Abstract
In this work, poly(α-methyl styrene) (PAS) was synthesized by free radical photo-polymerization. The tacticity and microstructure of PAS were studied by 13C nuclear magnetic resonance spectroscopy (13C NMR). Assignments of all stereo sequences were carried out at pentad and hexad levels of quaternary aliphatic, quaternary aromatic, and methylene carbons using 13C NMR in deuterated chloroform at 20 °C and 50 °C, respectively. It was found that by increasing the NMR acquisition temperature, higher resolution and higher splitting of the peaks were achieved for all mentioned carbons. Bernoullian and first-order Markov statistics were used for all carbons and the results were compared with experimental data by statistical method. It was shown that Bernoullian statics model fit slightly better than first-order Markov statics model for the assigned sequences. The results indicated that corresponding values of probability (Pm) was equal to 0.234, suggesting that the racemic addition was almost higher than the meso-one. The effect of the deuterated solvent on the peak resolution and the splitting of syndiotactic and isotactic sequences was investigated by replacing deuterated tetrahydrofuran with deuterated chloroform. It was observed that the peak resolution and the splitting in deuterated chloroform are much more than deuterated tetrahydrofuran.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last decades, α-methyl styrene has been extensively interested by researchers in the blending modification, adhesives formulation, and paper coating due to its low cost and withstanding performance [1,2,3]. Poly(α-methyl styrene) (PAS) is one of the most promising target capsule materials in inertial confinement fusion because of its appropriate thermal degradable [4]. In addition, PAS is used for enhancing the HDT, glass temperature and mechanical properties of PVC [5].
Homopolymerization of α-methyl styrene usually carried out via ionic polymerization [6, 7]. The free-radical polymerization of α-methyl styrene is slow and predominantly yields low molecular weight due to the low ceiling temperature (Tc = 61 °C). The ceiling temperature is related to the strong steric repulsions between substituents in consecutive monomer units in the polymer backbone [8, 9]. Thereupon, the bulk and the solution free radical polymerization of α-methyl styrene gives the polymer with low conversion and molecular weight. In order to minimize these problems, researchers proposed an emulsion technique to synthesize high molecular weight PAS [10,11,12]. However, the emulsion polymerization provides the heterogeneous reactions which afford the presence of impurities in the polymer. These disadvantages could be overcome through the radiation polymerization [13, 14]. Garrett and coworkers [15] report an ESR study of free radicals formed in irradiated PAS by gamma radiation. An electron beam lithographic study of PAS and PAS-Maleic anhydride copolymer was studied by Sharma et al. [16]
Knowledge of polymer microstructures and molecular dynamic motion is crucial to acquire information concerning the relation between structure and properties as well as development industries. It is well known that NMR is one of the best analytical methods by which to attain information on the chemical structure. 13C NMR has prepared stereochemical information on a large number of polymers [17, 18]. Heatley and his coworkers [19, 20] have investigated 13C NMR spectra for several vinyl polymers such as isotactic and atactic polystyrenes. Using 13C NMR, qualitative and quantitative analysis of tacticity can be implemented. Ziaee et al. [22] have studied the effect of temperature on the tacticity of bulk thermal polymerized polystyrene.
Many authors have investigated the stereochemical configuration of PAS by means of 1H NMR. Because of three different configurations, the methyl protons of PAS are split into three peaks [23, 24]. Brownstein et al. [24] assigned these three methyl peaks to isotactic, heterotactic, and syndiotactic triads. The 13C NMR of polystyrene and PAS was investigated by Inoue et al. [25]. They reported that the spectra split related to a triad, tetrad, and partially pentad placements. Comparison between 13C NMR and 1H NMR spectra affirmed the effect of nuclear Overhauser on the relative intensities of the peaks assigned to chemically equivalent carbons except for stereochemical configuration [26,27,28].
This study is designed to investigate PAS tacticity synthesized by photo-polymerization at room temperature. Analysis of all stereo sequences at pentad and hexad levels of the quaternary type of aliphatic, aromatic and methylene carbon was carried out using 13C NMR in different acquisition temperature. Finally, the effect of deuterated solvent on the tacticity of PAS is observed.
