Abstract
A series of symmetrical triblock copolymers containing crystallizable high-trans-1,4-polybutadiene (HTPB) were synthesized by sequential anionic polymerization of 1,3-butadiene (Bd) with isoprene (Ip) (or styrene (St)) using barium salt of di(ethylene glycol) ethyl ether/triisobutylaluminium/dilithium (BaDEGEE/TIBA/DLi) as initiation system. The microstructures of the symmetrical triblock copolymers were determined by IR, 1H NMR, and 13C NMR. The results indicated that polyisoprene-block-high-trans-1,4-polybutadiene-block-polyisoprene (IBI) contained HTPB segments and medium 3,4-polyisoprene (PI) segments, and polystyrene-block-HTPB-block-polystrene (SBS) contained HTPB and atatic-polystyrene (PS) segments. The DSC analysis revealed that SBS tended to phase separate but IBI did not. The cold crystallization was observed in IBI but not in SBS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Block copolymers often exhibit unique and useful properties in solution and in solid states as a consequence of the general thermodynamic incompatibility of blocks which results in microphase separation into domains [1, 2].
Block copolymers containing polybutadiene (PB) segments are among the most important rubbery materials and are industrial produced by emulsion [3] and solution polymerizations [4]. Due to the development of the catalysts which are effective in controlling the stereoregularity of PB, polystyrene (PS), or polyisoprene (PI) segments, it is possible to synthesize stereoregular block copolymers containing PB segments. Recent achievements in the stereospecific copolymerization of St and Bd with transition-metal catalysts have controlled the cis-1,4 selectivity of PB segments [5–10]. However, none of these catalysts are able to effectively control the stereoregularity of PS segments. Further more, copolymers with both cis-1,4-PB segments and syn-PS segments were achieved by Naga [11] and Zambelli [12], who used CpTiCl3/methylaluminoxane (MAO; Cp = cyclopentadienyl, indenyl, or pentamethylcyclopentadienyl) as catalysts. Nevertheless, because of the lack of livingness in these systems, no true block products were obtained. In fact, syn-PS-b-cis-1,4-PB copolymers were achieved by Ban et al. [13], who used C5Me5TiMe3/B(C6F5)3/Al(oct)3 as catalyst, and Caprio et al. [14], who used CpTiX3 (Cp = C5H5, X = Cl, F; Cp = C5Me5, X = Me) and TiXn (n = 3, X = acetylacetonate (acac); n = 4, X = t-BuO) activated by MAO.
To sum up, because of the industrial importance [15], many studies have been devoted to cis-1,4-PB while trans-1,4-PB has received rather limited attention [16, 17]. More recently, late transition metal based catalysts have been achieved for the synthesis of trans-1,4-PB with high selectivity (>95%) and narrow molecular weight distributions [16, 18]. The discovery of such single-site catalysts has renewed the interest in synthesis and use of trans-1,4 selective catalysts also for their outstanding performances in the copolymerization of Bd with olefins [19–21]. For example, high trans-1,4-Bd/Ip copolymers were synthesized with a TiCl4/MgCl2-Al(i-Bu)3 catalyst system [22]. Iso-PS-co-trans-1,4-PB with an unprecedented architecture, covering a wide range of compositions (x S = 0.15-0.97), were also obtained with octahedral Titanium catalyst [23]. However, because of the lack of livingness, only block-like or multiblock copolymers were obtained under specific polymerization conditions.
Our strategy for synthesizing block copolymers containing high-trans-1,4-PB (HTPB) segments is derived from the development of a new living anionic polymerization initiation system that is able to polymerize Bd to HTPB [24]. The initiation system is comprised of (a) an organolithium compound, (b) a group IIA metal salt, (c) an organoaluminium compound, and (d) an amine compound.
In this study, symmetrical triblock copolymers were first prepared by sequential anionic polymerization of Bd with Ip or St (Scheme 1) using barium salt of di(ethylene glycol) ethyl ether/triisobutylaluminium/dilithium (BaDEGEE/TIBA/DLi) as initiation system. The copolymers contained crystallizable HTPB segments and hard segments (PS) or soft segments (PI). The microstructures and macromolecular architectures were determined by IR, NMR, and size exclusion chromatography (SEC). The thermal behaviors were recorded by differential scanning calorimetry (DSC).
