Abstract
Waterborne polyurethane dispersions used in surface coatings have recently gained a lot of interest as the need for more and more alternatives in the case of chemical components has arisen. Nevertheless, WPUDs can be used to obtain coatings with superior qualities. Hence, a study of various research papers, articles, and other literature is being presented in the following review article. We have studied various aspects of improvement and advancement in the field of WPUDs. We have focused mainly on thermal and mechanical property elevation brought about by physical and chemical modifications. We have also described raw materials and the basic chemistry of WPUDs taking into consideration how properties can be achieved by alterations in these two aspects as well.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyurethanes (PU) are a growing class of polymers used in many day-to-day life activities all over the world. Since their discovery in 1937 by Otto Bayer [1], many areas of applications like building insulations [2], refrigerators and freezers [3], furniture and bedding [4], footwear [5], automotive parts [6], coatings [7] and adhesives [8], etc., have opened up for effective use of PU. Based on the field of application, polyurethanes can be classified into the following main types;
-
Flexible PU foam [9]
-
Rigid PU foam [10]
-
Coatings, adhesives, sealants [11] and elastomers [12] (CASE)
-
Thermoplastic polyurethane (TPU) [13]
-
Products of reaction injection molding (RIM) [14]
-
Emulsion binders [15]
-
Waterborne polyurethane dispersions (WPUDs) [16]
The percentage of application of various PU types is given in Fig. 1
WPUDs consist of a PU backbone dispersed in water instead of organic solvents as are in solvent-based PUs (SPUDs) [17]. Many organic solvents like toluene, xylene, formaldehyde are considered to be volatile organic compounds or VOCs [18], and due to environmental concerns in recent times, the use of organic solvents has been reduced in polyurethane dispersions. WPUDs have various advantages over SPUDs like high tensile strength [19], high abrasion resistance [20], high flexibility [21], good adhesion ability [22] and good low-temperature resistance [23]. WPUDs are used in various applications like antibacterial coatings [24], flame-retardant coatings [25], anticorrosive coatings [26], self-healing coatings [27], automotive coatings [28], adhesives [29], biomedical applications [30], etc. Although the end product and the application of each type of polyurethane are different, the basic chemistry to synthesize the polyurethane linkage remains the same.
The basic chemistry of WPUDs
Polyurethane reaction involves the formation of urethane linkage with the help of two functional monomers, the -OH functionality of a polyol and NCO functionality of a diisocyanate compound generally in the presence of metal catalysts like DBTDL [31], dibutyltin dichloride, zinc or bismuth-based catalysts or amine catalysts like dimethyl cyclohexylamine (DMCHA), dimethylethanolamine (DMEA), 1, 4-diazabicyclo[2.2.2]octane (DABCO) and triethylenediamine (TEDA). Not only catalysts may accelerate the process, but by DSC measurements it has also been proven that autocatalysis carried out majorly by primarily formed urethane bonds also helps in the reaction [32]. The polyol acts as a soft segment providing flexibility to the polymer, and the diisocyanate compound acts as a hard segment giving toughness to the polymer chain.
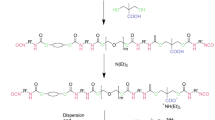
For WPUDs specifically, along with the two basic monomers, a solvent like acetone, THF, ethanol further with a dispersing agent is necessary to create a dispersion of the polymer chain in water. Dispersing agents like dimethyl propionic acid (DMPA), N-methyl diethanolamine, etc., are used in WPUD synthesis widely. Depending upon the types of dispersing agents used, WPUDs are classified into two main types—cationic WPUDs and anionic WPUDs. As evident by the name itself, in cationic WPUDs, a cationic dispersing agent like N-methyl diethanolamine is used, and in anionic WPUDs, an anionic dispersing agent like DMPA is used. In addition to this, chain extenders like ethylenediamine (EDA), hydrazine or ethylene glycol are added to the prepolymer to increase the molecular weight of the polymer. The chain extenders react with the NCO-terminated prepolymer to achieve high molecular weights and high crosslinking which are important factors for the mechanical and barrier properties of the WPUD films. The dispersing agents being ionic, the chain-extended polymer solution needs to be neutralized to create a stable dispersion, and hence, counter ions like triethylamine (TEA) and acetic acid are used for anionic and cationic WPUDs, respectively. The final stage involves the synthesized prepolymer dispersion in water by phase inversion as presented in Fig. 2.
Tailoring the thermal and mechanical properties by varying the raw materials
Polyols
One of the most dominant components in the synthesis of polyurethanes is the polyol on which the properties of PU depend. Polyols are usually of two types—polyether polyols and polyester polyols. Polyether polyols are made by the reaction of epoxides with active hydrogen atom, while polyester polyols are the products of a polycondensation reaction of multifunctional carboxylic acids and polyhydroxy compounds. One of the most characteristic and interesting features of polyol-based polyurethanes is the occurrence of phase-segregated morphology that yields polyol-rich domains (soft phase) and urethane (or urethane-urea) ones (hard phase) [33, 34]. This morphology is used in thermoplastic polyurethane elastomers, and specific segment interactions influence the behavior of these materials even in solution [35]. As a renewable biomass resource, vegetable oils, biodiesel-derived crude glycerol, etc., have attracted more attention in recent years because of their low price, potential biodegradability and functional diversity [36,37,38]. Vegetable oil-based PU films showed comparable or better thermal and mechanical properties than petroleum-based polyols [39]. Figure 3 shows the different vegetable oil-based polyols used to prepare WPUDs. Various efficient strategies have been devised for converting vegetable oils into different types of polyols having various properties like thermal stability, flame retardancy, water resistance, etc., which can be reflected in the final WPUD product [40].
The properties of PU depend on various factors of polyols like the molecular weight, hydroxyl number, etc., of the polyol. Crosslinking results in the increased molecular weight and viscosity of the product. Generally, after crosslinking, the material is losing crystallinity. The Tg of the polymer may or may not depend on its molecular weight, but it is affected by various other factors like polar groups, additives, crosslinking, crystallinity, etc., for example, increasing the long-range order, resulting from the crystalline structure, decreases the ability of the material to fluctuate easily and thus results in increasing the Tg. The final polymer particle size is determined by the inherent polymer aggregation behavior. The particle size has an important effect on the composite toughness, which can be improved or reduced with increasing particle size, whereas the modulus remains constant. However, the strength increases with decreasing particle size.
We can draw a comparison between the properties of WPUDs prepared from crude glycerol and vegetable oil polyols. Shengjun et al. [41] studied the preparation of polyols and waterborne polyurethane dispersions (WPUDs) from biodiesel-derived crude glycerol (CG) which is a low value and inexpensive byproduct as compared to vegetable oils. These (CG-WPUDs) were prepared by thermochemical conversion of CG to polyols in anionic form using DMPA, whereas cationic WPUDs were prepared using TOP (tung oil-based polyol), PEG (polyethylene glycol), IPDI (isophoronediisocyanate) and MDEA (N-methyl diethanol amine) [42]. The resulting films of both had higher glass transition temperatures (Tg), particle sizes and mechanical strengths, but the reasons for this increase differed. The relatively high hydroxyl number and high functionality of the polyol, leading to gelation and high crosslinking of polyurethane networks, caused good thermal stability compared to their analogs derived from vegetable oils [43,44,45]. In the case of TOP-based WPUDs, the average particle size was affected by various factors, including crosslinked structure, degree of neutralization and molecular chain flexibility. These films displayed high gel content, indicating that the resultant WPUD films possessed good solvent resistance. Hydrophobicity increased with TOP content. Whereas in the environment-friendly vegetable oil-based WPUDs synthesized from a series of methoxylated soybean oil polyols (MSOLs), hydrophilicity played a key factor in influencing the particle size [46]. The Tg value, storage modulus increased due to the higher crosslinking in the soft segment because of an increase in the MSOL –OH functionality. The functionality of the MSOLs, prepolymer viscosity, ionic group position, chain rigidity and the chemical structure of the soft segment played an important role in controlling the thermophysical and mechanical properties of the S-WPUD (soybean WPUD) film.
Similarly, hydrophilicity also affected another type of WPUDs. The cottonseed being one of the cheapest renewable resources in WPUD manufacturing, Sashivinay et al. [47] used phosphorylated polyols (phosphols) derived from cotton-seed oil to synthesize DMPA-free, catalyst-free WPUDs by a sol–gel process. The vegetable oil-based phosphorylated polyols were synthesized by ring-opening hydrolysis of epoxidized vegetable oils in the presence of ortho-phosphoric acid [48]. The particle sizes of the WPUDs increased due to two factors: (i) with an increase in the –OH functionality of the phospols resulting in higher crosslinking, (ii) with the decrease in hydrophilicity when the amount of counterion was lower making it difficult for water to penetrate it. The tensile strength, Young’s modulus, storage modulus, Tg value and thermal stability of the WPUDs increased as silica content increased because of the increasing siloxane crosslinking resulting from the higher –OH functionality of phospol. Hence, phosphols are considered to be promising materials for the synthesis of WPUDs for a wide range of applications.
Similarly, coatings made from castor oil as a polyol resource are attracting a lot of recognition in recent years replacing oil from fossil fuels [49, 50]. According to Xiang et al. [51] crude glycerin which is a byproduct of the biodiesel process can be converted to polyols to produce WPUDs. These polyols showed less dependence on fossil fuel and lower CO2 emission. Shengjun et al. [52] did a similar study that focused on the development of biobased polyols and polyurethane from crude glycerol and lignocellulosic biomass. Guo et al. [53] prepared a WPUD/epoxy resin composite coating by crosslinking an HDI (hexamethylenediisocyanate) with an anionic polyol dispersion which showed high gloss, impact strength, adhesion, flexibility, thermal stability and blocking resistance properties. In recent years, the use of waterborne coating systems like ultraviolet (UV)-curable WPUDs has increased due to their rapid curing features and environmental safety [54, 55]. Chen et al. [48] synthesized the UV-curable WPUDs using polyester polyols which showed that water absorption of the membranes significantly reduced by the use of these polyols. Table 1 shows the effect on thermal and mechanical properties of WPUDs caused by the polyols used.
Diisocyanates and compounds used in isocyanate-free formulations
Wei and Co-workers [56] designed a brand-new synthetic route that originated from the specific reaction mechanism, for the synthesis of aromatic-diisocyanate-based waterborne polyurethanes which was tightly related to the chemical structure of the prepolymer. Neutralized ionized polyoxyethylated n-butylamines (NPEO) segments were located in the middle of the prepolymer which possessed hydrophilic properties. The urethane hard segments were present at the ends of the prepolymer, which usually imparts hydrophilicity. Films cast from the emulsions showed considerable mechanical properties. The films showed an increase in Young’s modulus with higher polytetrahydrofuran (PTMO) content. Similarly, Cornille and colleagues [57] reported new synthesis routes for isocyanate-free polyurethanes. Synthesis of non-isocyanate polyurethanes (NIPUs) by rearrangement of acyl azides (Curtius rearrangement), carboxamides (Hoffman rearrangement), or hydroxamic azides (Lossen rearrangement) was discussed which has isocyanates being produced in situ. These methods allowed the formation of polymers that could be obtained at room temperature and which possessed high tensile strength and higher chemical stability (Fig. 4).