Experimental
Material
The α-methyl styrene (containing 4-tert-butylpyrocatechol) was supplied from Merck, Germany. Purification of the monomer was performed by vacuum distillation. Benzophenone (≥ 99%) was purchased from Sigma-Aldrich and was used as received.
Polymerization
The polymerization of α-methyl styrene was carried out by photo-polymerization using ultraviolet light with a wavelength of 254 nm at 20 °C under vacuum condition. For this purpose, after purification, the photo-initiator (BP) and monomer were injected into Pyrex glass ampoules and degassing was performed under vacuum to remove impurities such as oxygen. Then, the solution with low conversion was precipitated into methanol for removing unreacted monomers. The precipitated polymer was dried in a vacuum oven at 40 °C for 24 h.
Characterization
All NMR experiments performed in this work were carried out on a Bruker Avance 400 MHz NMR spectrometer (DMX-400, Germany). The polymer solution were prepared by dissolving 20 mg PAS in 0.5 ml of deuterated solvents. The acquisition temperature set at 20 and 50 °C. The pulse width was 90°, relaxation delay time, 2 s, acquisition time, 1.37 s, number of scans, 20,000, and data points, 64 K.
The molecular weight of PAS was determined by GPC in THF using Agilent 110 (USA). The system was calibrated with narrow molecular weight polystyrene standards ranging from 200 to 106 g/mol.
Results and discussion
In order to microstructure study and sequence determination, the polymer chain length must be long enough. Consequently, the GPC analysis was used to investigate the molecular weight of PAS. According to the GPC results, the number average molecular weight and the weight average molecular weight was equal to 2.25 × 104 g/mol and 4.85 × 104 g/mol, respectively.
13C NMR of PAS
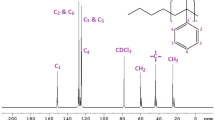
In 13C NMR spectrum, each peak can be correlated to a specific sequence with a special chemical shift. Figure 1 demonstrates these chemical shifts attributed to the α-methyl carbon, methylene carbon, quaternary aliphatic carbon, quaternary aromatic C1 carbon, C2, C3 and C4 carbons. These carbon atoms are surrounded by different isomers in the sequences. Thereupon, the neighbor carbon atoms affect each other. In this work, the study of stereospecificity was carried out on the methylene, quaternary aliphatic, quaternary aromatic and C1 carbons. These assignments were adapted from the work of Elgert et al. [29].
Expanded 13CNMR of quaternary aliphatic carbon
The quaternary aliphatic carbon of PAS is asymmetric and susceptible to the monomer insertion along the molecular chains. Furthermore, the resonance of this carbon represents distinct chemical shifts because of the microstructure. Figure 2a and b shows 13C NMR spectrum of the quaternary aliphatic carbon in CDCl3 at 20 and 50 °C, respectively. As it is seen from Fig. 2a, the quaternary aliphatic carbon split into 6 pentad sequences. The upfield region is rich in isotactic sequences, while the downfield region is rich in syndiotactic ones. In other words, the peaks with assignment number of 1, 2, and 3 are syndiotactic, 4 and 5 are atactic, and 6 is isotactic parts of chain. The experimental data was tested for Bernoullian and first-order Markov statics models of chain propagation and the results are shown in Table 1 and 2. As evident in Fig. 2b, by increasing the NMR acquisition temperature, higher resolution and higher splitting of the peaks are achieved. The upfield region is rich in isotactic and the downfield region is rich in syndiotactic. In the isotactic section, the number of peaks is higher at 50 °C than 20 °C, hence in this section, the heptad sequence is calculated. In statistical subject, probability of meso-(Pm) and racemic (Pr) sequences are defined by Eqs. (1) and (2), respectively. The mm, mr, and rr triad sequences have isotactic, atactic and syndiotactic parts of chain, respectively.
The relations between pentad, triad, and diad are attained by Eqs. (3–5) which finally enable to determine the probability of meso-sequences.
