Abstract
The objective of this work was to produce thermoplastic starch (TPS) and poly (butylene co-terephthalate adipate) (PBAT) incorporated with free and microencapsulated oregano essential oil (OEO) by blown extrusion and compare their properties. The OEO was microencapsulated by spray drying using arabic gum and maltodextrin as wall materials. The films were characterized in terms of physical, optical, morphological, thermal and antioxidant properties, and the OEO diffusion coefficient was determined in different food simulants. Regarding water vapor permeability (2.04–2.05 × 10−7 g m−1 Pa−1 h−1) and water solubility (6.25–9.65%), no significant difference (p > 0.05) was observed. Morphological images revealed that films with OEO microparticles (FM) showed greater roughness that caused a reduction in tensile strength, Young's modulus and elongation. FM film showed better thermal stability, significant concentration of phenolic compounds (3.6 mg EGA gfilm−1) and antioxidant capacity, and higher diffusion coefficient in ethanol 10% (aqueous food simulant, 1.3109 × 10–11 cm2 s−1) and 95% (non-aqueous food simulant, 39.8623 × 10–11 cm2 s−1). The results demonstrate the use potential of microencapsulated OEO in the development of biodegradable antioxidant films for food applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The current trend in the food industry is the development of active biodegradable packaging that can assist in food preservation. Active packaging may contain antioxidants and antimicrobials in its composition that can be released and interact with food, helping to preserve and minimize its addition to food formulation [1,2,3].
Among the substances that can be used in the production of active packaging, oregano (Origanum vulgare L.) essential oil stands out because of the high content of phenolic compounds in its composition, such as carvacrol and thymol, and a broad spectrum of antimicrobial and antioxidant action [4,5,6,7,8]. As a result of the chemical structure of OEO phenolic compounds, a part can interact with the constituents of the film (polymer and plasticizer) and reduce the diffusion of bioactive compounds to the packaged product [9]. The other aspect to be highlighted is the volatility of OEO, which during the production of films by extrusion can be degraded because of the direct exposure to heat, pressure and oxygen.
Considering the aspects above, the microencapsulation of the OEO is interesting because it can provide isolation, protection, transport, and control of the release of this compound in the product it is applied to, preventing its degradation [10]. Among the encapsulation methods, spray drying is an interesting process that consists of spraying the liquid in a compartment that receives a hot air flow, so the rapid evaporation of the water allows the temperature of the particles to remain low. Spray drying is the most common and economical technique for producing microencapsulated food materials, and the obtained free-flowing powders are easier to handle and incorporate into dry food systems, and biodegradable films produced by extrusion [11,12,13].
Biodegradable films based on blends of thermoplastic starch (TPS) and PBAT are already being produced on a pilot scale by blown extrusion. The obtained materials have interesting mechanical and barrier properties that can be used as food packaging [14,15,16,17]. Therefore, the TPS and PBAT film can be a promising matrix for incorporating microencapsulated OEO, enabling the production of active biodegradable packaging for food. OEO has been successfully incorporated in different biopolymer-based materials [18,19,20]. The objective of this work was to develop biodegradable films of cassava starch and PBAT by blown extrusion incorporated with free and microencapsulated OEO and to evaluate its physical, thermal, optical and morphological properties, as well as its antioxidant capacity and diffusion of OEO in the food simulant.
Material and methods
Material
The microparticles were produced with oregano essential oil (Origanum vulgare L.) (Sigma-Aldrich Co., USA), gum arabic (Nexira, Brazil), maltodextrin (DE 20, Cargill, Brazil) and paprika oleoresin (Citromax, Brazil). The films were produced using PBAT (Basf, Brazil), cassava starch (Pinduca, Brazil), citric acid and glycerol (Dinâmica, Brazil).
Microencapsulation of OEO by spray drying
Initially, an emulsion of OEO, gum arabic and maltodextrin (1:1) was prepared. The solids’ concentration of the emulsion was fixed at 30% (w/w) and the OEO content at 10% (w/w) concerning the weight of the solids. Paprika oleoresin (2% in relation to the OEO weight) was used to stain the microparticles and facilitate visualization. The mixture was homogenized with Ultra-Turrax at 12,000 rpm for 3 min, and the emulsion obtained was sprayed with the aid of a double-fluid nozzle (0.7 mm diameter) in the spray dryer chamber, whose process conditions were: temperatures of 130 °C for inlet air and 88 °C for the exhaust air; feed flow of 600 mL min−1; airflow of 1.65 m3 min−1 and compressed air pressure of 35 L min−1. The experimental conditions to obtain OEO microparticles were determined by preliminary tests.
Encapsulation efficiency and physical characteristics of microparticles
The encapsulation efficiency was determined in duplicate, using the Clevenger-type apparatus and steam distillation extraction method [20, 21]. The encapsulation efficiency (%) was calculated by the ratio of the amount of oil extracted from the microparticles and the initial amount of oil, multiplied by 100.