Experimental
Materials
1,3-Butadiene (Bd, polymerization grade, Beijing Yanshan Petrochemical Co., China) was purified with a small amount of sec-butyllithium (s-BuLi) and then vaporized to keep the water content below 10 ppm.
Styrene (St, polymerization grade, Beijing Yanshan Petrochemical Co., China) was dried over CaH2 for 12 h and distilled under reduced pressure before use.
Isoprene (Ip, polymerization grade, Puyang Xinyu Petrochemical Industry Co., Ltd., China) was refluxed for about 1 h over CaH2, and then distilled and stored over molecular sieves (5Å) under highly purified nitrogen (N2).
s-BuLi (1.3 M solution in cyclohexane/hexane (92/8), Acros Organics Co., Geel, Belgium) and triisobutylaluminium (TIBA, 1.1 M solution in toluene, Acros Organics Co., Geel, Belgium) were diluted in dry cyclohexane under highly purified N2, respectively. The concentration of s-BuLi was calibrated by Gilman double titration method [25]. The concentration of TIBA was calibrated by EDTA complexation titration method [26].
Cyclohexane (analytical reagent, Liaoyang Petrochemical Co., China) was dried and kept over molecular sieves (5Å) to keep water content below 5 ppm, and then it was purged with highly purified N2 for more than 15 min prior to use to keep oxygen content below 10 ppm.
Barium salt of di(ethylene glycol) ethyl ether (BaDEGEE) was synthesized according to the literature procedure [27].
Synthesis of dilithium
The synthesis of dilithium was described in the literatures [28, 29]. Further more, several Ip units were added to C–Li+ bond to enhance the solubility of the dilithium in cyclohexane (as shown in Scheme 2). The dilithium we adopted in the polymerization was simplified as DLi. The concentration of DLi was approximately 0.05 mol/L determined by the double titration method of Gilman.
Polymerization procedure
All the operations were conducted under highly purified N2 atmosphere. A typical anionic polymerization reaction was performed in a glass reactor connected to the Schlenk line and equipped with an inert gas (N2) inlet and a rubber septum. The glass assembly was dried with 3 cycles of a flaming/N2-purging/evacuating, before adding all the reagents.
The trans-specific living polymerization of Bd was conducted in a 300 mL, press-resistant glass reactor at 50 °C with cyclohexane as solvent. The sequential block copolymerization of Bd with St (or Ip) was conducted in a 300 mL, pressure-resistant glass reactor at 50 °C for the first step and at 70 °C for the second step with cyclohexane as solvent. A typical Bd and St (or Ip) sequential block polymerization was carried out as follows. A measured amount of Bd (10.4 g) in cyclohexane (200 mL) was introduced into the reactor, the polymerization was started by the addition of BaDEGEE (0.52 mmol), TIBA (2.08 mmol), and DLi (2.08 mmol) in that order at 50 °C. After 3 h of polymerization, a small mount of the polymer solution was taken out for the evaluation of microstructures and molecular weights of the corresponding polymers. Then the polymerization temperature was lowered to 20 ± 1 °C and 10.0 g of St (or Ip), which had been purified by s-BuLi, was added to the reactor. The polymerization proceeded for another 3 h at 70 °C. After finishing the polymerization, the symmetrical triblock polymers were terminated by a small amount of degassed isopropanol containing 2.5% w/v 2,6,4-antioxidant, precipitated by the addition of an excess amount of neat ethanol, and then dried in a vacuum oven at 40 °C.
Characterizations
IR. Infrared (IR) spectra of the polymers were recorded on a Nicolet FTIR spectrophotometer (USA) with films on NaCl discs.
DSC. The differential scanning calorimetry (DSC) measurements were conducted with a NETZSCH DSC 204 instrument (Germany). The calorimeter was calibrated with indium standard. About 8–10 mg samples were used at a heating rate of 10 °C min−1 under a flow of N2 (20 mL min−1).