By integrating sequences areas in Fig. 2 and using Eqs. (1–5) the calculated value of Pm for all samples is equal to 0.234, indicating that synthesis PAS samples have some deviation from the ideal random case (Pm = 0.5) and the degree of racemic addition is constantly higher than the meso-addition. This could be related to the stereohindrance of pendant group which tend to stay away from each other and form racemic sequences [30]. However, stereoregularity of chains can be changed by increasing the polymerization temperature and variation of solvent polarity. It is encouraging to compare this finding with that found by Inoue and coworkers [25]. They reported that Pm was, respectively, equal to 0.325 and 0.125 for PAS synthesized via anionic polymerization at −78 °C and cationic polymerization at −50 °C. The value of Pm in these three polymerization methods (< 0.5) suggests that for PAS the racemic addition is almost higher than the meso-one. Therefore, due to the fact that the photo-polymerization at ambient temperature is more straightforward than the anionic and cationic polymerization, this method is recommended for the synthesis of PAS.
In accordance with Bernoullian and first-order Markov statistical model, the polymeric chain formation could be predicted. In first-order Markov model, the new monomer is added to the prior sequence which governs its configuration. first-order Markov probability is given by Eqs. 6 and 7 [22, 31].
Pm/r designates that meso-diad effects the next diad situation as a racemic diad. For instance, Bernoullian and first-order Markov statistics for mrrmm are calculated as 2Pm3Pr3 and 2Pm Pm/r Pr/m Pm/m Pm/r Pr/r, respectively [31, 32]. By using sum of squared difference between two models and experimental data, adjustment of Bernoullian and first-order Markov models can be comprised for all the carbons. According to the tables, for all carbons, Bernoullian statics model can predict formation of monomers in polymer propagation more effectively.
Expanded 13C NMR of quaternary aromatic carbon
13C NMR spectrum of quaternary aromatic carbon in CDCl3 at 20 and 50 °C is shown in Fig. 3a and b, respectively. The quaternary aromatic carbon of PAS is symmetric with different stereospecificity. As it can be seen from Fig. 3a, quaternary aromatic carbon split into 6 pentad sequences. The upfield region in the quaternary aromatic carbon of PAS is rich in isotactic sequences and the downfield region is rich in syndiotactic ones. The peak with assignment number of 1 and 2 are syndiotactic, 3 and 4 are atactic, and 5 is isotactic [33]. Normalized data for the pentad sequences at 20 and 50 °C are presented in Tables 3 and 4, respectively. It can be seen from Fig. 3b that an increase in NMR acquisition temperature, led to an enhancement of peak number from 5 to 8. The upfield region is rich in isotactic and the downfield region is rich in syndiotactic. Also, the overlapping is reduced and the atactic and isotactic sequences split more separately. The sum squared difference between observed and calculated data are highlighted in Tables 3 and 4. The results showed that Bernoullian propagation is fitted pretty well by experimental results.
Expanded 13C NMR of methylene carbon
Figure 4a and b represents 13C NMR spectrum of the methylene carbon (a symmetric carbon) in CDCl3 at 20 and 50 °C, respectively. According to Fig. 4a, methylene carbon split into 11 hexad sequences. As it can be seen from Fig. 4b, by increasing the NMR acquisition temperature, higher resolution and higher splitting of the peaks are attained. Normalized data for the pentad sequences at 20 and 50 °C along with the sum squared difference between observed and calculated data are shown in Tables 5 and 6, respectively. It is found that the measured data is well consistent with calculated values based on Bernoullian statistics.
Effect of THF-d8 solvent
Several parameters can influence the degree of splitting by solvent such as relaxation time, viscosity and solubility. It is worth noting that by increasing relaxation time, higher resolution, and higher peak splitting are achieved. The same phenomenon happened by reducing solvent viscosity and increasing solubility. Figure 5 a and b shows the comparison 13C NMR spectrum of the quaternary aromatic carbon of PAS in CDCl3 and THF-d8, respectively. As can be seen, contrary to the splitting of the quaternary aromatic carbon in THF-d8, in CDCl3 at the syndiotactic section, the peak with assignment number of 2 split into two sequences. Furthermore, peak resolution and the splitting in deuterated chloroform is much more than deuterated tetrahydrofuran. These results are contrary to a similar study on the polystyrene by Ziaee et al. [30] which have reported that splitting of the methylene carbon in THF-d8 is higher in the syndiotactic sequences. However, in this study, it can be concluded that methylene carbon has better splitting in CDCl3 in all isotactic, syndiotactic and atactic sequences than THF-d8. Table 7 shows normalized data for the pentad sequences in THF-d8 at 50 °C. It is suggested that the measured results are in good agreement with Bernoullian statistics values.