The average diameter and size distribution of the microparticles were determined by light scattering (Horiba, LV950 model, Japan) using ethanol as a dispersing medium. The average particle diameter was expressed in terms of average diameter (D50), and polydispersity was given by the span index and calculated according to the equation: Span = (D90 – D10)/D50. where D10, D50, and D90 correspond to 10%, 50% and 90% of the cumulative distribution, respectively.
The morphology of the microparticles was evaluated using a scanning electron microscope (Philips, FEI Quanta 200 model, Japan) with an acceleration power of 20 kV and an observation magnitude of 5000× .
Production of the films by blown extrusion
For the films’ production, three formulations were used (Table 1), named as: control (FC) with OEO microparticles (FM) and with free OEO (FO). The addition of 10% microparticles was determined by preliminary tests. From the determination of encapsulation efficiency analysis, it was possible to establish that in 50 g of microparticles there was approximately 3.28 g of OEO, and this amount of OEO was added in FO.
For the production of the films, the ingredients (Table 1) were weighed, mixed and processed in a pilot single-screw extruder (BGM, model EL-25, Brazil) with a diameter of 25 mm, length of 28D, and configured to operate under the heating profile of 90/120/120/100 °C and screw rotation of 35 rpm. The cylindrical profiles were pelletized and processed again in the same extruder to form the film with the following conditions: temperature profile of 90/120/120/130/130 °C, screw rotation of 35 rpm and a film-blowing diameter die of 50 mm. The winding speed and airflow in the matrix that formed the balloon were adjusted for each formulation to allow the formation of the balloon without tearing or cracking.
Film characterization
Morphology
The films were previously conditioned for 14 days in a desiccator containing silica gel to remove residual moisture [22]. After that, the films were fractured in liquid nitrogen, fixed in stubs with carbon tape, and covered with gold in a Sputter Coater (BAL-TEC, SCD-050 model, Balzers, Liechtenstein). The fragile fracture and surface of the films were visualized in a scanning electron microscope (Philips, FEI Quanta 200 model, Japan), with an acceleration power of 20 kV. The magnitude of observation was 1600 × for the fracture area and 800 × for the surface.
Thickness
The thickness of the films was determined using a digital micrometer. Ten measurements were taken at random on the surface of the film.
Color and opacity
The color was measured using a colorimeter (Konica Minolta, CR-400 model, Japan). The films were placed on the equipment's sensor to measure the color parameters L* luminosity (black/white), a* (green/red) and b* (blue/yellow). The apparent opacity (Yap) was calculated based on the ratio between the luminosity measured on a black background and a white background according to the equation: Yap = (L*b/L*w) × 100, where Yap is the apparent opacity, L*b is the luminosity measured against a black background, and L*w is the luminosity measured against a white background. The values of Yap were divided by the sample thickness and expressed on an arbitrary scale (0–1% μm−1).
Mechanical properties
For the tensile tests, a texturometer (Stable Micro Systems, TA-TX2 model, England) was used. The films were fixed to the equipment's grips with an initial distance of 30 mm and a speed of 0.8 mm s−1. The properties determined were tensile strength (MPa), elongation at break (%), and Young's modulus (MPa). The test was performed on 10 films of each formulation [23].
Solubility in water and water vapor permeability
To determine water solubility [24], the films (2 × 2 cm) were oven-dried at 105 °C for 24 h and weighed (initial mass). These samples were placed in conical flasks containing 200 mL of distilled water and kept under agitation at 25 °C for 24 h. Then, the films were removed from the water and dried again in an oven at 105 °C for 24 h and weighed (final mass). Solubility was expressed by the difference in weight between the initial and final dry matter divided by the initial dry matter. Water vapor permeability (WVP) was determined by the gravimetric method [25]. All tests were performed in triplicate.
Thermogravimetric analysis
The thermogravimetric analysis (TGA) (PerkinElmer, STA-6000, USA) of the films was performed under nitrogen flux (20 mL min−1), with heating from 25 to 600 °C at a rate of 10 °C min−1.
Diffusion of OEO in food simulant fluid
The diffusion of the OEO from the film was performed according to [26], using ethanol 10% (v/v) as an aqueous food simulant and 95% (v/v) ethanol as a fat food simulant. In a conical flask, the films (6 cm × 10 cm) were immersed in 50 mL of simulant and kept under agitation (100 rpm) at 25 °C. At different times, an aliquot of 2 mL of solution was removed and the concentration of OEO released was quantified by UV–Vis spectroscopy (230 nm).
The mass diffusion of the OEO was modeled through the second Fick's law. For the film, a Cartesian geometry shall be considered, and the solution to Fick’s equation results can be given by using the Laplace transform (Eq. 1) or method of separation of variables (Eq. 2)
where Mt/M∞ is the amount of OEO released at each time t relative to the amount released at equilibrium, L is the half-thickness of the film, D is the diffusion coefficient, t is the time.