SEC. The molecular weight (Mn) and molecular weight distribution (PDI) of the samples were measured by size exclusion chromatography (SEC) in tetrahydrofuran (THF) at 30 °C. The measurements were performed using a Viscotek TDA-302 SEC (Viscotek Co., Houston, TX, USA), equipped with tetra detectors [refractive index (RI), UV, viscosity (VISC), and two-angle laser light scattering (7ο and 90ο, laser wavelength, λ = 670 nm)]. And the molecular weight was determined by light scattering. PS sample (Viscotek Co.) with a stated peak molecular weight of 99,500 g mol−1 and a PDI of 1.03 was used to calibrate the instrument. The value of dn/dc and intrinsic viscosity of this standard are 0.185 and 0.477 dL g−1, respectively. Two Viscogel-mixed bed columns (GMMXL, GMLXL, with a linear range of molecular weights from 103 to 107 g mol−1) were employed; THF was used as the mobile phase at a flow rate of 1.0 mL min−1, and the column temperature was 30 °C. The samples were dissolved in THF with the sample concentrations of 2.0–3.0 mg mL−1, and 100 μL of such solution was injected to start data collection. The data obtained were analyzed using OmniSEC software version 4.5 (Viscotek Co.).
NMR. The polymers were analyzed by 1H NMR and 13C NMR spectroscopy on a Varian Inova (USA) 400 MHz NMR spectrometer in CDCl3 at ambient temperature at a concentration of 4% w/v (for 1H NMR) and 15% w/v (for 13C NMR), respectively. The chemical shifts were recorded as δ values (ppm) relative to internal tetramethylsilane (TMS) in CDCl3.
Results and discussion
The synthesis of the symmetrical triblock copolymers
Polyisoprene-block-high-trans-1,4-polybutadiene-block-polyisoprene (PI-b-HTPB -b-PI, simplified as IBI) with different PI mass fractions (x I) was synthesized. The results are shown in Table 1. Polystyrene-block-high-trans-1,4-polybutadiene-block-polystyrene (PS-b-HTPB-b-PS, simplified as SBS) with different PS mass fractions (x S) was synthesized. The results are shown in Table 2.
Figure 1 shows the SEC traces of HTPB-1, IBI-5, HTPB-2, and SBS-5. From Fig. 1, we can conclude that well-defined symmetrical triblock copolymers were obtained.
The x I was designed at 10, 20, 30, 40, and 50 wt%, and the value was determined by 1H NMR [22] and SEC, respectively. The x S was designed at 10, 20, 30, 40, and 50 wt%, and the value was determined by IR [30] and SEC, respectively. According to Canto [30], the value determined by SEC was calculated based on Eq. 1.
Figure 2 shows the comparison of the values of x I (or x S) determined by 1H NMR (or IR for x S) and SEC. The values of x I determined by 1H NMR are bigger than that determined by SEC, but the values of the two methods agree well with each other. The values of x S (except sample SBS-1) determined by IR are smaller than that determined by SEC; however, the values of the two methods coincide well with each other.
The IR spectra of typical IBI and SBS, as well as that of the starting homopolymer HTPB are presented in Fig. 3. The absorption peaks in copolymers spectra are the combination of those of the two corresponding components in them. As marked in Fig. 3, the bands at 740, 911, and 966 cm−1 represent cis-1,4, 1,2, and trans-1,4 of Bd units, respectively, the bands at 840 and 889 cm−1 represent 1,4 and 3,4 of Ip units, respectively, and the bands at 699 and 750 cm−1 represent St units. As shown in Fig. 3, the relative peak intensity of trans-PB is high in all polymers, which indicates high content of trans-PB. As in SBS, the absorption at 966 cm−1 could be used to analyze x S because the contents of trans-PB of different types of SBS are very close [30] (Table 2).
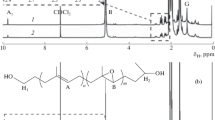
The 1H NMR spectra of typical IBI and SBS as well as that of the starting homopolymer HTPB are presented in Fig. 4. As shown in Fig. 4, the peaks appearing at 4.85–4.98 ppm indicate the few content of 1,2-Bd units, and the peaks appearing at 5.35–5.41 ppm indicate the high content of 1,4-Bd units. The chemical composition of IBI was determined by 1H NMR. The absent peak at 5.70 ppm which represent 1,2-Ip units indicates that IBI dose not contain any 1,2-Ip units. The peaks at 4.60–4.80 and 5.00–5.20 ppm represent 3,4-Ip and 1,4-Ip units, respectively. Judging from the intensity ratio of the olefinic protons, we estimated that the sample IBI-5 contained about 51 wt% Ip units and 49 wt% Bd units. It can conclude from Fig. 4 that the appearance of the peaks at 6.50 and 7.10 ppm indicates the block nature of SBS [31].