Conclusion
The aim of the present research was to synthesize PAS by free radical photo-polymerization and investigate the tacticity and microstructure of PAS using 13C NMR in different temperatures and solvents. The sequences assignments were performed at pentad and hexad levels of quaternary aliphatic, quaternary aromatic and methylene carbons. The results highlight the importance of acquisition temperature on the resolution and the splitting of the sequences. The increase in acquisition temperature led to an enhancement of resolution and number of peaks. Comparison between experimental data and calculated one by Bernoullian and first-order Markov statistics models revealed that Bernoullian statistics fit better than first-order Markov statistics for the assigned carbons. The probability of meso-addition for PAS was equal to 0.234, indicating a deviation from the random ideal case and a higher degree of racemic addition. A comparison between deuterated solvent showed that the peak resolution and the splitting of syndiotactic and isotactic sequences is CDCl3 is much more than THF-d8.
Change history
15 July 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00289-021-03823-6
References
Zimmel JM, Mansour AS, Juers S, Axer V, Holtin U, Nahkala AR, Fuller HB Co, (2019) Hot melt adhesive composition for bonding packs of metal containers. US Patent 10,351,298
Chambers T, Goodyear Tire and Rubber Co, (1974) Adhesive composition containing an aba block copolymer, poly alpha-methyl styrene and a tackifying resin. US Patent 3,784,587
Hu S, Sun W, Fu J, Zhang L, Fan Q, Zhang Z, Wu W, Tang Y (2017) Reactive molecular dynamics simulations on the thermal decomposition of poly alpha-methyl styrene. J Mol Model 23(6):179
Zhang Z, Chen S, Zhang J (2012) A study on properties of poly (vinyl chloride)/poly (α-methylstyrene-acrylonitrile) binary blends. J Macromol Sci Part B 51(1):22–34
Gupta B, Gautam D, Ikram S (2013) Preparation of proton exchange membranes by radiation-induced grafting of alpha methyl styrene–butyl acrylate mixture onto polyetheretherketone (PEEK) films. Polym bull 70(10):2691–2708
Halasa AF, Seo KS (2014) Anionic solution copolymerization of α-methylstyrene with conjugated dienes above the ceiling temperature of α-methylstyrene. Eur Polym J 51:80–86
Banerjee S, Paira TK, Mandal TK (2013) Control of molecular weight and tacticity in stereospecific living cationic polymerization of α-methylstyrene at 0 °C Using FeCl3-based initiators: effect of tacticity on thermal properties. Macromol Chem Phys 214(12):1332–1344
Wang W, Lu W, Goodwin A, Wang H, Yin P, Kang NG, Hong K, Mays JW (2019) Recent advances in thermoplastic elastomers from living polymerizations: macromolecular architectures and supramolecular chemistry. Prog Polym Sci 95:1–31
Li Y, Knauss DM (2018) Sequential bulk anionic polymerization of α-methylstyrene and isoprene to form diblock and triblock copolymers. Macromol Chem Phys 219(1):1700449
Nogueira RF, Tavares MIB (2001) Carbon-13 NMR study of poly (alpha–methylstyrene). Polym Test 20(4):379–382
Chen Q, Liu M, Pan D, Chen S, Shi R, Qi X, Zhang Z, Li B (2018) Effects of n-hexadecane on sphericity of poly-α-methylstyrene shells. Colloid Surface A 554:1–8
Shangguan X, Chen S, Ma S, Liu M, Tang C, Yi Y, Zhang Z (2017) Effect of molecular weight on the quality of poly (alpha-methylstyrene) mandrel. J Rheol 2(4):197–203
Gubler L, Slaski M, Wallasch F, Wokaun A, Scherer GG (2009) Radiation grafted fuel cell membranes based on co-grafting of α-methylstyrene and methacrylonitrile into a fluoropolymer base film. J Membrane Sci 339(1–2):68–77