For short-term diffusion, Mt/M∞ < 0.3 – 0.5, the series in Eq. (1) tends to zero, and the following equation is obtained:
The linear regression was fitted with Mt/M∞ versus t1/2, and the diffusion coefficient was obtained.
For long-term diffusion, Mt/M∞ > 0.5 – 0.7, the first term in the series of Eq. (2) is preponderant, and thus, the series can be truncated in the first term, resulting in:
The diffusion coefficient can be obtained through a graph composed of the terms log ((Mt – M∞)/M∞) versus t.
Phenolic compounds and antioxidant capacity
The extraction of phenolic compounds was according to [21] with minor modification. For this, 2 g of films and 20 mL of 80% (v/v) ethanol were mixed and homogenized on a tube shaker (Phoenix, Brazil) for 20 h at room temperature. The mixture was centrifuged, and the supernatant was used to determine total phenolic compounds according to the Folin–Ciocalteu method [27] and measurement of the antioxidant capacity by the DPPH [28] and ABTS [29] radical capture methods and by the iron reduction method (FRAP) [30].
Statistical analysis
The results obtained were compared after variance analysis (ANOVA), and the statistical differences between the means were identified by the Tukey test (p < 0.05) using Statistica® 12.0 (Statsoft, USA).
Results and discussion
OEO microparticle characterization
The encapsulation efficiency (EE) of OEO microparticles obtained by spray drying was 65.7 ± 2.3%. EE of 61.8% was obtained by Fernandes et al. [31] encapsulating rosemary essential oil using maltodextrin and modified starch as wall material. However, EE of 86.2% was obtained by Toledo Hijo et al. [32] encapsulating the OEO. The differences in EE are due to the nature of the wall materials that interfered in the retention of volatile compounds [31].
The morphology of the OEO microparticle observed by SEM (Fig. 1) showed irregular spherical shape with a concave and a rough surface. These morphological characteristics may be due to the rapid evaporation of water during the spray-drying process [11, 33]. Similar morphological structures of microparticles were observed by Fernandes et al. [31] and Toledo Hijo et al. [32] after spray drying.
The average diameter of the OEO microparticles was 9.45 ± 0.51 μm, and proximal values were observed by Teodoro et al. [34] in rosemary essential oil microparticles and by Toledo Hijo et al. [32] in OEO microparticles all produced by spray drying. The polydispersion or span index of the OEO microparticles was 1.69 ± 0.16; this value is considered high because it indicates that there was no homogeneity in the size of the samples. The non-homogeneity was observed because, during the spraying of the emulsion by the double-fluid nozzle, the drops did not have a uniform size. Also, the high span value corroborated the SEM image that showed a variation in the size of the OEO microparticles.
Visual aspect and morphology of the films
The films FC, FM, and FO had a white color, except for the FM film with OEO microparticles, which had a slightly pale orangish color, due to the presence of the paprika oil in microparticles. Nevertheless, films presented homogeneous characteristics, were easy handling, and were without bubbles or cracks (Fig. 2).
The morphology of the FC, FM, and FO films was evaluated by SEM, and the images of the surface and fracture are shown in Fig. 2. In the images of the surfaces (FCs, FMs, and FOs), it is observed that the FMs film presents a rougher surface and with a greater presence of granules with different sizes that may result from the addition of microparticles to the film. Similar behavior was observed in the work of de Medeiros et al. [20], when working with TPS/PBAT films and OEO microparticles produced by ionic gelation. Fracture images (FCf, FMf, and FOf) show granules of non-gelatinized starch with different sizes dispersed in the matrix. According to da Silva et al. [35], the presence of starch granules indicates that there has not been complete destructuring of the starch during the thermomechanical process. Additionally, the heterogeneous fracture image confirms the incompatibility between TPS and PBAT, also reported by other authors [15, 36] due to the hydrophobic character of PBAT. FM film presented a fracture with greater irregularity and roughness, which can be associated with the presence of microparticles, which seemed to behave as a filling in the film matrix.
Color, opacity and thickness
The color parameters of the FC and FO films were similar (Table 2). However, the FM film showed a significant difference in the L* and b* color parameters compared to the FC and FO films, which can be justified by the addition of the paprika oleoresin, which has an orangish color and was added to facilitate the visualization and distribution of the microparticles on the film. This was also confirmed by the overall aspect of the film, as shown in Fig. 2.
Opacity is a desirable parameter in packaging material to reduce light passage through to photosensitive food [37]. The opacity of the films FC, FM and FO was between 0.29 and 0.60% µm−1. Similar values of opacity (0.20–0.65% µm−1) were reported by Olivato et al. [15] in TPS/PBAT films produced by blown extrusion. Higher values of opacity (0.344–0.439% µm−1) were observed by Garcia et al. [36] in TPS/PBAT films with itaconic acid and sodium hypophosphite due to higher compactation between polymeric chains promoted by esterifying reactions. The films produced by blown extrusion favored the formation of crystalline zones that can reduce the transparency of the films because of the biaxial elongation of the molten material. The addition of microencapsulated OEO caused a significant increase in opacity and may be associated with the presence of paprika oleoresin in the microparticles. In PBAT films incorporated with OEO produced by extrusion, Cardoso et al. [38] observed that the increase in opacity was due to the concentration of OEO, and this occurred because lipid fractions caused a light scattering as a result of the distribution of fat droplets.
