gao N, Liu WQ, Yan ZL, Wang ZF (2013) Synthesis and properties of transparent cycloaliphatic epoxy–silicone resins for opto-electronic devices packaging. Opt Mater 35:567–575. https://doi.org/10.1016/j.optmat.2012.10.023
Ye H, Zhang XS, Li YJ, Wang T (2012) Synthesis and cationic photopolymerization of phenyl epoxy-silicone monomers. J Polym Res 19:1409–1412. https://doi.org/10.1007/s10965-012-0019-y
Chauhan BP, Rathore JS (2005) Regioselective synthesis of multifunctional hybrid polysiloxanes achieved by Pt-nanocluster catalysis. J Am Chem Soc 127:5790–5791. https://doi.org/10.1021/ja042824c
Huang W, Zhang Y, Yu Y, Yuan Y (2007) Studies on UV-stable silicone–epoxy resins. J Appl Polym Sci 104:3954–3959. https://doi.org/10.1002/app.26188
Smith KM, Browne SE, Jayaraman S, Bleickardt CJ (2014) Effect of dimethylpolysiloxane liquid on the cryogenic tensile strength and thermal contraction behavior of epoxy resins. Cryogenics 61:63–69. https://doi.org/10.1016/j.cryogenics.2014.01.014
Zhao M, Feng Y, Li G, Li Y, Wang Y (2014) Synthesis of an adhesion-enhancing polysiloxane containing epoxy groups for addition-cure silicone light emitting diodes encapsulant. Polym Adv Technol 25:927–933. https://doi.org/10.1002/pat.3327
Ma SQ, Liu WQ, Gao N, Yan Z, Zhao Y (2011) Synthesis and properties of LED-packaging epoxy resin toughened by a novel polysiloxane from hydrolysis and condensation. Macromol Res 19:972–979. https://doi.org/10.1007/s13233-011-0911-z
Ma SQ, Liu WQ, Wei ZJ (2011) Mechanical and thermal properties and morphology of epoxy resins modified by a silicon compound. J Macromol Sci Part A Pure Appl Chem 47:1084–1090. https://doi.org/10.1080/10601325.2010.511522
Yang SC, Kwak SY, Jin JH, Kim JS (2012) Thermally resistant UV-curable epoxy–siloxane hybrid materials for light emitting diode (LED) encapsulation. J Mater Chem 22:8874–8880. https://doi.org/10.1039/C2JM16355A
Jung KH, Bae JY, Park SJ (2010) High performance organic-inorganic hybrid barrier coating for encapsulation of OLEDs. J Mater Chem 21:1977–1983. https://doi.org/10.1039/C0JM02008G
Murias P, Maciejewski H, Galina H (2012) Epoxy resins modified with reactive low molecular weight siloxanes. Eur Polym J 48:769–773. https://doi.org/10.1016/j.eurpolymj.2012.01.009
Romo-Uribe A, Santiago-Santiago A, Reyes-Mayer M-F (2017) Functional PDMS enhanced strain at fracture and toughness of DGEBA epoxy resin. Eur Polym J 89:101–118. https://doi.org/10.1016/j.eurpolymj.2017.01.041
Zhao F, Sun Q, Fang DP, Yao KD (2000) Preparation and properties of polydimethylsiloxane-modified epoxy resins. J Appl Polym Sci 76:1683–1690
Yang X, Huang W, Yu Y (2011) Synthesis, characterization, and properties of silicone–epoxy resins. J Appl Polym Sci 120:1216–1224. https://doi.org/10.1002/app.33108
Lewis LN, Lewis N (1986) Platinum-catalyzed hydrosilylation—colloid formation as the essential step. J Am Chem Soc 108:7228–7231. https://doi.org/10.1021/ja00283a016
Sakaki S, Mizoe N, Musashi Y (1999) Platinum-catalyzed hydrosilylation of ethylene A theoretical study on the reaction mechanism involving cis-trans isomerization of PtH(SiH3)(PH3)2. J Mol Struct THEOCHEM 461:533–546. https://doi.org/10.1016/S0166-1280(98)00432-1
Cullen WR, Evans SV, Han NF (1987) Platinum(II) complexes of ferrocenylphosphines as hydrosilylation catalysts Crystal structure of (P-N) PtCl2 (P-N = Fe (.eta.5-C5H5) (.eta.5-C5H3(P(CHMe2)2) CHMeNMe2-1,2). Inorg Chem 26:514–519. https://doi.org/10.1021/ic00251a007
Sakaki S, Mizoe N, Sugimoto M (1998) Theoretical study of platinum(0)-catalyzed hydrosilylation of ethylene. Chalk-Harrod mechanism or modified Chalk-Harrod mechanism. Organomet 17:2510–2523. https://doi.org/10.1021/om980190a
Zhang B, Rui L, Luo J (2018) Epoxy-silicone copolymer synthesis via efficient hydrosilylation reaction catalyzed by high-activity platinum and its effect on structure and performance of silicone rubber coatings. Polym Bull 1:2105–2124. https://doi.org/10.1007/s00289-017-2127-7
Albinati A, Caseri WR, Pregosin PS (1987) Hydrosilylation with platinum complexes. preparation, low-temperature nmr spectra, and x-ray crystal structure of the novel bis-olefin catalyst cis-ptcl2(phch=ch2)2. Organometallics 6(4):788–793. https://doi.org/10.1021/om00147a017
Mengzhong C, Liu L, Kim J (2012) Infrared spectroscopy evidence for the hydrosilylation of rubbers and hydrogen-containing polysiloxane via heat processing. J Appl Polym Sci 125:3000–3005. https://doi.org/10.1002/app.35647
Pan Z, Zhu S, Huang B (2019) Synthesis of high-refractive-index epoxy-modified vinyl methyl phenyl silicone rsesins for encapsulation of LEDs. J Electron Mater 48:2865–2875. https://doi.org/10.1007/s11664-019-07015-x
ISO 7619: 1986 Rubber—Determination of indentation hardness by means of pocket hardness meters
GB/T 1040–92 Plastics—determination of tensile properties
ASTM D570–98 Standard test methods for water absorption of plastics
Acknowledgements
This project was supported by Department of Education’s Production-Study-Research combined innovation Funding -- “Blue fire plan (Huizhou)” (Grant No. CXZJHZ201718) and Guangdong Basic and Applied Basic Research Foundation (Grant No. 2021A1515010945).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pan, Z., Chen, M., Zeng, K. et al. Synthesis and application of cyclotetrasiloxane modified with epoxy resins. Polym. Bull. 79, 7177–7192 (2022). https://doi.org/10.1007/s00289-021-03847-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03847-y