The 13C NMR spectrum (olefinic region) of typical SBS is presented in Fig. 5. The observation of the weak peaks at 114.6 and 143.0 ppm indicates that the polymer contains low 1,2-Bd units [32, 33]. However, the peaks (at 129.5 and 130.2 ppm) assigned to cis-PB and trans-PB are predominantly observed, and the intensity of trans-PB is much larger than that of cis-PB, which represents high trans-1,4 content. The pentad aromatic carbon derived from the aromatic ring is an asymmetric carbon and is sensitive to the monomer insertion in the macromolecular chains. Three main peaks with small peaks and shoulders are seen in Fig. 5. The assignments of the pentad sequences as shown in Fig. 5 very nearly obey Bernoullian statistics [34]. It is clear to identify the sample SBS-5 as an atactic PS (ata-PS) according to Fig. 5.
Basic thermal behavior
The thermal behaviors of IBI block copolymers and corresponding homopolymer HTPB are shown in Fig. 6 and Table 3. The molecular mobility can be well characterized by the position of glass transition temperature (T g) of the components. It is obvious that a significant shift of T g of IBI toward higher temperature occurs in the order IBI-1 → IBI-2 → IBI-3 → IBI-3 → IBI-5. Generally, the main factors affecting the values of T g are molecular weight and polymer structure [35]. In our case, the molecular weight of IBI is far above 10,000 g mol−1 where the molecular weight has only a negligible influence on the position of T g. Therefore, the clarification of the shifting of T g should be found in the difference of interfacial structures. The components tend to phase miscible because of the thermodynamic compatibility (solubility parameter δPB = 8.38 and δPI = 8.35 (cal cm−3)0.5) [36]. Due to the irregular structures at the interface, a part of PB segments is seriously restricted in mobility and hence as flexible components only at elevated temperatures. The shift of the glass transition should be explained by an increased amount of PI segments (higher T g than that of PB segments) that are ‘dissolved’ in the PB matrix [37].
The melting temperature (T m) of HTPB-1 is observed at about 3 °C, which indicates the crystallization of the polymer. An exothermic peak corresponding to cold crystallization (T cc) is observed above T g when x I is bigger enough. It has been reported that high cis-PB (more than 95% cis-1,4 content) exhibited cold crystallization [38, 39]. Crystallization of a polymer in its melt state involves transport and rearrangement of entangled chains in the vicinity of the crystal front. The strong tendency of induced nucleation might be related to the strain of entangled chains at the crystal front in a manner similar to strain-induced crystallization of cross-linked rubber [38]. Therefore, the cold crystallization peaks are introduced by the entanglement of crystallizable HTPB segments and soft PI segments. When the sample is quenched, there is no enough time for the crystallizable chain to reorganize. After being heated above T g, the sample exhibits rubbery and the frozen chains become free. The crystallizable HTPB segments become crystal because of being induced by soft PI (with x I bigger enough) to reorganize into regular structure. The soft PI segments promote the segmental motion of crystallizable HTPB segments.
The thermal behaviors of SBS block copolymers and corresponding homopolymer HTPB are shown in Fig. 7 and Table 4. As shown in Fig. 7, the glass transition temperature of PB phase (T g,B) remains unchanged, while it appears ambiguous as the x S increased. It is obvious that the glass transition temperature of PS phase (T g,S) appears when x S is bigger than 29% (sample SBS-3, SBS-4, and SBS-5). A significant shift of T g,S toward higher temperature occurs in the sequence SBS-3 → SBS-4 → SBS-5. The components tend to phase separate because of the thermodynamic incompatibility (solubility parameter δPB = 8.38 and δPs = 9.10 (cal cm−3)0.5) [36]. The shift of T g,S toward lower temperature is due to the increasing intermixing of soft PB segments into the PS domains.
In addition, no cold crystallization is observed in SBS in Fig. 7. The absence of cold crystallization in SBS can be explained as the reason for the formation of cold crystallization in IBI. When the hard PS segments are used instead of soft PI segments, the crystallizable HTPB segments are seriously restricted in mobility and hence can not reorganize to regular structure.