The mean thickness of the films FC, FM and FO was 184 µm, 116 µm and 190 µm, respectively, and there were no significant differences (p > 0.05) in thickness between FC and FO films. The different thickness of the FM film may be associated with the incorporation of OEO microparticles, which allowed a greater stretching of the film during the formation of the balloon, resulting in films thinner than the others. The cassava starch and PBAT films incorporated of free OEO and OEO encapsulated by ionic gelation [20] presented greater thickness (247 μm and 219 μm, respectively) than the films produced in the present work. This difference may be related to the higher concentration of PBAT (40%) in the film formulations of this work, which provided greater stretching during the formation of the balloon, making them thinner.
Mechanical properties
The stress and strain curve (Fig. 3) of the FC, FO, and FM films was typical of flexible film. During the stretching of the film, the maximum force reached followed by the order FC > FO > FM, and after yielding the strain developed continuously, indicating that films presented a ductile characteristic. The fracture point of the FC and FO film was close and higher than FM film. Based on the stress and strain curve, the mechanical properties of the films in terms of the tensile strength (T), elongation at break (ELO), and Young's modulus (YM) were determined, and the results are shown in Table 3. The films of FC, FM and FO showed significant differences with respect to T and YM, and the highest value was found for FC, followed by FO and FM. The elongation at break (ELO) of the FM film was lower compared to the FC and FO films.
The reduction on the T and YM values of the FM and FO films may be associated with the decrease in the polymer–polymer interaction that formed a less cohesive structure and also with the plasticizing effect of OEO, as previously evidenced in films incorporated of OEO [5, 20, 21, 39]. The film containing microparticles showed the lowest values in all parameters, possibly due to the presence of the microparticles that generated the stress concentration points that consequently decreased and interfered in the mechanical properties of the film [20]. The SEM images reinforce this fact since it presented a morphology with a high presence of granules, greater roughness and irregularities. Also, it should be pointed out that mechanical properties for filled systems, such as FM, depend on the state of the polymer–particle interface since when there is a good adhesion between filler and matrix, an enhanced stress transfer occurs at the interface [40]. In this study, microparticles did not act as an effective filler, and the lack of reinforcement observed in TPS/PBAT film may be due to weak interaction between the matrix and microparticles, which was incorporated at high concentration (10%).
Although the presence of OEO microparticles has affected the mechanical properties of TPS/PBAT film, this active film is an interesting alternative for applications where films with high tensile strength and elongation at break are not required but instead show an efficient bioactive property, such as film used to separate pastry dough [41,42,43].
Water solubility and water vapor permeability (WVP)
The solubility of the FC, FM and FO films did not show significant differences (Table 3), and therefore, the average was 7.65%. The water solubility of the TPS and PBAT films incorporated of curcumin was evaluated by de Campos et al. [14], and greater solubility values (between 20 and 40%) were associated with the incorporation of curcumin that increased film hydrophilicity. Films based on TPS and PBAT with the incorporation of pine nut extract also showed higher values of solubility (between 24 and 28%) [16] in relation to the films of FC, FM and FO. The differences in solubility can also be related to the higher concentration of PBAT (40%) used in the formulation of the FC, FM and FO films, which has more hydrophobic character and contributed to the reduction in water solubility.
The WVP is one of the most important parameters to determine during development of films for food packaging applications because the diffusion of water vapor inside the packaging may contribute to food deterioration, consequently reducing shelf life. For this reason, the production of films with low WVP is interesting, and this property is substantially dependent of film matrix [44, 45]. The WVP of the FC, FM and FO films did not show a significant difference (p > 0.05), and therefore, the main value was 2.05 × 10–7 g m−1 h−1 Pa−1 (Table 3). Proximate values of WVP (from 6.67 to 15.22 × 10–11 g m−1 s−1 Pa−11) were reported by Zhai et al. [17] in modified starch, PBAT and nanoclay films produced by blown extrusion and by Garcia et al. [36] (from 4.882 to 6.389 × 10–11 g m−1 s−1 Pa−11) in starch and PBAT blown films incorporated with itaconic acid and sodium hypophosphite. Lower values of WVP (from 4.36 to 4.55 × 10–16 g m−1 s−1 Pa−1) in cassava starch and PBAT incorporated of OEO were observed by de Medeiros et al. [20]. These difference can be attributed to the formation of a more compact and homogeneous matrix that hindered the diffusion of water vapor.