In [58] catalytic synthesis of toluene-2,4-diisocyanate was discussed. A new phosgene-free route for catalytic synthesis of TDI had been researched in two steps. These routes showed higher thermal stability of the obtained product. Also, Brocas and Team [59] prepared poly(allyl glycidyl ether) and poly(allyl glycidyl ether co-epichlorohydrin) by monomer-activated anionic polymerization. Ethers with low dispersity, high molar mass and high thermal stability were obtained. Yilgor and co-workers [60] prepared novel segmented polyurethanes with hard segments based on a single diisocyanate molecule with no chain extenders. It was found that the kinetics of the hydrogen bonding between urethane hard heavily depended on the chemical structure and the symmetry of the diisocyanate. It was demonstrated that NIPUs based on crosslinks to prepare specific networks can partially fill in these gaps. This can pretty much lead to WPUs with higher mechanical and thermal properties. Likewise, Schmitz and coworkers [61] examined various physicochemical methods (zeta potential, adsorption, desorption) and did paint application tests with numerous binders and pigments. It was observed that zeta potential would reduce due to the presence of polyfunctional polymeric additives with a limited number of ionic groups, while the presence of non-ionic oleo-ABCs did not significantly influence the zeta potential. It was seen that electrostatic stabilization is the dominant and effective stabilization mechanism. It was seen that a hydrophobic additive (low HLB) showed strong adsorption. The short hydrophilic chain on the other hand can only provide limited steric stabilization. A mono-oleo-ABC with medium HLB gives good color strength also in dispersant mixtures even if the other components by themselves do not give good results concerning color strength [62,63,64]. We also saw that the properties, high gloss, excellent color strength and color stability are often linked to individual agents, while in solvent-borne systems often one additive fulfilled all requirements. Water-based coatings, therefore, need several different additives or sophisticated combinations of them.
In another work, Janvier and others [65] prepared a five-membered syringaresinol cyclic carbonate (SYR-CC) by carbon dioxide addition to syringaresinol bis-epoxy monomer (SYR-EPO) derived from syringaresinol. The FT-IR analysis of bisphenol A cyclic carbonate (BPA-CC) which was polymerized at various temperatures showed better conversion at a higher temperature. NIPU thermosets were also prepared by coupling SYR-CC with tris(2-aminoethyl)amine (TREN), which had thermal properties similar to BPA-based resins. Furthermore, Subramani and coworkers [66] prepared and characterized water-dispersible/reducible anionic methyl ethyl ketoxime (MEKO), dimethylolpropionic acid (DMP) and ε-caprolactam-blocked aromatic-diisocyanate toluene 2,4-diisocyanate (TDI) and 4,4-methylene biphenyl isocyanate (MDI) dispersion. It was seen that the molecular weight of MDI-based adducts was higher than TDI-based adducts. Also, all dispersions prepared were stable for more than 6 months in case of storage stability. All the dispersions were in liquid form and miscible (compatible) with WPUDs (polyether and polyester-based) and acrylic dispersions. The gelation test results showed that MDI-based dispersions de-block at the higher temperature and cure at a faster rate than TDI-based dispersions. This may be because the degree of crosslinking was related to the de-blocking reaction, which increased by increasing the de-blocking temperature.
Sheth et al. [67] researched the time-dependent morphology development in a segmented polyurethane. A segmented polyurethane with monodisperse hard segments based on the symmetric 1,4-phenylene diisocyanate was able to develop a microphase-separated morphology. Its hard phase, amounting to 14 wt.%, was hydrogen-bonded, crystalline and extensively percolated. Thus, the material exhibited a high plateau storage modulus, which is an important requirement for elastomers intended for structural applications.
So in general it was seen that presence of urethane hard segments at the ends of the prepolymer the films so the cast had high mechanical properties [56], whereas the synthesis of NIPUs with the in situ process, NIPUs based on crosslinks which partially fill the gaps in prepolymer [60] showed higher tensile strength and chemical stability [57] and NIPUs prepared by coupling SYR-CC with TREN showed high thermal properties [65]. Similarly, a phosgene-free route [58] and monomer-activated anionic polymers [59] gave high thermal stability (Fig. 5).
Furthermore, it was seen that MDI-based adducts had higher molecular weight than TDI-based adducts whose dispersions had more than 6 months of storage stability [66]. A segmented polyurethane with microphase-separated morphology exhibited a high plateau storage modulus [67].
Emulsifiers
Emulsifiers play an important role in influencing the properties of both polyurethane dispersions (PUDs) and the resulting films. The addition of emulsifiers helps solids to be dispersed in liquids or insoluble liquids with other liquids. Hence, the introduction of hydrophilic groups of ionic or non-ionic nature during the synthesis of polymer and eventually becoming a part of the main chain is more desirable. Polyurethanes are hydrophobic and require the use of emulsifiers to disperse them in water. The introduction of internal emulsifiers in the polymer is found to be more advantageous than using external emulsifiers. Environment-friendly characteristics of waterborne polyurethanes such as flexibility, good strength, abrasion resistance are achieved by the introduction of internal emulsifiers. Internal emulsifiers are reactive ionic groups including carboxylate, sulfonate or quaternary ammonium salt, or non-ionic groups like poly (ethylene oxide) that can be incorporated into the backbone chain [68]. Figure 6 shows the structures of various emulsifiers used in the synthesis of WPUDs.
It is seen that one of the main factors getting affected by the emulsifier content is the stability of the resulting PUDs (polyurethane dispersions). Increase in the content of emulsifiers like DMBA, DHSA, DHA, NMDEA and some biobased emulsifiers which are used as substitutes for DMPA leads to a decrease in the final particle size of the PUDs. Variations are observed in the properties such as Tg, water absorption, crosslinked density, tensile strength, toughness, elongation at break, Young’s modulus depending on the type of emulsifier used. For example, the use of NMDEA [69] emulsifier causes an increase in hydrophilicity, whereas the bio-based waterborne polyurethane dispersion (BPUD) prepared by using an emulsifier made from castor oil and 3-mercaptopropionic acid [70] showed good hydrophobic properties. It was seen that the chain of cWPU had the more hydrophilic block in the chain because of the EG (ethylene glycol) content effect. The more EG content, the more hydrophilic hard segment was seen in the class of isocyanate because of the high possibility of reaction between isocyanate-terminated chain and the active short-chain hydroxyl group of EG.
We can make comparisons between the properties of WPUDs obtained by using DHSA (dihydroxystearic acid), DMBA (dimethylol butanoic acid) with DMPA (dimethylol propionic acid). While the incorporation of DHSA had a significant effect on the particle size and viscosity of the palm oil-based WPUDs [71], DMBA exhibited a higher rate with NCO as compared to DMPA with NCO during the synthesis of castor oil-based WPUDs [72]. The reason being (i) high melting point and low solubility of DMPA, (ii) the extra methylene in the side chain of DMBA decreased the steric hindrance for the reactions between its OH and NCO groups in IPDI. When 25% of DHSA was introduced, the viscosity and the particle size of the WPUDs increased, whereas, when the DHSA content was taken up to 50%, the particle size and viscosity decreased. The films prepared by DHSA and DMBA have some contrasting properties like DHSA being a bigger molecule due to long carbon chain length, which gives flexibility and softness to the films. Whereas the film becomes stiffer as the DMBA emulsifier content increases [73] due to (i) the slightly higher hard segment content of the films from DMBA than those from DMPA, (ii) the large side chain of DMBA and high crosslinking reduced chain mobility making chains less easy to align to recrystallize. Another difference is the water absorption of the WPUD films. It increases in the case of DMBA but decreases when the amount of DHSA increases. DHSA lowered the reaction temperature and time at the initial stage of synthesis. DMBA had higher crosslinking densities, storage modulus and glass transition temperature (Tg) but lower thermal stability and contact angle of the films than those from DMPA.
Properties of the films obtained using vegetable oil-based emulsifiers can also be compared. Dihydroxy acid (DHA) was synthesized from sunflower oil (SFO) by an epoxidized reaction of SFO and a mixture of formic acid and hydrogen peroxide to prepare diol using the methanolysis process and the saponification of diol leading to dihydroxy acid [74]. Lingxiao et al. [75] successfully prepared a novel, fully biobased emulsifier from soybean oil and glutaric acid through a solvent-free and catalyst-free method which exhibited similar properties to those of DMPA and DMBA. The glass transition temperature (Tg) decreased in both cases with increasing emulsifier content due to dangling chains of DHA that act as plasticizers in the first case, while the appearance of the resulting PUDs became more transparent, but the crosslinking density decreased and the rigid carbon ring content resulted in the low Tgs of the PU films. Another similarity is the small particle size. The mobility of the chains of the ionic groups of DHA influenced the stability of dispersions that led to smaller nanoparticles, whereas in the SFO-based emulsifier, due to the higher surface charge density of the particles, they were not easily aggregated due to electronic repulsion, leading to small particle size [76] along with that the increases in emulsifier content led to higher phase stability [77], high thermal stability, high tensile strength, toughness and water contact angles but low elongation at break.
While anionic WPUDs were prepared using the above emulsifier, Nathapong et al. [69] used N-methyl diethanolamine (NMDEA) as an emulsifier in the synthesis of novel cationic waterborne polyurethane dispersions (cWPUDs). Cationic WPUs are usually composed of NMDEA [73, 78] where the particle size decreases, while the hydrophilicity increases with increasing NMDEA content owing to the decrease in its water contact angle. The effect of neutralized NMDEA content by acetic acid made the more positive charge in the system leading to the more hydrophilic property. The NMDEA content below 1.5 mol could not be stable and coagulated due to emulsifier insufficiency [79].
(DMPA) is an internal emulsifier that has been widely used as a hydrophilic chain extender in the synthesis of anionic WPUDs. But it also has some drawbacks like high melting point, low solubility leading to heavy use of solvents [77, 80]. Hence the development of liquefiable emulsifiers with compatible reactivity with polyols has attracted much attention. Wu and Chen [81] prepared liquefiable DMPA (LDMPA) by condensation reaction between DMPA and ε- caprolactone and successfully synthesized a series of PUDs. With the addition of LDMPA, the content of the polar group (–COOH) increased which resulted in better mechanical properties. Tatai et al. [82] synthesized a biodegradable emulsifier from ethylene glycol and lactic acid. The incorporation of this emulsifier did not have a considerable effect on mechanical or thermal properties, but the molecular weight was lower presumably due to its low reactivity. Fu et al. [70] prepared a new hydrophilic emulsifier from castor oil and 3-mercaptopropionic acid. Karak and colleagues [83] reported the synthesis of environment-friendly PUDs derived from a biobased chain extender prepared from citric acid and glycerol.
Tailoring the thermal and mechanical properties by chemical modifications in the ingredients of WPUDs
Various studies have been done in past for the enhancement of thermal and mechanical properties of WPUDs to increase their already wide scope of applications. Along with changes in raw materials like polyols, diisocyanates and dispersing agents, many studies have tried chemically modifying WPUDs by adding additional reagents along with the major monomers to bring about a chemical change in the polymer backbone, thus improving certain aspects of WPUDs. Compounds based on elements like phosphorous, nitrogen, silicon, sulfur and also bio-based compounds like chitosan [84,85,86,87,88] are used to chemically modify WPUDs as given in Table 2.
Silicon compounds
Among all these types, silicon-based compounds have been used widely in various studies to determine their effects on the thermal and mechanical properties of WPUDs.
Wei et al. [89] used polyurethane/silicone dispersions to synthesize a hydrophobic and weather-resistant coating. Polysiloxane diols (PESI) along with polytetrahydrofuran polyether diols (PTMG) were used as a soft segment, while methyltriethoxysilane (ETMS) was used as a termination agent and a chain extender. It was found that the thermal stability of the films increased with an increase in the PESI/PTMG ratio as the crosslinking reaction between PESI and ETMS causes the system to have certain crosslinkages which inevitably improve the weather resistance ability of the PUSD films. Also, the tensile strength of the films as the PESI/PTMG weight ratio increases. This is because the addition of organic siloxanes enhances the degree of crosslinking in the films which in turn improves the tensile strength. On similar grounds, Zhang et al. [90] used a novel approach to synthesize chemically modified WPUDs. Along with PTMG and IPDI as the main monomers, modified monomers like hydroxy-terminated polydimethoxy silane (PDMS) and dialcohol-terminated perfluoropolyether (E10-H) were used as modified monomers in the preparation of WPUDs. PDMS and E10-H were introduced in the system in various amounts. Some samples had only PDMS in varying amounts, some only had E10-H, while the rest of the samples consisted of both PDMS and E10-H in different amounts. The tensile strength for the sample with PDMS (~ 17.5 MPa) was greater than the tensile strength of the samples with E10-H (~ 15 MPa). The extent of strain hardening increased as the amount of PDMS and E10-H was increased, which was attributed to the increase in microphase separation due to the addition of silicon and fluorine. However, when PDMS and E10-H were simultaneously introduced into the polyurethane system, a synergistic effect was observed as the tensile strength of the films became ~ 27 MPa. It was found that microphase separation could increase the tensile strength of the films, but it decreased when an excess amount of PDMS or E10-H was added. The films were also analyzed for thermal properties by TGA. The decomposition rate reduced when the PDMS and E10-H were introduced into the main chains, indicating that the thermal stability of the WPU films had improved. Improvement in thermal and mechanical properties due to chemical modifications in various polyols is mentioned in Fig. 7.