Conclusion
The sequential anionic copolymerization of Bd and Ip (or St) was achieved with ternary initiation system BaDEGEE/TIBA/DLi to give trans-1,4-Bd units, medium 3,4-Ip units, and ata-PS units. Since soft PI segments could promote the mobility of cystallizable trans-PB segments, the cold crystallization was observed in IBI. However, there was no cold crystallization in SBS because the hard PS segments could not induce the trans-PB segments to crystallize. The thermodynamic incompatibility of PB and PS segments made SBS tend to phase separate. In contrary, the thermodynamic compatibility of PB and PI segments made IBI tend to phase miscible.
References
Hsieh HL, Quirk RP (1996) Anionic polymerization: principles and practical applications. Marcel Dekker, Inc., New York
Bates FS, Fredrickson GH (1990) Block copolymer thermodynamics: theory and experiment. Annu Rev Phys Chem 41:525–557
Lovell PA, El-Aasser MS (1997) Emulsion polymerization and emulsion polymers. Wiley, New York
Szwarc M (1998) Living polymers. Their discovery, characterization, and properties. J Polym Sci A 36:9–15
Zambelli A, Proto A, Longo P, Oliva L (1994) Binary copolymerizations of styrene and conjugated diolefins in the presence of cyclopentadienyltitanium trichloride-methylaluminoxane. Macromol Chem Phys 195:2623–2631
Kobayashi E, Hayashi N, Aoshima S, Furukawa J (1998) Homo- and copolymerization of butadiene and styrene with neodymium tricarboxylate catalysts. J Polym Sci A 36:241–247
Endo K, Matsuda Y (1999) Copolymerization of styrene and butadiene with Ni(acac)2-methylaluminoxane catalyst. J Polym Sci A 37:3838–3844
Kobayashi E, Kaita S, Aoshima S, Furukawa J (1995) Copolymerization of butadiene and styrene with a gadolinium tricarboxylate catalyst. J Polym Sci A 33:2175–2182
Nakamura N, Yamaguchi Y, Endo K (2003) Synthesis of high molecular weight copolymer of styrene and butadiene bearing high 1,4-cis butadiene unit from copolymerization with CpTiCl3/methylaluminoxane catalyst. J Appl Polym Sci 88:2942–2946
Kaita S, Hou Z, Wakatsuki Y (2001) Random- and block-copolymerization of 1,3-butadiene with styrene based on the stereospecific living system: (C5Me5)2Sm(μ-Me)2AlMe2/Al(i-Bu)3/[Ph3C][B(C6F5)4]. Macromolecules 34:1539–1541
Naga N, Imanishi Y (2003) Copolymerization of styrene and conjugated dienes with half-sandwich titanium(iv) catalysts: the effect of the ligand structure on the monomer reactivity, monomer sequence distribution, and insertion mode of dienes. J Polym Sci A 41:939–946
Zambelli A, Grassi A, Caprio M, Bowen DE (2000) European Patent Appllication EP 1,013,683
Ban HT, Tsunogae Y, Shiono T (2005) Stereospecific sequential block copolymerizations of styrene and 1,3-butadiene with a C5Me5TiMe3/B(C6F5)3/Al(oct)3 catalyst. J Polym Sci A 43:1188–1195
Caprio M, Serra MC, Bowen DE, Grassi A (2002) Structural characterization of novel styrene-butadiene block copolymers containing syndiotactic styrene homosequences. Macromolecules 35:9315–9322
Porri L, Giarrusso A, Ricci G (1991) Recent views on the mechanism of diolefin polymerization with transition metal initiator systems. Prog Polym Sci 16:405–441
Nakayama Y, Baba Y, Yasuda H, Kawakita K, Ueyama N (2003) Stereospecific polymerizations of conjugated dienes by single site iron complexes having chelating n,n,n-donor ligands. Macromolecules 36:7953–7958
Colamarco E, Milione S, Cuomo C, Grassi A (2004) Homo- and copolymerization of butadiene catalyzed by an bis(imino)pyridyl vanadium complex. Macromol Rapid Commun 25:450–454
Gromada J, le Pichon L, Mortreux A, Leising F, Carpentier JF (2003) Neodymium alk(aryl)oxides-dialkylmagnesium systems for butadiene polymerization and copolymerization with styrene and glycidyl methacrylate. J Organomet Chem 683:44–45