Thermogravimetric analysis (TGA)
The thermal stability of the FC, FM and FO films was evaluated by TGA, and the thermal degradation curves (Fig. 4) presented different characteristics. The degradation of the FC film occurred in three stages: the first peak at a temperature below 100 °C is related to moisture loss, the second peak is related to TPS degradation (≃ 320 °C) and the third peak represents the degradation of PBAT (≃ 400 °C) [46, 47]. For the FM and FO films, it was observed that the degradation occurred in four stages, with an additional peak in the range of ≃ 160 to 170 °C that may be associated with OEO degradation [38, 48].
According to the derivative weight loss curves (DTG), the film containing OEO microparticle presented higher degradation temperature of OEO, starch and PBAT, suggesting an improvement of its thermal stability in relation to the FC and FO films. The increase in the degradation temperature of FM film may be due to the materials in the microparticle wall (gum arabic and maltodextrin). The considerable increase in OEO degradation temperature from 161 °C in FO to 175 °C in FM suggests that the incorporation of OEO in the film in encapsulated form contributed to the thermal stability of the essential oil, minimizing its degradation during the thermoplastic extrusion process. The results obtained are consistent to the findings of Cardoso et al. [38], which observed a reduction in the thermal stability of PBAT films incorporated with free OEO.
Diffusion of OEO in simulated fluid
The OEO diffusion coefficients (D) of the FO and FM films (Table 4) in short- and long-term migration were about 10–11 cm2 s−1 and 10–12 cm2 s−1, in ethanol 10% and 95% at 25 °C, respectively. There was a reduction in the D values for FM and FO films in the two food simulant (10% and 95% ethanol) over time. This reduction occurred because the flow or release of the OEO is proportional to the gradient of concentration in the system. It was observed that in the initial stage, there is a low concentration of OEO in the simulant fluid, and this caused a large concentration gradient between the film and food simulant, which provided greater mass transport, resulting in a high mass diffusion coefficient at early stages. During the long-term diffusion stage, D decreased due to the increase in OEO in the food simulant and its subsequent decrease in the film matrix [26].
Considering the mode addition of the OEO to the TPS and PBAT film, the FM film with microencapsulated OEO presented a higher D in relation to the FO film with free OEO, both for short- and for long-term release in 10 and 95% ethanol. The lower diffusion of the OEO of FO may be related to the weak interactions between the phenolic compounds of the OEO with the film that hindered their mobility through the polymeric chains toward the simulant. Also, it was possible to verify that in the encapsulated and powdered form, there was a better distribution of OEO in the film, which may have contributed to its release in the fluids investigated.
Comparing the food simulant, the D value was higher in 95% ethanol for FM and FO. This may be associated with the greater affinity of OEO with the solvent that has a more hydrophobic characteristic [49]. The 10% ethanol, with a hydrophilic characteristic, showed a double-release mechanism in which the hydrophilic film matrix became swollen when in contact with water, causing the chains to relax and modify the interaction between the film and the OEO. In this way, water was less available for the release of OEO, and therefore, the D value showed a tendency for reduction.
Regardless of whether the food is aqueous or fat and based on the D values (Table 4), it is recommended to apply FM in foods that require greater diffusion of OEO. On the other hand, the application of FO would be recommended for foods, which require slower diffusion of OEO. It is important to highlight that active compounds should migrate to the middle or to the surface of the food, where they can interact with other food ingredients in order to improve the properties of the final product. Also, the release of the active compound was dependent on the morphology, microstructure and material of the film, as well as the polarity and chemical structure of the active ingredient, and the nature of the food product [50].
Phenolic compounds and antioxidant capacity
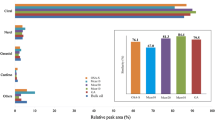
The phenolic compounds and antioxidant capacity, evaluated by the FRAP, DPPH and ABTS methods of the FC, FM and FO films (Fig. 5), showed significant differences. The incorporation of OEO, whether in free or microencapsulated form, significantly increased the content of phenolic compounds and, consequently, the antioxidant capacity of the films. OEO phenolic compounds such as carvacrol, thymol and γ-terpinene are mainly responsible for the antioxidant capacity [6]. The antioxidant capacity of biodegradable films incorporated with OEO was also reported by other researches [21, 38].
The content of phenolic compounds in FM was higher (p < 0.05) than in FC and FO. However, the antioxidant capacity (measured by FRAP, DPPH and ABTS) of FM and FO did not show significant differences. Considering the higher diffusion coefficient of OEO in FM (Table 4), it can be deduced that, by the extraction method employed, there was a greater extraction of phenolic compounds from FM. However, not all the extracted compounds showed antioxidant activity. Terpinc et al. [51] also did not find a positive correlation between the different antioxidant activity assays and total phenolic contents in oil cake extracts, and this occurred due to the presence of non-phenolic compounds, which are known to react with Folin–Ciocalteu reagent but are not effective as free radical scavengers (citric acid, ferrous sulfate, polysaccharides).