Wu et al. [91] synthesized superhydrophobic films from siloxane-modified two-component waterborne polyurethane. The thermal properties of the nanocomposite films were assessed by DSC curves, and it was seen that the Tg of the films increased from 55 to 66 °C when nano-SiO2 content increased from 0 to 20%. The reason for the same was most nano-silica was wrapped in the PU network and acted as another point for crosslinking due to the formation of hydrogen bonds and chemical grafting between the residual hydroxyl groups of the nano-SiO2 and PU matrix. Zhang et al. [92] made waterborne UV-curable polyurethane nanocomposites using side chain triethoxysilane and colloidal silica via inverse emulsification process. The Young’s modulus and the tensile strength of the films significantly increased from 3.7 to 6.6 MPa and 6.7 to 11.6 MPa, increasing the silica content to 10 wt%, respectively. This was attributed to larger crosslinking density as HEA-APTES content increases making the films more rigid, thus improving their mechanical properties. Hui et al. [93] also synthesized novel silica-modified WPUD. Silica modified with N-(β-aminoethyl)-γ-aminopropyltriethoxysilane in water was used as a chain extender. The mechanical properties of the films were tested by tensile tests, and the results revealed that as compared to normal WPUD, the WPUDs modified with 2.0 wt % silica showed an improvement in tensile strength from 6.1 to 7.1 MPa. This was because the modified SiO2 particles and aqueous PU were graft polymerized and hence there existed a certain amount of crosslinking between them. The existence of covalent bonds between WPU and silica imposed more restraint on the chain movement, thereby increasing the tensile strength of the films with the addition of modified silica.
Modified castor oil-based PU-silica nanocomposite dispersions were prepared by Fu et al. [94]. MACO or carboxyl castor oil was synthesized from the reaction of castor oil and 3-mercaptopropionic acid (MPA), and MSCO or alkoxysilane castor oil was synthesized with the help of castor oil and 3-mercaptopropyl trimethoxysilane (MPTS). Both these compounds were prepared through a thiol-ene coupling reaction as shown in Fig. 8. The TGA curves and the data extracted proved that with an increase in the silicon content, the thermal properties of the PU-silica dispersions improved. The increase in thermal stability was justified based on the strong hydrogen bonding between castor oil-based PU and colloidal silica. Because of the increased crosslinking density, the Tg values of all SiWPU films were higher than the regular WPU films. Another kind of modified diol was synthesized and used by Gaddam et al. [45] The phosphorylated polyols were employed as ionic soft segments in the synthesis of DMPA-free WPUD by sol–gel process, and APTES was used as a chain extender. The Tg values of WPUDs with higher silicon groups were higher as it increased the siloxane crosslinking density, thereby increasing the thermal stability of the WPUD. PUD-P5 showed the highest Young’s modulus and increased tensile strength because of the enhanced crosslink density resulting from the higher OH functionality of the phosphol -P5 has used in the WPUD. Cheng et al. [95] synthesized a novel linseed oil-based WPUD by using (3-aminopropyl)trimethoxysilane (APTMS) as a crosslinking agent. The TGA results of the hybrid films showed that there was an increase in the thermal stability of the films with an increase in the amount of APTMS from 1 to 5 wt %. This was attributed to the Si–O–Si crosslinked network which was formed in the composite matrix. The Tg of the WPUDs also increased with an increase in APTMS content. The tensile strength of the films increased from 18.04 to 30.50 MPa with an increase in APTMS content; also, the elongation at break decreased significantly. Thus, the films had excellent mechanical properties (Table 3).
The silicon-based WPUDs have shown great promise in the area of thermal and mechanical property improvement. Silicon has been used in all types of raw materials right from polyols to chain extenders. It leads to greater thermal stability and tensile strength, mainly due to the crosslinking effect and the excess packing density. The highest tensile strength increase (18.04–30.5 MPa) was seen when the silicon-based crosslinking agent (APTMS) was used in WPUD synthesis [95]. Similarly, the greatest increase in the Tg of the WPUD was seen when nano-silica was incorporated in the synthesis, which subsequently restricted the degrees of freedom and hence the thermal stability of the film increased.
Phosphorous and nitrogen-based compounds
Improvement in chemical and physical properties of WPUDs based on phosphorous and nitrogen-based compounds has gained a lot of importance in recent times as phosphorous provides innate thermal stability to the films.
Wang et al. [85] synthesized a phosphorous and nitrogen-containing intumescent flame-retardant, pentaerythritol di-N-hydroxyethyl phosphamide (PDNP), using phosphorous oxychloride, ethanolamine and pentaerythritol as raw materials. This flame retardant was used as a chain extender to make waterborne polyurethane dispersions. The results obtained from TGA clearly show that the thermal stability of the PUDs has increased after the addition of PDNP. The char yields of all the samples show that conjugation of PDNP into waterborne polyurethane increased the amount of char residue. Char residue formation helps in blocking any kind of heat or flame from the polymeric material beneath it and hence increases the durability of the films in extreme heat conditions, thus enhancing the thermal stability of WPU films at the higher temperature. Another type of flame retardant, tri (N,N-bis-(2-hydroxy-ethyl) acyloxoethyl) phosphate (TNAP), was synthesized by Wang et al. [96] and incorporated into WPUDs as a chain extender. The TGA of the films showed that at 6000C, the residual char of TNAP was 34.98 wt %. Thus the high residues indicated that TNAP exhibited high thermal stability at high temperatures. This was due to the presence of phosphorous which provided innate durability toward heat. The combination of TNAP and PDNP increased the formation of the charring layer, thereby blocking the polymeric materials from heat and flame. Also, with the addition of TNAP, the tensile strength of the films increased from 9.89 to 25.70 MPa which was attributed to the branched structure of TNAP. This branched structure resulted in a highly crosslinked structure in the linear polyurethane, thereby increasing the crosslink density in the PU prepolymer and hence improving the tensile strength of the films. On similar grounds, Zhang et al. [97] synthesized a novel diol chain extender bearing a cyclic phosphoramidate pendant group, namely 2-(5,5-dimethyl-2-oxo-2–1,3,2-dioxaphosphinan-2-ylamino)-2-methyl-propane-1,3-diol (PNMPD). The thermal stability of the PNWPU films was evaluated by TGA. The degradation of phosphoester bond can promote the formation of polyphosphoric acid or its derivatives, which catalyze the decomposition of polyurethane to produce additional thermally stable phosphorous-rich carbonaceous layers at elevated temperature. The char yield of the films increased from 0.71 to 5.44 wt%, indicating good thermal stability at high temperatures. Though the thermal properties of the films were good, the mechanical properties of the films like tensile strength deteriorated systemically with an increasing amount of PNMPD. When PNMPD is incorporated into PNWPU, the lateral chains in the former compound cause a steric hindrance which disrupts the crystallization of the hard segments. Also, the organophosphate flame retardant has an internal plasticization effect on the PU matrix, which leads to soft resins and thereby the decrease of tensile strength.
Wu et al. [98] synthesized a series of flame-retardant polyurethanes with diethylbis(2-hydroxyethyl)aminomethylphosphonate (fyrol-6) as a chain extender and 1,1,1-tris(hydroxymethyl)propane (TMP) as a chain extender and crosslinking agent. The thermal analysis of the films showed an increase in the residual carbon from 8.9 to 19.8%. The amount of residual carbon indicated the flame resistance of the material and hence with an increase in fyrol-6 content the flame resistance of the films and also their thermal stability increased. The crosslinking modification due to TMP has good effects on the thermal stability of the films but not much. The tensile strength and impact resistance of the fyrol-6-induced WPUDs decreased due to the long molecular chain of the flame retardant which provided flexibility to the films. As opposed to this, when the films were incorporated with TMP as a crosslinking agent, the tensile strength and the impact resistance of the films increased by a high margin of 103.5% and 33.2%, respectively. This was attributed to the high crosslinking density of the films. Thus, the films showed good mechanical properties. Yin et al. [99] used Fyrol-6 as a hard segment modifier along with halogen-free polyphosphate (OP550) as a soft segment modifier for the synthesis of WPUDs. As Fyrol-6 was used as a hard segment modifier, it reinforced the hardness of the films, and the mechanical properties of the film like tensile strength, impact resistance were high. But when the amount of OP550 was increased, the tensile strength deteriorated as the number of soft segments increased providing flexibility to the film. Thus, the optimum amount of Fyrol-6 and OP550 needs to be added to get the best possible mechanical properties.
Phosphorous and nitrogen are usually added in a WPUD for flame retardancy but with it comes to the inherent thermal stability provided by phosphorous. From the above-mentioned references, it can be seen that using the phosphorous-based material as a chain extender in the WPUD leads to improved thermal and mechanical properties. This is because of the increase in crosslinking density brought about by the hard segment (phosphorous-based material). The highest increase in tensile strength (9.89–25.70 MPa) was observed when TNAP and PDNP were both used in the WPUD synthesis [96].
Sulfur compounds
Wan et al. [100] synthesized a self-healing waterborne polyurethane with the help of disulfide bonds. 2-Hydroxyethyl disulfide (HEDS) was used as the chain extender. The TGA of the films showed that the temperature at the maximum mass loss rate point (Tmax) increased with an increase in HEDS content which indicated that the overall thermal stability of the samples increased. The exchange reaction between disulfide bonds promoted the crosslinking effect of different polyurethane chains, leading to an increase in the overall thermal stability of the samples. The Tg value of the films increased with an increase in HEDS content but further decreased when the HEDS content was too high. Yin et al. [101] synthesized a hydrophilic curing agent from 2-((2-aminoethyl)amino)ethanesulfonic acid (AAS salt) and incorporated it into WPUDs. The tensile strength of the WPUDs with AAS salt in them (MAWPUs) was 1.5 times that of the WPUDs without the salt (MWPUs). The reason was the presence of –SO3− and –CH2CH2SO3 in the MAWPU macromolecule chain, which increased the relative molecular mass of the hard segment unit of the PU. The larger the number of small steric hindrance groups, the better the tensile strength.
Polyester-based WPUDs modified with hydroxypropyl-terminated polydimethoxysilane (HPDMS) and sulfonate polyester diol (SPD) were synthesized by Dai et al. [102] When only HPDMS was present in the WPUDs, the water resistance of the films improved due to the migration of HPDMS segment to the surface, but the mechanical properties of the films deteriorated; with addition of ionic groups, like sulfonate ion, in the soft segments (SPD) improvement in the mechanical properties was observed. As the amount of HPDMS decreased from 3 to 0.5%, the tensile strength increased from 58.4 to 62.6 MPa owing to the sulfonate group of SPD and hydrogen-bonding interaction between the carbonyl groups. Wu et al. [103] used aliphatic diamine sulfonate (AAS-Na) as a chain extender for the synthesis of WPUDs. The thermal properties of the sample did not improve, but the mechanical properties were enhanced greatly. The tensile strength of the films increased from 19.0 to 36.0 MPa as the –COOH/–SO3Na molar ratio varied from 10:0 to 3:7. With an increasing amount of –SO3Na, the micro-phase separation of the material increased and hence the mechanical properties of the films improved.