Hu G, Wang Y, Ma J, Qiu J, Peng J, Li J, Zhai M (2012) A novel amphoteric ion exchange membrane synthesized by radiation-induced grafting α-methylstyrene and N, N-dimethylaminoethyl methacrylate for vanadium redox flow battery application. J Membrane Sci 407:184–192
Garrett RW, Hill DJ, Le TT, O'Donnell JH, Pomery PJ (1992) An ESR study of free radicals formed in irradiated poly (alpha-methyl styrene) by gamma-radiation. Radiat Phys Chem 39(2):215–221
Sharma VK, Affrossman S, Pethrick RA (1984) Poly (α-methylstyrene) and α-methylstyrene-maleic anhydride copolymer: an electron beam lithographic study. Polymer 25(8):1087–1089
Zhang X, Zhang C, Wang Y, Li Y (2010) Synthesis and characterization of symmetrical triblock copolymers containing crystallizable high-trans-1, 4-polybutadiene. Polym bull 65(3):201–213
Ziaee F, Ronagh-Baghbani M, Jozaghkar MR (2019) Microstructure characterization of low molecular weight polybutadiene using the chain end groups by nuclear magnetic resonance spectroscopy. Polym Bull 77:2345–2365
Britton D, Heatley F, Lovell PA (1998) Chain transfer to polymer in free-radical bulk and emulsion polymerization of vinyl acetate studied by NMR spectroscopy. Macromolecules 31(9):2828–2837
Ahmad NM, Heatley F, Britton D, Lovell PA (1999) Chain transfer to polymer in emulsion polymerization. Macromol Sy 143(1):231–241
Britton D, Heatley F, Lovell PA (2001) 13C NMR spectroscopy studies of branching and sequence distribution in copolymers of vinyl acetate and n-butyl acrylate prepared by semibatch emulsion copolymerization. Macromolecules 34(4):817–829
Ziaee F, Nekoomanesh M, Mobarakeh HS, Arabi H (2008) The effect of temperature on tacticity for bulk thermal polymerization of styrene. E-Polymers. https://doi.org/10.1515/epoly.2008.8.1.466
Brownstein S, Bywater S, Worsfold DJ (1961) The proton resonance spectra of poly-α-methylstyrenes. Macromol Chem Phys 48(1):127–134
Sakurada Y, Matsumoto M, Imai K, Nishioka A, Kato Y (1963) Proton magnetic resonance spectra of poly-α-methylstyrene. J Polym Sci Pol Lett 1(11):633–639
Inoue Y, Nishioka A, Chûjô R (1972) Carbon-13 nuclear magnetic resonance spectroscopy of polystyrene and poly-α-methylstyrene. Macromol Chem Phys 156(1):207–223
Parella T, Espinosa JF (2013) Long-range proton–carbon coupling constants: NMR methods and applications. Prog Nucl Mag Res Sp 73:17–55
Yazgan I (2020) NMR-based structural characterization of common aromatic poly(amic acid) polymers. Polym Bull 77(3):1191–1203
Vögeli B (2014) The nuclear overhauser effect from a quantitative perspective. Prog Nucl Mag Res Sp 78:1–46
Elgert KF, Wicke R, Stützel B, Ritter W (1975) On the structure of poly (α-methylstyrene): 13C nmr spectra at 67.88 and at 90.51 MHz. Polymer 16(6):465–467
Ziaee F, Salehi MH (2011) Effect of temperature on polystyrene tacticity through para aromatic carbon splitting in 13C NMR spectroscopy. Iran Polym J 20(3):213–221
Odian G (2004) Principles of polymerization. Wiley, New York
Tonelli AE (2017) From NMR spectra to molecular structures and conformation. stereochemistry and global connectivity: the legacy of ernest L. Eliel 2:161–190
Ziaee F, Bouhendi H (2009) NMR study of polyacrylamide tacticity synthesized by precipitated polymerization method. Iran Polym J 18(12):947–956
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jozaghkar, M.R., Ziaee, F. & Azar, A.S. Investigation of poly(α-methyl styrene) tacticity synthesized by photo-polymerization. Polym. Bull. 78, 5303–5314 (2021). https://doi.org/10.1007/s00289-020-03381-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03381-3