Comparing the antioxidant capacity assays, different orders of magnitude were observed being FRAP > ABTS > DPPH. The same behavior was observed in our previous study [21, 22], and it is related to difference in the ability of antioxidant compounds in the film extracts to capture ABTS+ and DPPH free radical and to reduce ferric iron in in vitro systems.
Conclusion
The microencapsulation of OEO by spray drying and production of active TPS and PBAT films by blown extrusion on a pilot scale was feasible. Considering mechanical properties, the microparticles did not act as an efficient filler in the TPS and PBAT matrix because it was observed a reduction around 55, 70, 68% in the T, YM and ELO, respectively, but did not alter the WVP of TPS/PBAT films. On the other hand, the incorporation of OEO microparticles improved the thermal stability of OEO in films, provided significant antioxidant activity, and a higher coefficient of diffusion in ethanol 10% (1.3109 × 10–11 and 6.8133 × 10–12 cm2 s−1 for short and long terms) and 95% (39.8623 × 10–11 and 54.715 × 10–12 cm2 s−1 for short and long term). Finally, the films obtained in this work can be used in the production of active biodegradable packaging, and the application of these materials in food will depend on the need for more or less diffusion of OEO, regardless of whether the food is aqueous or fat.
Availability of data and material
Data and material available if necessary.
References
Gómez-Estaca J, López-de-Dicastillo C, Hernández-Muñoz P et al (2014) Advances in antioxidant active food packaging. Trends Food Sci Technol 35:42–51. https://doi.org/10.1016/j.tifs.2013.10.008
Riaz A, Lei S, Akhtar HMS et al (2018) Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int J Biol Macromol 114:547–555. https://doi.org/10.1016/j.ijbiomac.2018.03.126
De Conto D, dos Santos V, Zattera AJ, Santana RMC (2020) Swelling of biodegradable polymers for the production of nanocapsules and films with the incorporation of essential oils. Polym Bull. https://doi.org/10.1007/s00289-020-03465-0
Bonfanti C, Iannì R, Mazzaglia A et al (2012) Emerging cultivation of oregano in Sicily: sensory evaluation of plants and chemical composition of essential oils. Ind Crops Prod 35:160–165. https://doi.org/10.1016/j.indcrop.2011.06.029
Solano ACV, de Gante CR (2012) Two different processes to obtain antimicrobial packaging containing natural oils. Food Bioprocess Technol 5:2522–2528. https://doi.org/10.1007/s11947-011-0626-3
Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol 94:223–253. https://doi.org/10.1016/j.ijfoodmicro.2004.03.022
Castilho PC, Savluchinske-Feio S, Weinhold TS, Gouveia SC (2012) Evaluation of the antimicrobial and antioxidant activities of essential oils, extracts and their main components from oregano from Madeira Island, Portugal. Food Control 23:552–558. https://doi.org/10.1016/j.foodcont.2011.08.031
Ribeiro-Santos R, Andrade M, Sanches-Silva A, de Melo NR (2018) Essential oils for food application: natural substances with established biological activities. Food Bioprocess Technol 11:43–71. https://doi.org/10.1007/s11947-017-1948-6
Ruiz-Navajas Y, Viuda-Martos M, Sendra E et al (2013) In vitro antibacterial and antioxidant properties of chitosan edible films incorporated with Thymus moroderi or Thymus piperella essential oils. Food Control 30:386–392. https://doi.org/10.1016/j.foodcont.2012.07.052
Ribeiro-Santos R, Andrade M, Sanches-Silva A (2017) Application of encapsulated essential oils as antimicrobial agents in food packaging. Curr Opin Food Sci 14:78–84. https://doi.org/10.1016/j.cofs.2017.01.012
Frascareli EC, Silva VM, Tonon RV, Hubinger MD (2012) Determination of critical storage conditions of coffee oil microcapsules by coupling water sorption isotherms and glass transition temperature. Int J Food Sci Technol 47:1044–1054. https://doi.org/10.1111/j.1365-2621.2012.02939.x
Gharsallaoui A, Roudaut G, Chambin O et al (2007) Applications of spray-drying in microencapsulation of food ingredients: an overview. Food Res Int 40:1107–1121. https://doi.org/10.1016/j.foodres.2007.07.004
Ré MI (1998) Microencapsulation by spray drying. Dry Technol 16:1195–1236
de Campos SS, de Oliveira A, Moreira TFM et al (2019) TPCS/PBAT blown extruded films added with curcumin as a technological approach for active packaging materials. Food Packag Shelf Life 22:100424. https://doi.org/10.1016/j.fpsl.2019.100424
Olivato JB, Grossmann MVE, Yamashita F et al (2012) Citric acid and maleic anhydride as compatibilizers in starch/poly(butylene adipate-co-terephthalate) blends by one-step reactive extrusion. Carbohydr Polym 87:2614–2618. https://doi.org/10.1016/j.carbpol.2011.11.035