Sulfur is mainly added in WPUDs as it gives self-healing properties to the film. Added in various forms like sulfonate ions, sulfide linkages, sodium salt of sulfur compounds, it provides the WPUD with good thermal stability and tensile strength. The highest increase in tensile strength (19–36 MPa) was seen when aliphatic diamine sulfonate (AAS-Na) was added as a chain extender [103]. This was since the more the amount of –SO3Na present in the film, the more was the micro-phase separation and hence the mechanical properties improved.
Chitosan-based compounds
Chitosan is a linear polysaccharide composed of randomly distributed de-acetylated and acetylated D-glucosamine unities and has been widely used in biomaterials as well as nanoparticles designed for drug release [104,105,106].
Javaid et al. [88] synthesized chitosan (CS) and montmorillonite (MMT) clay for making modified PU bio-nano-composites. CS was used as a chain extender along with 1, 4-butanediol (1, 4-BDO) in the series of different WPUDs. In some WPUDs, only 1, 4-BDO was; some had a combination of CS and 1, 4-BDO in various molar ratios, and some WPUDs only had CS as a chain extender. It was observed that chitosan formed of D-glucosamine monomers and the linked glucosamine rings on chitosan had better solubility in the hard segments of PUs which resulted in the formation of intermolecular forces between –NH groups in the hard segments and soft segments. Chitosan itself is thermally stable up to 320 °C, and hence, all the films containing CS started to degrade only above this temperature indicating the thermal stability of the films at high temperatures. Similarly, Ren et al. [107] used a novel phenolic acid-modified chitosan for the preparation of WPUDs. Oligochitosan was grafted with protocatechuic acid (PA-g-OCS) and gallic acid (GA-g-OCS) and was used as a crosslinking agent in the synthesis of WPUDs. The TGA of the PA-g-OCS and GA-g-OCS showed that apart from the general two-stage decomposition of WPUD films, there was an additional degradation stage around 240–270 °C which corresponded specifically to the phenolic acid-grafted oligochitosan. This indicated an improvement in the thermal stability of the WPUD films. The high thermal stability of phenolic acid-grafted oligochitosan was imparted by aromatic moieties, ester linkages, heterocyclic ring system, which is beneficial to the thermal properties of the films. The mechanical properties of the films too had increased to a great extent with the incorporation of PA-g-OCS and GA-g-OCS. The tensile strength of the PA-g-OCS added WPUD films was found to be 23.2 MPa and that of GA-g-OCS-added films was 29.4 MPa as compared to the 13 MPa tensile strength of unmodified WPUD films. The enhanced mechanical properties of the two modified WPU films were attributed to the introduction of phenolic acid-grafted oligochitosan copolymer into the waterborne polyurethane, leading to the conversion of linear chains into a three-dimensional network. Besides, the phenolic acid-grafted oligochitosan had a large amount of –OH and –NH2, which can react with –NCO to form urethane and urea groups, constituting the hard segment of PU film. This hard segment contributes to the rigidity of the films which in turn improves the overall mechanical properties. Improvement in thermal and mechanical properties by chemical modifications in various chain extenders is given in Fig. 9.
Though not used traditionally for property improvement in WPUDs, studies have shown that the use of organic materials like chitosan helps in improving the thermal and mechanical properties of the WPUD films. Chitosan being inherently stable up to 320 °C brings about phenomenal improvement in the thermal stability of the WPUD films. Also, due to the H-bonding between chitosan and the hard segment of WPUD, the mechanical properties of the films were seen to improve.
Tailoring the thermal and mechanical properties by introducing fillers
Zhou and Co [108] synthesized three aqueous WPUDs with different molar ratios of isocyanate groups to oligomer hydroxyl groups and tested for change in viscosity and particle size with variation in aging time. The viscosity, mean particle size and specific surface area values of the polyurethane dispersion after 30 days of aging were calculated. It was observed that particle size of the sample with high DMPA (Sample B1) and one with high PPG (Sample B5) content decreased and that with high IPDI (Sample B2) content increased drastically and it remained constant even after increasing the content of 1,4-BDO (Sample B3) and TEA (Sample B4). The mean particle size of all samples increased in the following order: B2 > B1 > B5 > B3 > B4. Also the solid content of all samples increased in the following order: (B5 > B2 > B3 > B1 > B4). The solid content order of all samples was almost the same as that of the mean particle size (B5 > B1 > B2 > B3 > B4). The initial viscosity of all samples increased in the following order: B1 > B4 > B5 > B3 > B2 Looking at the orders we can say that the order of the initial viscosity had no similarity with the order of solid content and mean particle size. Therefore we consider that the initial viscosity of the polyurethane dispersion has no influence on the solid content and mean particle size. The excess ionic groups in the polyurethane dispersion contributed to the improvement of phase mixing between the hard and soft segments, leading to a limitation to the movement of the polymer molecular chain, [109] which in turn suppressed the aggregation of nanoparticles to a different magnitude. During storage, the intermolecular force and hydrogen bond suppress the flexible inter-tangling chain to generate smaller particles. This kinetic process ends up when a kinetic equilibrium is achieved after several days of aging. Due to this inter-/internal-particle force and the aggregation of particles of different size magnitude, the particle size distribution is narrower after aging time. We can say that the initial viscosity of the polyurethane dispersion depended largely on the particle size distribution but not on the magnitude of particle size and the higher the hydrophilicity of the prepolymer, the smaller the mean particle size. Hence, films with better appearance and mechanical properties could be obtained. Christopher & others [110] studied for the changes in contact angle, surface morphology and dispersion stability of waterborne polyurethane reinforced with a synthetic polymer of polyvinyl alcohol (PVA) modified with GO/zinc oxide (GO/ZnO) and functionalized carbon black/ZnO (CB/ZnO). FT-IR results suggested that the incorporation of PVA and carbon materials supported ZnO which formed a more phase mixed structure in WPU dispersion. Though there was no significant increase in the surface wettability of the films due to nanofiller content, the final output concluded WPU/PGZ (GO) with 0.3% dosage gave better dispersion, minimum anti-settling nature and better corrosion resistance than WPU/PCZ (CB). Since both the carbon-based materials enhanced the corrosion protection, GO was much better than CB. Chen & colleagues [111] coated the GO nano-sheet with poly(dopamine) on its interface and then facilely incorporated it into the W-PU matrix by water solution blending. Graphene oxide with dopamine then incorporated into waterborne polyurethane via water solution blending. It was found that Young’s modulus and elongation at break of the W-PU nano-composites are readily improved by loading of D-GO (dopamine-coated filler), compared with that of neat W-PU or PU with the same loading of GO. The DGO also acted as an excellent free radical scavenger that greatly slowed down UV-induced and thermo-oxidative degradation of W-PU owing to its phenol group in coating materials, showing much less drop of tensile mechanical properties for PU/D-GO composites after UV irradiation.
Zhou & co-workers [112] prepared a novel waterborne polyurethane dispersion with a unique nanoparticle appearance through the system-water generating process. Both the samples are amorphous from the XRD results, while the field emission scanning electron microscope (FE-SEM) indicated that the novel polyurethane exhibited some cylindrical self-assembled aggregates. This led to better mechanical properties. According to the results of DSC and DMA, the number of amorphous domains in polyurethane is decided by the interaction of hard and soft segments. A larger number of hard segments had solubilized in the soft segments in PUD2 comparing with PUD1, resulting in higher thermal stability and more sufficient hard and soft segment interaction in PUD1, corresponding to the results of TGA. Similarly, Fuensanta & Co [113] prepared several waterborne polyurethane urea dispersions (WPUUs) by mixing different amounts of two waterborne polyurethane urea dispersions made with polyester (WPUU-Polyester) and polycarbonate diol (WPUU-PCD). It was found that PUU-Polyester is more thermally stable than PUU-PCD, and the increase of the WPUU-PCD content in WPUUs mixture gave less thermally stable PUUs as compared to PUU-Polyester. The addition of 25 wt% of WPUU-Polyester imparted crystallinity to the polyurethane urea due to the interactions between the carbonate groups in the soft segments. The differences in the degree of phase separation and crystallinity of PUU films made from WPUU-Polyester + WPUU-PCD mixtures were evidenced by the increase in the glass transition temperature associated with the alpha relaxation of the soft segments and the higher modulus at the crossover between the storage and loss moduli. Kim & friends [114] prepared an easily peelable coating using silane-terminated polyurethane dispersions (SPUDs) and UiO-66 catalyst (zirconium(IV)-based metal–organic framework), to capture and decompose the nerve agent simulant, methyl paraoxon (MPO), at room temperature. SPUDs were used as a binder. The peel strength test showed that SPUDs have great adhesion to many surfaces including PVC, glass and steel substrates. In the case of tensile properties, as the catalyst content increased, Young’s modulus increased, but the elongation decreased. (Fig. 10).
Clay fillers
Liao & Co. [115] incorporated three silylated nanoclay which was platelet-like, rod-like, and tubular into jatropha curcas oil base WPU matrix via in situ polymerization. Platelet-like montmorillonite (MMT), rod-like attapulgite (AT), tubular halloysite nanotubes (HT), have been used to modify polymers. FTIR results indicated that the interaction between clay particles and polymer matrix was very strong. The thermal stability of nanocomposites increased due to the formation of an intercalated structure in WPU/SMT. The interactions between clays and polymer matrix increased the microphase separation degree of WPU/clay nanocomposites, and WPU/SHT had the highest microphase separation degree, which could be assigned to the fact that the silylated halloysite nanotubes formed strong interplays with hard segments but weak interactions with soft segments of WPU. The tensile strength and elongation at breakage of WPU/SAT and WPU/SHT also increased. Surface properties like surface roughness and hydrophobicity were improved due to the introduction of nanoclays, and the enhancement varied with the types of nanoclays. Stefanovic and Friends [116] synthesized a series of the polyurethane nanocomposites based on 4,4′-methylenediphenyldiisocyanate and 1,4-butanediol as the comonomers of the HS and poly(propylene oxide)-b-poly(dimethylsiloxane)-b-poly(propylene oxide) as the part of the SS and OMt (Cloisite 30B®, 1 wt%). OMt was oriented toward both, hard and soft segments. XRD and TEM analysis showed a homogeneous dispersion and existence of mixed exfoliated/intercalated morphology of the OMt within the PU matrix. AFM analysis confirmed that PUNC had a microphase separation structure that was more evident in nanocomposites with higher HS content. The addition of OMt in the amount of only 1 wt% led to the improvement of the thermal stability of the PUNC. Storage modulus, Young's modulus and tensile strength also improved after the reinforcement of the PU matrix with OMt nanoparticles. Ni & Co-Workers [117] successfully prepared high-performance WPU-based composite films by using fibrous palygorskite (PAL) and rhombohedral dolomite (DOL) together. It was observed that with an addition of 4 wt% PAL and 6 wt% DOL, the tensile strength of this ternary composite film increased by 178% in comparison with neat WPU. Using 10 wt% of PAL or DOL only, the tensile strength of both WPU/PAL and WPU/DOL composite films even slightly reduced by 17% and 14.3%, respectively. This happens due to the formation of a 3D-conjugated filler network with 1D PAL inserted into the 3D rhombohedral DOL crystal network. Such networks also improve the thermal stability of the composites. The tensile strength, as well as the thermal stability, decreases with a too high amount of MIX filler incorporation due to its aggregation.