Bonnet F, Visseaux M, Barbier-Baudry D, Dormond A (2002) Copolymerization of isoprene with nonconjugated α,ω-dienes using a single component samarocene catalyst. Macromolecules 35:1143–1145
Bonnet F, Visseaux M, Pereira A, Bouyer F, Barbier-Baudry D (2004) Stereospecific polymerization of isoprene with Nd(BH4)3(THF)3/MgBu2 as catalyst. Macromol Rapid Commun 25:873–877
Thuilliez J, Monteil V, Spitz R, Boisson C (2005) Alternating copolymerization of ethylene and butadiene with a neodymocene catalyst. Angew Chem Int Ed 44:2593–2596
He A, Huang B, Jiao S (2003) Synthesis of a high-trans-1,4-butadiene/isoprene copolymers with supported titanium catalysts. J Appl Polym Sci 89:1800–1807
Milione S, Cuomo C, Capacchione C, Zannoni C, Grassi A, Proto A (2007) Stereoselective polymerization of conjugated dienes and styrene-butadiene copolymerization promoted by octahedral titanium catalyst. Macromolecules 40:5638–5643
Halasa AF, Hsu W-L, Jasiunas CA, Zuppo JR (2008) US Patent, US 7,321,017
Gilman H, Haubein AH (1944) The quantitative analysis of alkyllithium compounds. J Am Chem Soc 66:1515–1516
Yuan L, He R (1979) Organo aluminium compounds. People Press, Bejing
Zhang X, Zhang C, Li Y, Guo H, Song S, Wang Y (2009) Synthesis of trans-polybutadiene by BADEGEE/TIBA/n-BuLi initiation system. Petrochem Technol 38:316–321
Tung LH, Lo GY-S (1994) Studies on dilithium initiators. 1. Hydrocarbon-soluble initiators 1,3-phenylenebis(3-methyl-1-phenylpentylidene)dilithium and 1,3-phenylenebis[3-methyl-1-(methylphenyl)pentylidene]dilithium. Macromolecules 27:2219–2224
Lo GY-S, Otterbacher EW, Pews RG, Tung LH (1994) Studies on dilithium initiators. 4. Effect of structure variations. Macromolecules 27:2241–2248
Canto LB, Mantovani GL, DeAzevedo ER, Bonagamba TJ, Hage E, Pessan LA (2006) Molecular characterization of styrene-butadiene-styrene block copolymers (SBS) by GPC, NMR, and FTIR. Polym Bull 57:513–524
Kato N, Harada M, Miyagi A (1991) Styrene sequence distribution of styrene-butadiene copolymers. Anal Sci 7:1605–1608
Van der Velden G, Didden C, Veermans T, Beulen J (1987) New method for the microstructure determination of polybutadiene with cis-1,4, trans-1,4, and vinyl-1,2 units by 13C NMR. Macromolecules 20:1252–1256
Wang HT, Bethea TW, Harwood HJ (1993) Carbon-13 NMR spectra of isomerized polybutadienes. Macromolecules 26:715–720
Kawamura T, Toshima N, Matsuzaki K (1994) Comparison of 13C NMR spectra of polystyrenes having various tacticities and assignment of the spectra. Macromol Rapid Commun 15:479–486
Andrews RJ, Grulke EA (1999) Glass transition temperatures of polymers. In: Brandrup J, Immergut H (eds) Polymer handbook, 4th edn. Wiley, New York
Grulke EA (1999) Solubility parameter values. In: Brandrup J, Immergut H (eds) Polymer handbook, 4th edn. Wiley, New York
Pochan JM, Beatty CL, Pochan DF (1979) Different approach for the correlation of the Tg of mixed amorphous systems. Polymer 20:879–886
Cheng TL, Su AC (1993) Spherulites of long-chain branched cis-1,4-polybutadiene. Macromolecules 26:7161–7166
Su T-K, Mark JE (1977) Effect of strain-induced crystallization on the elastomeric properties of cis-1,4-polybutadiene networks. Macromolecules 10:120–125
Acknowledgments
This work was financially supported by the National Nature Science Foundation of China (NNSFC 20674007 & 20774015) and China National Petroleum Corporation Innovation Fund (No. 05E7006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X., Zhang, C., Wang, Y. et al. Synthesis and characterization of symmetrical triblock copolymers containing crystallizable high-trans-1,4-polybutadiene. Polym. Bull. 65, 201–213 (2010). https://doi.org/10.1007/s00289-009-0192-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-009-0192-2