da Silva TBV, Moreira TFM, de Oliveira A et al (2019) Araucaria angustifolia (Bertol.) Kuntze extract as a source of phenolic compounds in TPS/PBAT active films. Food Funct 10:7697–7706. https://doi.org/10.1039/C9FO01315F
Zhai X, Wang W, Zhang H et al (2020) Effects of high starch content on the physicochemical properties of starch/PBAT nanocomposite films prepared by extrusion blowing. Carbohydr Polym 239:116231. https://doi.org/10.1016/j.carbpol.2020.116231
Cruz-Tirado JP, Barros Ferreira RS, Lizárraga E et al (2020) Bioactive Andean sweet potato starch-based foam incorporated with oregano or thyme essential oil. Food Packag Shelf Life 23:100457. https://doi.org/10.1016/j.fpsl.2019.100457
de Espíndol Sobczyk A, Luchese CL, Faccin DJL, Tessaro IC (2021) Influence of replacing oregano essential oil by ground oregano leaves on chitosan/alginate-based dressings properties. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2021.03.084
de Medeiros JAS, Blick AP, Galindo MV et al (2019) Incorporation of oregano essential oil microcapsules in starch-poly (butylene adipate co-terephthalate) (PBAT) films. Macromol Symp 383:1–7. https://doi.org/10.1002/masy.201800052
dos Santos PI, Galindo MV, de Medeiros JAS et al (2019) Comparative study of the properties of soy protein concentrate films containing free and encapsulated oregano essential oil. Food Packag Shelf Life 22:100419. https://doi.org/10.1016/j.fpsl.2019.100419
de Souza KC, Correa LG, da Silva TBV et al (2020) Soy protein isolate films incorporated with Pinhão (Araucaria angustifolia (Bertol.) Kuntze) extract for potential use as edible oil active packaging. Food Bioprocess Technol 13:998–1008. https://doi.org/10.1007/s11947-020-02454-5
ASTM (2001) Standard test methods for tensile properties of thin plastic sheeting D882-00. In: Annual book of ASTM. American Society for Testing and Materials, Philadelphia, PA
Gontard N, Guilbert S, Cuq J-L (1993) Water and glycerol as plasticizers affect mechanical and water vapor barrier properties of an edible wheat gluten film. J Food Sci 58:206–211. https://doi.org/10.1111/j.1365-2621.1993.tb03246.x
ASTM (2000) Standard test methods for water vapor transmission of materials ASTM E96 - 00. In: Annual book of ASTM. American Society for Testing and Materials, Philadelphia, PA
Ke J, Xiao L, Yu G et al (2019) The study of diffusion kinetics of cinnamaldehyde from corn starch-based film into food simulant and physical properties of antibacterial polymer film. Int J Biol Macromol 125:642–650. https://doi.org/10.1016/j.ijbiomac.2018.12.094
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Mensor LL, Menezes FS, Leitão GG et al (2001) Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phyther Res 15:127–130
Thaipong K, Boonprakob U, Crosby K et al (2006) Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compos Anal 19:669–675. https://doi.org/10.1016/j.jfca.2006.01.003
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76. https://doi.org/10.1006/abio.1996.0292
Fernandes RVDB, Borges SV, Botrel DA (2014) Gum arabic/starch/maltodextrin/inulin as wall materials on the microencapsulation of rosemary essential oil. Carbohydr Polym 101:524–532. https://doi.org/10.1016/j.carbpol.2013.09.083
Toledo Hijo AAC, Da Costa JMG, Silva EK et al (2015) Physical and thermal properties of oregano (Origanum vulgare L.) essential oil microparticles. J Food Process Eng 38:1–10. https://doi.org/10.1111/jfpe.12120
Sittipummongkol K, Chuysinuan P, Techasakul S et al (2019) Core shell microcapsules of neem seed oil extract containing azadirachtin and biodegradable polymers and their release characteristics. Polym Bull 76:3803–3817. https://doi.org/10.1007/s00289-018-2456-1
Teodoro RAR, de Barros Fernandes RV, Botrel DA et al (2014) Characterization of microencapsulated rosemary essential oil and its antimicrobial effect on fresh dough. Food Bioprocess Technol 7:2560–2569. https://doi.org/10.1007/s11947-014-1302-1
da Silva JBA, Santana JS, de Almeida LA et al (2019) PBAT/TPS-nanowhiskers blends preparation and application as food packaging. J Appl Polym Sci 136:1–10. https://doi.org/10.1002/app.47699
Garcia PS, Baron AM, Yamashita F et al (2018) Compatibilization of starch/poly(butylene adipate-co-terephthalate) blown films using itaconic acid and sodium hypophosphite. J Appl Polym Sci 135:14–19. https://doi.org/10.1002/app.46629
Orsuwan A, Sothornvit R (2018) Active banana flour nanocomposite films incorporated with garlic essential oil as multifunctional packaging material for food application. Food Bioprocess Technol 11:1199–1210. https://doi.org/10.1007/s11947-018-2089-2