Silica fillers
Cakić & others [118] prepared a series of waterborne PU–SiO2 hybrid dispersions from castor oil-based monomers and characterized them by different techniques. The phase separation of the polyurethane–silica hybrid dispersions was influenced by nanoparticle content. The thermal stability of hybrid film increased with increasing SiO2 content because of the increased formation of the hydrogen bond between the hydroxyl groups of SiO2 and polymer backbone. The hardness of obtained hybrid films is related to the crosslinking density which depended on the amount of nanofillers content and phase separation, resulting in the increase in rigidity/stiffness of the main chain of the CPU. SEM examinations of hybrid films showed that the nano-silica was well dispersed in the polyurethane hybrid films. The values for the adhesion and gloss film confirmed their excellent properties for potential application from adhesives to elastomeric or hard coatings in civil engineering, metal or polymer industry. Ding and Co [119] successfully prepared waterborne polyurethane-silica (WPUS) composites by a click chemistry method. Particle size analysis, SEM and TEM indicated that all the WPUS dispersions occurred when the content of SiO2 was below 2.5%. After a small amount of SiO2 was added to WPU, the mechanical properties of WPUS greatly improved. The water resistance of the WPUS nanocomposites was increased as the SiO2 increased due to the formation of a crosslinked network structure. The fabricated WPUS nanocomposite also demonstrated outstanding thermal stability by TGA. Han & Friends [120] obtained waterborne polyurethane (WPU) with high solid content (45%) by utilizing dimethylol propionic acid (DMPA) and ethoxylated capped polymeric diol as complex hydrophilic groups. After the addition of alkyl-grafted silica, the average particle size of waterborne polyurethane (WPU) dispersions enlarged, while the particle size distribution broadened. The emulsion viscosity increased with the increasing content of alkyl-grafted silica. The combination of thermal insulation ability and the tortuous path effect of silica particles led to better thermal stability. Compared to neat WPU, WPU/silica coatings showed higher storage modulus, Young’s modulus and tensile strength without much loss of ductility. Hassanajili & Others [121] explored how the addition and surface modification of nano-silica filler affected the structure and properties of polyesterurethane. Three different types of nano-silica, including unmodified silica and two different organically modified silicas utilizing octylsilane and polydimethylsiloxane, were used. The results of TGA showed that nanocomposites containing treated nanoparticles have higher thermal stability. In general, there were two reasons which led to improvements in nanocomposites properties: better dispersion of nanoparticles in the matrix and higher phase separation of TPU. The dispersion of nano-silica fillers in polyurethane matrix was analyzed using scanning electron microscopy (SEM); a uniform distribution is observed in all samples, except PU/Si-Un composite on which a large aggregation of silica nanoparticles was observed. It is known that fumed silica is inherently hydrophilic owing to its oxide nature as well as the presence of Si–OH groups on its surface. The observed effect can be ascribed to changes of surface free energy of fillers and, thus, its improved compatibility with soft segments. It was noted that silica nanoparticles modified with octylsilane (Si-OS) have a more prominent effect on disrupting the HDs than nano-silica modified with polydimethylsiloxane (Si-PDMS). Si-OS nanoparticles showed a better dispersion in TPU in comparison with Si-PDMS. Cakić & Co-workers [122] prepared and characterized a series of waterborne PU–SiO2 hybrid dispersions from castor oil-based monomers by different techniques. The castor oil-based monomers have been synthesized from depolymerized oligoester obtained from glycolysis of PET waste. The phase separation of the polyurethane–silica hybrid dispersions is influenced by nanoparticle content. The TGA results showed that the thermal stability of hybrid film increases with increasing of SiO2 content because of the increased formation of the hydrogen bond between the hydroxyl groups of SiO2 and polymer backbone. The hardness of obtained hybrid films is related to the crosslinking density which depends on the amount of nanofiller content and phase separation, resulting in the increase in rigidity/stiffness of the main chain of the CPU.
Boron based
Gao & Co [123] successfully modified WPU to improve its water resistance, mechanical and anti-corrosion properties, by Gr, GO and h-BN 2 D nanosheets via the in situ polymerization method. Results indicated that with the loading amount of 0.25 wt%, h-BN nanosheets achieved homogenous dispersion in PPG and WPU matrix. The water resistance, tensile strength and anti-corrosion properties of WPU also greatly improved by the incorporation of these 2D nanosheets, especially h-BN. In the presence of 0.25 wt% h-BN, the WAR of WPU decreased from 37.3 to 15.7%, and the tensile strength improved from 4.16 to 19.56 MPa. Furthermore, the incorporation of Gr, GO and h-BN nanosheets did not change the internal structure of WPU. Yin & Colleagues [124] used BP and BN nanosheets as a flame retardant for WPU. The SEM and mapping results indicated that the BP and BN nanosheets were distributed uniformly in the matrix WPU. The flame-retardant tests demonstrated that the PHRR of WPU decreased by 50.94% and the THR decreases by 23.92% at a BP/BN content of only 0.4 wt%. The LOI of the BP/BN/WPU composite increased from 21.7 to 33.8%, compared with pure WPU. The residue of BP/BN/WPU after the CC test was denser and approximately 10 times more than the residue of pure WPU, according to TG, SEM, XPS, TG-IR analysis of WPU during the combustion process.
Titanium dioxides
Da Silva & Others [125] synthesized nanocomposites by in situ polymerization. The results showed, in general, thermal properties (degradation temperature) and mechanical properties higher than the pure polymer. Despite showing a degradation temperature lower than the materials with higher amounts of TiO2, the additions of 2.0, 3.0 and 5.0% showed to be the most efficient in tensile tests. The increase in the Tg value indicated that there was a decrease in the mobility of polymer chains due to the action of the filler. This reduction can also be seen by the results of the creep-recovery test, which showed that the percentage of recovery after deformation is related to the major amount of the filler. There was, also, a slight increase in storage modulus in the rubbery region (above the Tg) as compared to the vitreous region (below the Tg). The stress–strain tests showed that the material with 2% of TiO2 improved in its Young’s modulus when compared to the pure polymer, indicating an increase in the mechanical strength. The SEM analysis showed that even with some agglomeration points.
Fullerene filler
Kausar [126] fabricated novel waterborne polyurethane adhesive and coating on nylon 12/fullerene composite film substrate to form mechanically and thermally stable as well as non-flammable materials. The dip-coating technique was employed as a proficient and cost-effective procedure to coat WPU on polyamide substrates. Fullerene-C60 nanofiller enhanced the adhesion of polyamide to polyurethane due to non-covalent interaction. Consequently, the composites depicted unique morphology due to fine compatibility between WPU, nylon 12 and fullerene C-60. The WPU coating as well as filler loading enhanced the tensile strength, Young’s modulus and toughness of the resulting composite. The thermal properties of WPU-layered Ny12/Full-C60 composite film were also found to increase with fullerene loading and dip coating. A remarkable effect of WPU coating was observed in the case of flammability tests. The WPU coating acted as a shield for polyamide material toward the flame. Fullerene nanoparticle loading also seemed to enhance the non-flammability of nylon 12.
Talc fillers
Dias & Friends [127] prepared ternary nanocomposites by in situ polymerization utilizing SSMMPs and SPR clay as fillers, proving that it is possible to blend these fillers. Structural analyses demonstrated that the fillers were well dispersed/exfoliated into the polymeric matrix leading to nanocomposites PU/ SSMMP/SPR with superior thermal and mechanical properties. Using blended fillers into a polyurethane matrix resulted in materials that could perform functions that required high thermal and mechanical performance. Dias & Co [128] synthesized new waterborne polyurethane nanocomposites using synthetic talc as a catalyst and as filler by in situ polymerization. FTIR confirmed that it is possible to produce WPU/synthetic talc nanocomposites without adding the commercial catalyst. Also, FTIR indicated hydrogen bonds between filler and polymer chains. The thermal stability of WPU nanocomposites increased when compared to pristine WPU. DSC indicated that the glass transition temperature of nanocomposites was affected by the addition of synthetic talc, probably due to good filler dispersion. Mechanical properties improvement can be associated with hydrogen bonding between filler and polymer and good filler dispersion. Prado & colleagues [129] prepared WPU/Si4(MgxNi1–x)3O10(OH)2 nanocomposites by physical mixing. Synthetic talc presented different chemical composition concerning Mg and Ni content occurring in the octahedral sheets of synthetic talc structure. FTIR, DRX and TEM analyses demonstrated good synthetic talc/WPU matrix interaction. FESEM and AFM showed a homogeneous distribution of talc particles for all samples. Improvements in nanocomposite’s mechanical properties about the pristine WPU matrix were observed for samples with higher synthetic talc content. The variation of the amount of Mg and Ni in synthetic talc composition altered the storage and loss module results being higher for nanocomposites obtained with synthetic talc with higher Ni content in their compositions. The Tg varied according to synthetic talc content. Santos & Others [130] presented a new way to obtain magnetic nanocomposites, using water-based environmentally and friendly materials. The hydrophilicity of Fe3O4-synthetic talc nanofillers resulted in a good filler dispersion into the polyurethane matrix even at high filler content as supported by XRD and TEM analyses. NMR indicates the interaction of filler OH groups with the matrix. For all nanocomposites, one can see a typical ferromagnetic behavior below Curie temperature (about 120 K) and a superparamagnetic behavior above this temperature. The use of Fe3O4-synthetic talc for obtaining magnetic nanocomposites resulted in improved materials with superior mechanical properties.
We saw that various fillers affect the thermal and mechanical properties of WPUDs. The presence of GO/zinc oxide in the dispersion did not increase the surface wettability of the films per se, but it did increase the corrosion resistance [110] and increase in break point during Young’s modulus test [112]. Silica fillers generally have high crosslinking density [118], outstanding thermal stability [119,120,121] when the concentration of the fillers is increased. This was because of better dispersion of nanoparticles in the matrix and higher phase separation of TPU. Boron-based fillers generally increased the water resistance, mechanical and anti-corrosion properties [123], and there was no change in the structure of the structure matrix by their addition [124] Addition of titanium dioxides [125], fullerene [126] and talc [127,128,129] fillers generally increased the mechanical properties like tensile strength by manifolds.
Conclusion
This review gives an overview of the effect of raw materials, chemical and physical modifications on the enhancement of thermal and mechanical properties of WPUDs. The particle size of the dispersions and the thermophysical and mechanical properties of the resulting films depend strongly on the polyol functionality and the hard segment content. Bio-derived polyols (mainly vegetable oils) and their structural modifications are capable of the production of dispersion coatings having excellent adhesion, scratch resistance, gloss, pencil hardness, flexibility and impact resistance to the final substrate. This paper described the use of elements like phosphorous, sulfur, silicon, nitrogen and bio-based compounds like chitosan to chemically modify WPUDs. In the physical modification, a simple blending of different polymers, forming interpenetrating networks, the addition of either a foreign moiety or changing the process design, in general, are some of the methods used to improve the properties of the dispersion. These improvements have broadened the potential and range of applications for WPUDs.
Abbreviations
- 1,4-BDO:
-
1, 4-Butanediol
- AAS Salt:
-
2-((2-Aminoethyl)amino)ethanesulfonic acid
- AAS-Na:
-
Aliphatic diamine sulfonate
- APTES:
-
3-Aminopropyltriethoxysilane
- APTMS:
-
(3-Aminopropyl)trimethoxysilane
- AT:
-
Attapulgite
- BN:
-
Boron nitride
- BP:
-
Black phosphorene
- CASE:
-
Coatings, adhesives, sealants and elastomers
- CB:
-
Carbon black
- CS:
-
Chitosan
- DABCO:
-
1, 4-Diazabicyclo[2.2.2]octane
- DBTDL:
-
Di-butyl tin dilaurate
- D-GO:
-
Dopamine-coated filler
- DMA:
-
Dynamic mechanical analysis
- DMCHA:
-
Dimethyl cyclohexylamine
- DMEA:
-
Dimethylethanolamine
- DMPA:
-
Dimethylolpropionic acid
- DOL:
-
Dolomite
- DSC:
-
Differential scanning calorimetry
- E10-H:
-
Dialcohol-terminated perfluoropolyether
- EDA:
-
Ethylenediamine
- ETMS:
-
Methyltriethoxysilane
- FE-SEM:
-
Field emission scanning electron microscope
- Fyrol-6:
-
Diethylbis(2-hydroxyethyl)aminomethylphosphonate
- GA-g-OCS:
-
Gallic acid-grafted OCS
- GO:
-
Graphene oxide
- Gr:
-
Graphene
- HEA:
-
2-Hydroxyethyl acrylate
- HEDS:
-
2-Hydroxyethyl disulfide
- HPDMS:
-
Hydroxypropyl-terminated polydimethoxysilane
- HS:
-
Hard segments
- HT:
-
Halloysite nanotubes
- IPDI:
-
Isophorone diisocyanate
- MACO:
-
Carboxyl castor oil
- MAWPU:
-
Two-component WPU with M12A4 as crosslinking agent
- MMT:
-
Montmorillonite
- MPA:
-
3-Mercaptopropionic acid
- MPTS:
-
3-Mercaptopropyl trimethoxysilane
- MSCO:
-
Alkoxysilane castor oil
- MWPU:
-
Two-component WPU with MHP15 as crosslinking agent
- NMR:
-
Nuclear magnetic resonance
- OCS:
-
Oligochitosan
- OMt:
-
Organically modified montmorillonite
- OP550:
-
Halogen-free polyphosphate
- PA-g-OCS:
-
Protocatechuic acid-grafted OCS
- PAL:
-
Palygorskite
- PCZ:
-
PVA-anchored functionalized CB-modified ZnO
- PDMS:
-
Polydimethoxysilane
- PDNP:
-
Di-N-hydroxyethyl phosphamide
- PESI:
-
Polysiloxane diols
- PGZ:
-
PVA-anchored GO-modified ZnO
- PNMPD:
-
2-(5,5-Dimethyl-2-oxo-2–1,3,2-dioxaphosphinan-2-ylamino)-2-methyl-propane-1,3-diol
- PNWPU:
-
Phosphorus–nitrogen-containing waterborne polyurethane
- PPG:
-
Polypropylene glycol
- PTMG:
-
Polytetrahydrofuran polyether diols
- PU:
-
Polyurethane
- PUD:
-
Polyurethane dispersion
- PUNC:
-
Polyurethane nanocomposites
- PUSD:
-
Polyurethane–silicone dispersion
- PVA:
-
Polyvinyl alcohol
- RIM:
-
Reaction injection molding
- SiWPU:
-
Silicon-based waterborne polyurethane
- SPD:
-
Sulfonate polyester diol
- SPR:
-
Organically modified commercial clay
- SPUD:
-
Solvent-based polyurethane dispersions
- SPUDs:
-
Silane-terminated polyurethane dispersions
- SS:
-
Soft segments
- SSMMP:
-
Silico-metallic mineral particles
- TEA:
-
Triethanolamine
- TEDA:
-
Triethylenediamine
- TEM:
-
Transmission electron microscopy
- TGA:
-
Thermogravimetric analysis
- THF:
-
Tetrahydrofuran
- TMP:
-
1,1,1-Tris(hydroxymethyl)propane
- TNAP:
-
Tri (N,N-bis-(2-hydroxy-ethyl) acyloxoethyl) phosphate
- TPU:
-
Thermoplastic polyurethane
- VOC:
-
Volatile organic compounds
- WPU:
-
Waterborne polyurethane
- WPUD:
-
Waterborne polyurethane dispersion
- WPU/SHT, WPU/SAT:
-
WPU/clay nanocomposites
- WPUU-PCD:
-
Waterborne polyurethane urea dispersions made with polycarbonate diol
References
Bayer O (1947) Polyurethane paper von bayer. Angew Chemie 59:257–288. https://doi.org/10.1002/ange.19470590901
Ding H, Xia C, Wang J (2016) Inherently flame-retardant flexible bio-based polyurethane sealant with phosphorus and nitrogen-containing polyurethane prepolymer. J Mater Sci 51:5008–5018. https://doi.org/10.1007/s10853-016-9805-y
Yang WJ, Lee GY, Park SH (2019) Analysis on chemical and physical behaviors of polyurethane foam for prediction of deformation of refrigerator panels. Int J Precis Eng Manuf 20:2041–2049. https://doi.org/10.1007/s12541-019-00159-0
Scarfato P, Di Maio L, Incarnato L (2017) Structure and physical-mechanical properties related to comfort of flexible polyurethane foams for mattress and effects of artificial weathering. Compos Part B Eng 109:45–52. https://doi.org/10.1016/j.compositesb.2016.10.041
Gnanasundaram S, Kannan S, Ranganathan M (2015) Preparation and characterization of footwear soling materials based on biodegradable polyurethane. Polym Plast Technol Eng 54:1585–1595. https://doi.org/10.1080/03602559.2015.1036443
Zain NM, Roslin EN, Ahmad S (2016) Preliminary study on bio-based polyurethane adhesive/aluminum laminated composites for automotive applications. Int J Adhes Adhes 71:1–9. https://doi.org/10.1016/j.ijadhadh.2016.08.001
Fridrihsone-Girone A, Stirna U, Misane M (2016) Spray-applied 100% volatile organic compounds free two component polyurethane coatings based on rapeseed oil polyols. Prog Org Coatings 94:90–97. https://doi.org/10.1016/j.porgcoat.2015.11.022
Turkenburg DH, van Bracht H, Funke B (2017) Polyurethane adhesives containing Diels–Alder-based thermoreversible bonds. J Appl Polym Sci 134:1–11. https://doi.org/10.1002/app.44972
Bernardini J, Cinelli P, Anguillesi I (2015) Flexible polyurethane foams green production employing lignin or oxypropylated lignin. Eur Polym J 64:147–156. https://doi.org/10.1016/j.eurpolymj.2014.11.039
Zieleniewska M, Leszczyński MK, Kurańska M (2015) Preparation and characterisation of rigid polyurethane foams using a rapeseed oil-based polyol. Ind Crops Prod 74:887–897. https://doi.org/10.1016/j.indcrop.2015.05.081
Ding H, Wang J, Wang C, Chu F (2016) Synthesis of a novel phosphorus and nitrogen-containing bio-based polyols and its application in flame retardant polyurethane sealant. Polym Degrad Stab 124:43–50. https://doi.org/10.1016/j.polymdegradstab.2015.12.006
Sobczak M (2015) Biodegradable polyurethane elastomers for biomedical applications—Synthesis methods and properties. Polym Plast Technol Eng 54:155–172. https://doi.org/10.1080/03602559.2014.955201
Liu H, Huang W, Yang X (2016) Organic vapor sensing behaviors of conductive thermoplastic polyurethane-graphene nanocomposites. J Mater Chem C 4:4459–4469. https://doi.org/10.1039/c6tc00987e
Tröltzsch J, Schäfer K, Niedziela D (2017) Simulation of RIM-process for polyurethane foam expansion in fiber reinforced sandwich structures. Procedia CIRP 66:62–67. https://doi.org/10.1016/j.procir.2017.03.285
Lei L, Xia Z, Ou C (2015) Effects of crosslinking on adhesion behavior of waterborne polyurethane ink binder. Prog Org Coatings 88:155–163. https://doi.org/10.1016/j.porgcoat.2015.07.002
Jiménez-Pardo I, Sun P, van Benthem RATM, Esteves ACC (2018) Design of self-dispersible charged-polymer building blocks for waterborne polyurethane dispersions. Eur Polym J 101:324–331. https://doi.org/10.1016/j.eurpolymj.2018.02.026
Yong Q, Nian F, Liao B (2015) Synthesis and characterization of solvent-free waterborne polyurethane dispersion with both sulfonic and carboxylic hydrophilic chain-extending agents for matt coating applications. RSC Adv 5:107413–107420. https://doi.org/10.1039/c5ra21471h
Kamal MS, Razzak SA, Hossain MM (2016) Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos Environ 140:117–134. https://doi.org/10.1016/j.atmosenv.2016.05.031
Saalah S, Abdullah LC, Aung MM (2015) Waterborne polyurethane dispersions synthesized from jatropha oil. Ind Crops Prod 64:194–200. https://doi.org/10.1016/j.indcrop.2014.10.046
Fan W, Du W, Li Z (2015) Abrasion resistance of waterborne polyurethane films incorporated with PU/silica hybrids. Prog Org Coatings 86:125–133. https://doi.org/10.1016/j.porgcoat.2015.04.022
Honarkar H, Barmar M, Barikani M (2016) New sulfonated waterborne polyurethane dispersions: preparation and characterization. J Dispers Sci Technol 37:1219–1225. https://doi.org/10.1080/01932691.2015.1028071
Lei L, Xia Z, Lin X (2015) Synthesis and adhesion properties of waterborne polyurethane dispersions with long-branched aliphatic chains. J Appl Polym Sci 132:1–8. https://doi.org/10.1002/app.41688
Zhou X, Fang C, Yu Q (2017) Synthesis and characterization of waterborne polyurethane dispersion from glycolyzed products of waste polyethylene terephthalate used as soft and hard segment. Int J Adhes Adhes 74:49–56. https://doi.org/10.1016/j.ijadhadh.2016.12.010
Lokhande GP, Chambhare SU, Jagtap RN (2017) Anionic water-based polyurethane dispersions for antimicrobial coating application. Polym Bull 74:4781–4798. https://doi.org/10.1007/s00289-017-1965-7
Patel R, Kapatel P (2018) Waterborne polyurethanes: a three step synthetic approach towards environmental friendly flame retardant coatings. Prog Org Coatings 125:186–194. https://doi.org/10.1016/j.porgcoat.2018.09.010
Cai K, Zuo S, Luo S (2016) Preparation of polyaniline/graphene composites with excellent anti-corrosion properties and their application in waterborne polyurethane anticorrosive coatings. RSC Adv 6:95965–95972. https://doi.org/10.1039/c6ra19618g
Mirmohseni A, Akbari M, Najjar R, Hosseini M (2019) Self-healing waterborne polyurethane coating by pH-dependent triggered-release mechanism. J Appl Polym Sci 136:1–12. https://doi.org/10.1002/app.47082
Boutar Y, Naïmi S, Mezlini S (2018) Fatigue resistance of an aluminium one-component polyurethane adhesive joint for the automotive industry: effect of surface roughness and adhesive thickness. Int J Adhes Adhes 83:143–152. https://doi.org/10.1016/j.ijadhadh.2018.02.012
Cristofolini L, Guidetti G, Morellato K (2018) Graphene materials strengthen aqueous polyurethane adhesives. ACS Omega 3:8829–8835. https://doi.org/10.1021/acsomega.8b01342
Shin EJ, Choi SM (2018) Advances in waterborne polyurethane-based biomaterials for biomedical applications. Adv Exp Med Biol 1077:251–283. https://doi.org/10.1007/978-981-13-0947-2_14
Saeedi S, Omrani I, Bafkary R (2019) Facile preparation of biodegradable dual stimuli-responsive micelles from waterborne polyurethane for efficient intracellular drug delivery. New J Chem 43:18534–18545. https://doi.org/10.1039/C9NJ03773J
Rodrigues JME, Pereira MR, De SAG (2005) DSC monitoring of the cure kinetics of a castor oil-based polyurethane. Thermochimica Acta 427:31–36. https://doi.org/10.1016/j.tca.2004.08.010
Monteiro EEC, Fonseca JLC (1997) Phase segregation and viscoelastic behavior of poly (ether urethane urea) s. J Appl Polym Sci 65(11):2227–2236
Characterisation P (1999) Stress relaxation of thermoplastic polyurethanes monitored by FTIR spectroscopy. Polymer testing 18:281–286
De VCL, Martins RR, Ferreira MO, Fonseca JLC (2002) Rheology of polyurethane solutions with different solvents. Polymer international 74:69–74. https://doi.org/10.1002/pi.800
Bullermann J, Friebel S, Salthammer T, Spohnholz R (2013) Novel polyurethane dispersions based on renewable raw materials—Stability studies by variations of DMPA content and degree of neutralisation. Prog Org Coatings 76:609–615. https://doi.org/10.1016/j.porgcoat.2012.11.011