Cardoso LG, Pereira Santos JC, Camilloto GP et al (2017) Development of active films poly (butylene adipate co-terephthalate)—PBAT incorporated with oregano essential oil and application in fish fillet preservation. Ind Crops Prod 108:388–397. https://doi.org/10.1016/j.indcrop.2017.06.058
Pelissari FM, Grossmann MVE, Yamashita F, Pineda EAG (2009) Antimicrobial, mechanical, and barrier properties of cassava starch−chitosan films incorporated with oregano essential oil. J Agric Food Chem 57:7499–7504. https://doi.org/10.1021/jf9002363
Ratanakamnuan U, Aht-Ong D (2006) Photobiodegradation of low-density polyethylene/banana starch films. J Appl Polym Sci 100:2725–2736. https://doi.org/10.1002/app.23048
Andrade-Molina TPC, Shirai MA, Grossmann MVE, Yamashita F (2013) Active biodegradable packaging for fresh pasta. LWT Food Sci Technol 54:25–29. https://doi.org/10.1016/j.lwt.2013.05.011
Rivero S, Giannuzzi L, García MA, Pinotti A (2013) Controlled delivery of propionic acid from chitosan films for pastry dough conservation. J Food Eng 116:524–531. https://doi.org/10.1016/j.jfoodeng.2012.12.025
Sousa GM, Yamashita F, Soares Júnior MS (2016) Application of biodegradable films made from rice flour, poly(butylene adipate-co-terphthalate), glycerol and potassium sorbate in the preservation of fresh food pastas. LWT Food Sci Technol 65:39–45. https://doi.org/10.1016/j.lwt.2015.07.054
Malmir S, Montero B, Rico M et al (2018) Effects of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) microparticles on morphological, mechanical, thermal, and barrier properties in thermoplastic potato starch films. Carbohydr Polym 194:357–364. https://doi.org/10.1016/j.carbpol.2018.04.056
Ghamari MA, Amiri S, Rezazadeh-Bari M, Rezazad-Bari L (2021) Physical, mechanical, and antimicrobial properties of active edible film based on milk proteins incorporated with Nigella sativa essential oil. Polym Bull. https://doi.org/10.1007/s00289-021-03550-y
Cyras VP, Manfredi LB, Ton-That M-T, Vázquez A (2008) Physical and mechanical properties of thermoplastic starch/montmorillonite nanocomposite films. Carbohydr Polym 73:55–63. https://doi.org/10.1016/j.carbpol.2007.11.014
Lendvai L, Apostolov A, Karger-Kocsis J (2017) Characterization of layered silicate-reinforced blends of thermoplastic starch (TPS) and poly(butylene adipate-co-terephthalate). Carbohydr Polym 173:566–572. https://doi.org/10.1016/j.carbpol.2017.05.100
Fraj A, Jaâfar F, Marti M et al (2019) A comparative study of oregano (Origanum vulgare L.) essential oil-based polycaprolactone nanocapsules/ microspheres: preparation, physicochemical characterization, and storage stability. Ind Crops Prod. https://doi.org/10.1016/j.indcrop.2019.111669
Sogut E, Ili Balqis AM, Nur Hanani ZA, Seydim AC (2019) The properties of κ-carrageenan and whey protein isolate blended films containing pomegranate seed oil. Polym Test 77:105886. https://doi.org/10.1016/j.polymertesting.2019.05.002
Ganiari S, Choulitoudi E, Oreopoulou V (2017) Edible and active films and coatings as carriers of natural antioxidants for lipid food. Trends Food Sci Technol 68:70–82. https://doi.org/10.1016/j.tifs.2017.08.009
Terpinc P, Čeh B, Ulrih NP, Abramovič H (2012) Studies of the correlation between antioxidant properties and the total phenolic content of different oil cake extracts. Ind Crops Prod 39:210–217. https://doi.org/10.1016/j.indcrop.2012.02.023
Acknowledgements
The authors thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (Project No. 420055/2018-5) for financial support, Multiuser Laboratory of Federal University—Paraná—Campus Londrina and Food Technology Institute (ITAL)—Campinas—São Paulo.
Funding
This work was financially supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (Project No. 420055/2018-5).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Ana Flávia Sampaio Paulo, Geane Cristiane Balan, Gylles Ricardo Ströher, Paulo Rodrigo Stival Bittencourt, Marly Sayuri Katsuda, Lyssa Setsuko Sakanaka and Fabio Yamashita. The first draft of the manuscript was written by Ana Flávia Sampaio Paulo and Marianne Ayumi Shirai, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paulo, A.F.S., Balan, G.C., Ströher, G.R. et al. Influence of free and microencapsulated oregano oil on starch and poly (butylene co-terephthalate adipate) active film properties. Polym. Bull. 79, 4859–4877 (2022). https://doi.org/10.1007/s00289-021-03743-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03743-5