Altuna FI, Ruseckaite RA, Stefani PM (2015) Biobased thermosetting epoxy foams: mechanical and thermal characterization. ACS Sustain Chem Eng 3:1406–1411. https://doi.org/10.1021/acssuschemeng.5b00114
Lligadas G, Ronda JC, Galiá M, Cádiz V (2010) Plant oils as platform chemicals for polyurethane synthesis: current state-of-the-art. Biomacromol 11:2825–2835. https://doi.org/10.1021/bm100839x
Hu S, Luo X, Li Y (2015) Production of polyols and waterborne polyurethane dispersions from biodiesel-derived crude glycerol. J Appl Polym Sci 132:1–8. https://doi.org/10.1002/app.41425
Lu Y, Larock RC (2008) Soybean-oil-based waterborne polyurethane dispersions: effects of polyol functionality and hard segment content on properties. Biomacromol 9:3332–3340. https://doi.org/10.1021/bm801030g
Shah MY, Ahmad S (2012) Waterborne vegetable oil epoxy coatings: preparation and characterization. Prog Org Coatings 75:248–252. https://doi.org/10.1016/j.porgcoat.2012.05.001
Guo Y, Hardesty JH, Mannari VM, Massingill JL (2007) Hydrolysis of epoxidized soybean oil in the presence of phosphoric acid. J Am Oil Chem Soc 84:929–935. https://doi.org/10.1007/s11746-007-1126-5
Man L, Feng Y, Hu Y (2019) A renewable and multifunctional eco-friendly coating from novel tung oil-based cationic waterborne polyurethane dispersions. J Clean Prod 241:118341. https://doi.org/10.1016/j.jclepro.2019.118341
Huang K, Liu Z, Zhang J (2014) Epoxy monomers derived from tung oil fatty acids and its regulable thermosets cured in two synergistic ways. Biomacromol 15:837–843. https://doi.org/10.1021/bm4018929
Gaddam SK, Raju Kutcherlapati SN, Palanisamy A (2017) Self-cross-linkable anionic waterborne polyurethane-silanol dispersions from cottonseed-oil-based phosphorylated polyol as ionic soft segment. ACS Sustain Chem Eng 5:6447–6455. https://doi.org/10.1021/acssuschemeng.7b00327
Tielemans M, Roose P, De Groote P, Vanovervelt JC (2006) Colloidal stability of surfactant-free radiation curable polyurethane dispersions. Prog Org Coatings 55:128–136. https://doi.org/10.1016/j.porgcoat.2005.08.010
Asif A, Shi W, Shen X, Nie K (2005) Physical and thermal properties of UV curable waterborne polyurethane dispersions incorporating hyperbranched aliphatic polyester of varying generation number. Polymer 46:11066–11078. https://doi.org/10.1016/j.polymer.2005.09.046
Bai CY, Zhang XY, Dai JB, Zhang CY (2007) Water resistance of the membranes for UV curable waterborne polyurethane dispersions. Prog Org Coatings 59:331–336. https://doi.org/10.1016/j.porgcoat.2007.05.003
Panda SS, Panda BP, Nayak SK, Mohanty S (2018) A review on waterborne thermosetting polyurethane coatings based on castor oil: synthesis, characterization, and application. Polym Plast Technol Eng 57:500–522. https://doi.org/10.1080/03602559.2016.1275681
Lu Y, Larock RC (2010) Soybean oil-based, aqueous cationic polyurethane dispersions: synthesis and properties. Prog Org Coatings 69:31–37. https://doi.org/10.1016/j.porgcoat.2010.04.024
Zhang X (2015) Thesis: Hybrid Eco-LCA of emerging cellulosic ethanol systems and crude glycerin based polyols. The Ohio State University
Hu S (2013) Thesis: Production and characterization of bio-based polyols and polyurethanes from biodiesel-derived crude glycerol and lignocellulosic biomass. The Ohio State University
Wu GM, Kong ZW, Chen J (2014) Preparation and properties of waterborne polyurethane/epoxy resin composite coating from anionic terpene-based polyol dispersion. Prog Org Coatings 77:315–321. https://doi.org/10.1016/j.porgcoat.2013.10.005
Madbouly SA, Xia Y, Kessler MR (2013) Rheological behavior of environmentally friendly castor oil-based waterborne polyurethane dispersions. Macromolecules 46:4606–4616. https://doi.org/10.1021/ma400200y
Liu K, Miao S, Su Z (2016) Castor oil-based waterborne polyurethanes with tunable properties and excellent biocompatibility. Eur J Lipid Sci Technol 118:1512–1520. https://doi.org/10.1002/ejlt.201500595
Wei X, Ying Y, Yu X (1998) A novel synthetic strategy to aromatic-diisocyanate-based waterborne polyurethanes. J Appl Polym Sci 70:1621–1626. https://doi.org/10.1002/(SICI)1097-4628(19981121)70:8%3c1621::AID-APP20%3e3.0.CO;2-O
Cornille A, Auvergne R, Figovsky O (2017) A perspective approach to sustainable routes for non-isocyanate polyurethanes. Eur Polym J 87:535–552. https://doi.org/10.1016/j.eurpolymj.2016.11.027
Wang Y, Zhao X, Li F (2001) Catalytic synthesis of toluene-2,4-diisocyanate from dimethyl carbonate. J Chem Technol Biotechnol 76:857–861. https://doi.org/10.1002/jctb.455
Brocas A-L, Cendejas G, Caillol S (2011) Controlled synthesis of polyepichlorohydrin with pendant cyclic carbonate functions for isocyanate-free polyurethane networks. J Polym Sci A Polym Chem. https://doi.org/10.1002/pola.24699
Yilgor I, Yilgor E, Guler IG (2006) FTIR investigation of the influence of diisocyanate symmetry on the morphology development in model segmented polyurethanes. Polymer 47:4105–4114. https://doi.org/10.1016/j.polymer.2006.02.027
Schmitz J, Frommelius H, Pegelow U (1999) New concept for dispersing agents in aqueous coatings. Prog Org Coatings 35:191–196. https://doi.org/10.1016/S0300-9440(99)00028-4
Aznar AC, Pardini OR, Amalvy JI (2006) Glossy topcoat exterior paint formulations using water-based polyurethane/acrylic hybrid binders. Prog Org Coatings 55:43–49. https://doi.org/10.1016/j.porgcoat.2005.11.001
Scrinzi E, Rossi S, Deflorian F, Zanella C (2011) Progress in organic coatings evaluation of aesthetic durability of waterborne polyurethane coatings applied on wood for interior applications. Prog Org Coatings 72:81–87. https://doi.org/10.1016/j.porgcoat.2011.03.013
Heilen W (2009) Additives for waterborne coatings. European Coating Tech Files, Vincentz, Evonik Industries. ISBN 978-3-86630-850-3
Janvier M, Ducrot PH, Allais F (2017) Isocyanate-free synthesis and characterization of renewable poly(hydroxy)urethanes from syringaresinol. ACS Sustain Chem Eng 5:8648–8656. https://doi.org/10.1021/acssuschemeng.7b01271
Subramani S, Park YJ, Lee YS, Kim JH (2003) New development of polyurethane dispersion derived from blocked aromatic diisocyanate. Prog Org Coatings 48:71–79. https://doi.org/10.1016/S0300-9440(03)00118-8
Sheth JP, Klinedinst DB, Pechar TW (2005) Time-dependent morphology development in a segmented polyurethane with monodisperse hard segments based on 1,4-phenylene diisocyanate. Macromolecules 38:10074–10079. https://doi.org/10.1021/ma051063a
Honarkar H, Barmar M, Barikani M (2015) Synthesis, characterization and properties of waterborne polyurethanes based on two different ionic centers. Fibers Polym 16:718–725. https://doi.org/10.1007/s12221-015-0718-1
Sukhawipat N, Saetung N, Pilard JF (2018) Synthesis and characterization of novel natural rubber based cationic waterborne polyurethane: effect of emulsifier and diol class chain extender. J Appl Polym Sci 135:17–19. https://doi.org/10.1002/app.45715
Fu C, Zheng Z, Yang Z (2014) A fully bio-based waterborne polyurethane dispersion from vegetable oils: From synthesis of precursors by thiol-ene reaction to study of final material. Prog Org Coatings 77:53–60. https://doi.org/10.1016/j.porgcoat.2013.08.002
Kosheeladevi PP, Tuan Noor Maznee TI, Hoong SS (2016) Performance of palm oil-based dihydroxystearic acid as ionizable molecule in waterborne polyurethane dispersions. J Appl Polym Sci 133:1–10. https://doi.org/10.1002/app.43614
Liang H, Wang S, He H (2018) Aqueous anionic polyurethane dispersions from castor oil. Ind Crops Prod 122:182–189. https://doi.org/10.1016/j.indcrop.2018.05.079
Xia Y, Larock RC (2011) Castor-oil-based waterborne polyurethane dispersions cured with an aziridine-based crosslinker. Macromol Mater Eng 296:703–709. https://doi.org/10.1002/mame.201000431
Shendi HK, Omrani I, Ahmadi A (2017) Synthesis and characterization of a novel internal emulsifier derived from sunflower oil for the preparation of waterborne polyurethane and their application in coatings. Prog Org Coatings 105:303–309. https://doi.org/10.1016/j.porgcoat.2016.11.033
Liu L, Lu J, Zhang Y (2019) Thermosetting polyurethanes prepared with the aid of a fully bio-based emulsifier with high bio-content, high solid content, and superior mechanical properties. Green Chem 21:526–537. https://doi.org/10.1039/c8gc03560a
Zhu Z, Li R, Zhang C, Gong S (2018) Preparation and properties of high solid content and low viscositywaterborne polyurethane-acrylate emulsion with a reactive emulsifier. Polymers. https://doi.org/10.3390/polym10020154
Bao LH, Lan YJ, Zhang SF (2006) Synthesis and properties of waterborne polyurethane dispersions with ions in the soft segments. J Polym Res 13:507–514. https://doi.org/10.1007/s10965-006-9073-7
Li J, Zhang X, Gooch J (2015) Photo- and pH-sensitive azo-containing cationic waterborne polyurethane. Polym Bull 72:881–895. https://doi.org/10.1007/s00289-015-1312-9
Noble KL (1997) Waterborne polyurethanes. Prog Org Coatings 32:131–136. https://doi.org/10.1016/S0300-9440(97)00071-4
Lee YM, Lee JC, Kim BK (1994) Effect of soft segment length on the properties of polyurethane anionomer dispersion. Polymer 35:1095–1099. https://doi.org/10.1016/0032-3861(94)90958-X
Wu J, Chen D (2016) Synthesis and characterization of waterborne polyurethane based on covalently bound dimethylol propionic acid to e-caprolactone based polyester polyol. Prog Org Coatings 97:203–209. https://doi.org/10.1016/j.porgcoat.2016.04.033
Tatai L, Moore TG, Adhikari R (2007) Thermoplastic biodegradable polyurethanes: the effect of chain extender structure on properties and in-vitro degradation. Biomaterials 28:5407–5417. https://doi.org/10.1016/j.biomaterials.2007.08.035
Chandra S, Karak N (2018) Environmentally friendly polyurethane dispersion derived from dimer acid and citric acid. ACS Sustain Chem Eng 6:16412–16423. https://doi.org/10.1021/acssuschemeng.8b03474
Wazarkar K, Kathalewar M, Sabnis A (2015) Improvement in flame retardancy of polyurethane dispersions by newer reactive flame retardant. Prog Org Coatings 87:75–82. https://doi.org/10.1016/j.porgcoat.2015.05.016
Wang S, Du Z, Cheng X (2018) Synthesis of a phosphorus- and nitrogen-containing flame retardant and evaluation of its application in waterborne polyurethane. J Appl Polym Sci 135:1–10. https://doi.org/10.1002/app.46093
Yue S, Zhang Z, Fan X (2015) Effect of 3-aminopropyltriethoxysilane on solvent resistance, thermal stability, and mechanical properties of two-component waterborne polyurethane. Int J Polym Anal Charact 20:285–297. https://doi.org/10.1080/1023666X.2015.1015931
Zhang Y, Shao L, Dong D, Wang Y (2016) Enhancement of water and organic solvent resistances of a waterborne polyurethane film by incorporating liquid polysulfide. RSC Adv 6:17163–17171. https://doi.org/10.1039/c5ra24574e
Javaid MA, Khera RA, Zia KM (2018) Synthesis and characterization of chitosan modified polyurethane bio-nanocomposites with biomedical potential. Int J Biol Macromol 115:375–384. https://doi.org/10.1016/j.ijbiomac.2018.04.013
Wei X, Zhang F (2018) Preparation of an ionic/nonionic polyurethane-silicone dispersion (PUSD) with a high solid content and low viscosity using complex soft segments. J Coatings Technol Res 15:1229–1237. https://doi.org/10.1007/s11998-018-0063-6
Zhang Y, Lin R, Shi Y (2019) Synthesis and surface migration of polydimethylsiloxane and perfluorinated polyether in modified waterborne polyurethane. Polym Bull 76:5517–5535. https://doi.org/10.1007/s00289-018-2666-6
Wu G, Liu D, Chen J (2019) Preparation and properties of super hydrophobic films from siloxane-modified two-component waterborne polyurethane and hydrophobic nano SiO2. Prog Org Coatings 127:80–87. https://doi.org/10.1016/j.porgcoat.2018.06.016
Zhang S, Chen Z, Guo M (2015) Synthesis and characterization of waterborne UV-curable polyurethane modified with side-chain triethoxysilane and colloidal silica. Coll Surfaces A Physicochem Eng Asp 468:1–9. https://doi.org/10.1016/j.colsurfa.2014.12.004
Ma H, Liu Y, Guo J (2020) Synthesis of a novel silica modified environmentally friendly waterborne polyurethane matting coating. Prog Org Coatings. https://doi.org/10.1016/j.porgcoat.2019.105441
Fu C, Hu X, Yang Z (2015) Preparation and properties of waterborne bio-based polyurethane/siloxane cross-linked films by an in situ sol-gel process. Prog Org Coatings 84:18–27. https://doi.org/10.1016/j.porgcoat.2015.02.008
Cheng Z, Li Q, Yan Z (2019) Design and synthesis of novel aminosiloxane crosslinked linseed oil-based waterborne polyurethane composites and its physicochemical properties. Prog Org Coatings 127:194–201. https://doi.org/10.1016/j.porgcoat.2018.11.020
Wang S, Du X, Jiang Y (2019) Synergetic enhancement of mechanical and fire-resistance performance of waterborne polyurethane by introducing two kinds of phosphorus–nitrogen flame retardant. J Coll Interface Sci 537:197–205. https://doi.org/10.1016/j.jcis.2018.11.003
Zhang P, Zhang Z, Fan H (2016) Waterborne polyurethane conjugated with novel diol chain-extender bearing cyclic phosphoramidate lateral group: synthesis, flammability and thermal degradation mechanism. RSC Adv 6:56610–56622. https://doi.org/10.1039/c6ra06856a
Wu L, Guo J, Zhao S (2017) Flame-retardant and crosslinking modification of MDI-based waterborne polyurethane. Polym Bull 74:2099–2116. https://doi.org/10.1007/s00289-016-1826-9
Yin X, Luo Y, Zhang J (2017) Synthesis and characterization of halogen-free flame retardant two-component waterborne polyurethane by different modification. Ind Eng Chem Res 56:1791–1802. https://doi.org/10.1021/acs.iecr.6b04452
Wan T, Chen D (2017) Synthesis and properties of self-healing waterborne polyurethanes containing disulfide bonds in the main chain. J Mater Sci 52:197–207. https://doi.org/10.1007/s10853-016-0321-x
Yin X, Dong C, Luo Y (2017) Effects of hydrophilic groups of curing agents on the properties of flame-retardant two-component waterborne coatings. Coll Polym Sci 295:2423–2431. https://doi.org/10.1007/s00396-017-4218-2
Dai M, Wang J, Zhang Y (2020) Improving water resistance of waterborne polyurethane coating with high transparency and good mechanical properties. Coll Surfaces A Physicochem Eng Asp 601:124994. https://doi.org/10.1016/j.colsurfa.2020.124994
Wu J, Chen D (2018) Synthesis and characterization of waterborne polyurethane based on aliphatic diamine sulphonate and liquefiable dimethylol propionic acid. Prog Org Coatings 118:116–121. https://doi.org/10.1016/j.porgcoat.2018.02.001
Stopilha RT, Xavier-júnior FH, De VCL (2019) Particles: bulk solids and aqueous dispersions. J Dispers Sci Technol. https://doi.org/10.1080/01932691.2019.1611440
De Lima CR, De Souza PR, Stopilha RT, De Morais WA (2017) Formation and structure of chitosan-poly (sodium methacrylate) complex nanoparticles. J Dispers Sci Technol. https://doi.org/10.1080/01932691.2017.1296772
De LCRM, Gomes DN, Filho JRDM (2018) Colloids and surfaces B : biointerfaces anionic and cationic drug sorption on interpolyelectrolyte complexes. Coll Surfaces B Biointerfaces 170:210–218. https://doi.org/10.1016/j.colsurfb.2018.05.071
Ren L, Guo X, Zhao Y, Qiang T (2019) Synthesis and properties of waterborne polyurethane incorporated with phenolic acid grafted oligochitosan. Prog Org Coatings 135:410–416. https://doi.org/10.1016/j.porgcoat.2019.06.030
Zhou X, Fang C, Yu Q (2015) Synthesis of polyurethane dispersions in nanoparticles and their properties that depend on aging time. J Dispers Sci Technol 36:1178–1189. https://doi.org/10.1080/01932691.2014.961195
Li Q, Sun DC (2007) Synthesis and characterization of high solid content aqueous polyurethane dispersion. J Appl Polym Sci 105:2516–2524. https://doi.org/10.1002/app.24627
Christopher G, Anbu Kulandainathan M, Harichandran G (2015) Comparative study of effect of corrosion on mild steel with waterborne polyurethane dispersion containing graphene oxide versus carbon black nanocomposites. Prog Org Coatings 89:199–211. https://doi.org/10.1016/j.porgcoat.2015.09.022
Chen F, Zhou D, Yang L, Sun J, Wu J (2018) Poly(dopamine) coated graphene oxide as multi-functional filler in waterborne polyurethane. Mat Res Exp 6(1):015308
Zhou X, Fang C, Lei W (2017) Thermal and crystalline properties of waterborne polyurethane by in situ water reaction process and the potential application as biomaterial. Prog Org Coatings 104:1–10. https://doi.org/10.1016/j.porgcoat.2016.12.001
Fuensanta M, Jofre-Reche JA, Rodríguez-Llansola F et al (2018) Structure and adhesion properties before and after hydrolytic ageing of polyurethane urea adhesives made with mixtures of waterborne polyurethane dispersions. Int J Adhes Adhes 85:165–176. https://doi.org/10.1016/j.ijadhadh.2018.06.002
Kim KM, Park HW, Shim GS (2020) Mechanical properties and decomposition performance of peelable coating containing UiO-66 catalyst and waterborne silane-terminated polyurethane dispersions. J Mater Sci 55:2604–2617. https://doi.org/10.1007/s10853-019-04184-2
Liao L, Li X, Wang Y (2016) Effects of surface structure and morphology of nanoclays on the properties of jatropha curcas oil-based waterborne polyurethane/ clay nanocomposites. Ind Eng Chem Res 55:11689–11699. https://doi.org/10.1021/acs.iecr.6b02527
Stefanović IS, Špírková M, Ostojić S (2017) Montmorillonite/poly(urethane-siloxane) nanocomposites: morphological, thermal, mechanical and surface properties. Appl Clay Sci 149:136–146. https://doi.org/10.1016/j.clay.2017.08.021
Ni L, Mao Y, Liu Y (2020) Synergistic reinforcement of waterborne polyurethane films using palygorskite and dolomite as micro/nano-fillers. J Polym Res 27:1–8. https://doi.org/10.1007/s10965-019-1995-y
Cakić SM, Ristić IS, M-Cincović M (2016) Preparation and characterization of waterborne polyurethane/silica hybrid dispersions from castor oil polyols obtained by glycolysis poly(ethylene terephthalate) waste. Int J Adhes Adhes 70:329–341. https://doi.org/10.1016/j.ijadhadh.2016.07.010
Ding X, Wang X, Zhang H (2020) Preparation of waterborne polyurethane-silica nanocomposites by a click chemistry method. Mater Today Commun 23:100911. https://doi.org/10.1016/j.mtcomm.2020.100911
Han Y, Hu J, Xin Z (2018) In-situ incorporation of alkyl-grafted silica into waterborne polyurethane with high solid content for enhanced physical properties of coatings. Polymers. https://doi.org/10.3390/polym10050514
Hassanajili S, Sajedi MT (2016) Fumed silica/polyurethane nanocomposites: effect of silica concentration and its surface modification on rheology and mechanical properties. Iran Polym J 25:697–710. https://doi.org/10.1007/s13726-016-0458-0
Cakić SM, Ristić IS, Stojiljković DT (2019) Effect of the silica nanofiller on the properties of castor oil-based waterborne polyurethane hybrid dispersions based on recycled PET waste. Polym Bull 76:1217–1238. https://doi.org/10.1007/s00289-018-2429-4
Gao X, Bilal M, Ali N (2020) Two-dimensional nanosheets functionalized water-borne polyurethane nanocomposites with improved mechanical and anti-corrosion properties. Inorg Nano-Metal Chem. https://doi.org/10.1080/24701556.2020.1749656
Yin S, Ren X, Lian P (2020) Synergistic effects of black phosphorus/boron nitride nanosheets on enhancing the flame-retardant properties of waterborne polyurethane and its flame-retardant mechanism. Polymers 12:1–14. https://doi.org/10.3390/polym12071487
Da Silva VD, Dos Santos LM, Subda SM (2013) Synthesis and characterization of polyurethane/titanium dioxide nanocomposites obtained by in situ polymerization. Polym Bull 70:1819–1833. https://doi.org/10.1007/s00289-013-0927-y
Kausar A (2016) Waterborne polyurethane-coated polyamide/fullerene composite films: mechanical, thermal, and flammability properties. Int J Polym Anal Charact 21:275–285. https://doi.org/10.1080/1023666X.2016.1147729
Dias G, Prado M, Ligabue R (2018) Hybrid PU/synthetic talc/organic clay ternary nanocomposites: thermal, mechanical and morphological properties. Polym Polym Compos 26:127–140. https://doi.org/10.1177/096739111802600201
Dias G, Prado M, Le Roux C (2020) Synthetic talc as catalyst and filler for waterborne polyurethane-based nanocomposite synthesis. Polym Bull 77:975–987. https://doi.org/10.1007/s00289-019-02789-w
Prado MA, Dias G, dos Santos LM (2020) The influence of Ni/Mg content of synthetic Mg/Ni talc on mechanical and thermal properties of waterborne polyurethane nanocomposites. SN Appl Sci. https://doi.org/10.1007/s42452-020-2852-7
dos Santos LM, Ligabue R, Dumas A (2018) Waterborne polyurethane/Fe3O4-synthetic talc composites: synthesis, characterization, and magnetic properties. Polym Bull 75:1915–1930. https://doi.org/10.1007/s00289-017-2133-9
Lyu J, Xu K, Zhang N (2019) In situ incorporation of diamino silane group into waterborne polyurethane for enhancing surface hydrophobicity of coating. Molecules. https://doi.org/10.3390/molecules24091667
Han Y, Hu J, Xin Z (2019) Facile preparation of high solid content waterborne polyurethane and its application in leather surface finishing. Prog Org Coatings 130:8–16. https://doi.org/10.1016/j.porgcoat.2019.01.031
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vaidya, S.M., Jadhav, S.M., Patil, M.J. et al. Recent developments in waterborne polyurethane dispersions (WPUDs): a mini-review on thermal and mechanical properties improvement. Polym. Bull. 79, 5709–5745 (2022). https://doi.org/10.1007/s00289-021-03814-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03814-7