Abstract
In the present study, bio-functional edible films were developed by incorporating different concentrations (0, 3, 6, 9, 12, and 15%) of orange peel powder (OPP) into the gelatin. The thickness, moisture content, and water vapor permeability of the gelatin films enhanced as the content of incorporated OPP increased. OPP-incorporated films showed lower L* value, but higher a*, b*, and opacity values. Films with higher concentration of OPP showed higher strengths and lower elongation properties. The surface and cross-sectional microstructure of films were also characterized by scanning electron microscopy. The antioxidant properties of films including total phenolic content, ABTS radical scavenging activity, and reducing power were significantly improved by enriching with OPP. The incorporation of OPP also increased the antimicrobial activity of gelatin films against Staphylococcus aureus and Escherichia coli. These results suggested that the orange peel has a good potential to be incorporated into gelatin to produce bioactive packaging films which will be helpful to maintain the quality of the packaged food products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In the last years, extensive studies are conducted to develop functional food film as it outstrips the basic containment and protection functions of the traditional packaging film [1]. In addition, it enables the interaction among the packaging, food product, and the internal and external environments for improving the organoleptic attributes, maintenance of food safety, and quality to extend the shelf life of foods [2, 3]. These purposes are achieved by incorporating an active ingredient such as antimicrobials and antioxidant compounds into the packaging materials. Moreover, most of the food packaging materials are made of non-degradable petrochemical polymers which represent serious environmental issues [4]. Therefore, biodegradable materials, polymers, and biopolymers have been increasingly suggested as suitable materials to substitute conventional plastic food packaging to overcome health concerns and environmental problems [5,6,7,8]. Among the biopolymers, proteins derived from various sources are widely employed for the fabrication of biodegradable films thanks to their compatibility, renewability, non-toxicity, and excellent film-creating character [9].
Fish gelatin, a natural polymer which is isolated from the hydrolysis of collagen can be obtained from the waste of skin and bone generated from the fish processing industry [10], is an interesting alternative of mammalian gelatin, because it does not have any safety matter such as spongiform encephalopathy related to the mammalian gelatin [11]. In particular, fish gelatin is a suitable biodegradable polymer for the fabrication of edible films thanks to its great film-forming characteristics [12] and excellent oxygen barrier property, which is the primary disadvantage of gelatin films when used as packaging material [13]. Moreover, fish gelatin films show good water barrier and hydrophobicity compared to the mammalian gelatin-based film which can be attributed to its amino acid blends [2, 14].
There has been an increasing interest in the incorporation of bioactive compounds from by-products such as apple skin phenols [15], tea polyphenol [16], and pomegranate peel powder [2], in biopolymers as alternative sources to improve the functionality of bio-based packaging materials. In this regard, in the present study the orange peel was used for enriching of the gelatin-based films. Million tons of oranges are produced every year around the world that produce peels and seeds as by-products of the industrial processing or human consumption, accounting for almost half of the entire fruit weight [17]. These by-products are considered as valuable sources of ingredients such as pectin and essential oils. Thus, further use of orange by-products may be effective in recycling valuable compounds and reducing environmental pollution [3]. Traditionally, orange peel has been processed for application in food, drug, and cosmetics [18]. The extracts of orange peels are a good source of vitamins and dietary fibers [19] as well as other biologically active ingredients such as flavonoids and phenolic acids, which demonstrate antioxidant, anti-inflammatory, anti-carcinogenic, and anti-atherosclerosis activities [20]. Prior investigations have also illustrated that orange peel contains active ingredients oils including limonene, myrcene, α-farnesene, γ-terpinene, α-pinene, β-pinene, and α-terpinolene showing antimicrobial activity [21].
To the best of our knowledge, there was no research in the literature on using orange peel powder (OPP) for the enrichment of edible films made of gelatin. Therefore, in this study, our challenge was to develop active antioxidant films with fish gelatin and OPP. Accordingly, the aim of the current study was to fabricate active films made of fish gelatin functionalized with different concentrations of OPP. The effect of enriching with OPP on the physicochemical, structural, mechanical, antioxidant, and antimicrobial characteristics of the resulting bio-functional films was also investigated by different techniques.
Materials and methods
Materials
The fish gelatin was obtained from Sigma-Aldrich (St. Louis, MO, USA). Glycerol (analytical grade), ABTS [2,2-azinobis(3-ethylbenzothiazoline-6-sulphonate)], Folin–Ciocalteu reagent, and gallic acid were all purchased from Merck Chemicals Co. (Darmstadt, Germany). All of the other chemicals also were supplied from Sigma-Aldrich and Merck.
Preparation of OPP
The orange fruit was obtained from a local supermarket in Karaj, Iran. The orange fruit was peeled off and chopped into smaller parts, then they were dried in an oven at 30 °C for 24 h, and dry peels were powdered using the grinder. The ground orange peel powder was then passed through 80-mesh sieve size to obtain fine particles. Finally, the OPP was kept in a freezer to further uses.
Films preparation
The film-forming solutions were prepared by dispersing 6% (w/v) gelatin in distilled water at 50 °C and a mechanical stirring of 500 rpm. After 30 min, glycerol was added at a constant concentration (30%, w/w based on the gelatin) to film solution and stirred for further 30 min. After that, the OPP with concentrations from 3 to 15% (w/w based on gelatin weight) was added into the film solutions and stirred at 400 rpm for an additional 30 min at room temperature. The film-forming solutions were sonicated for 3 min at 100 W and then were degasified for 5 min to remove air bubbles by using a vacuum pump. A film without OPP also was prepared as a control. Finally, 13 mL of film solution was cast onto plastic plates (diameter of 10 cm) and allowed to air-dry at room temperature for 2 days. Lastly, all films were kept at 23 ± 2 °C and 50 ± 5% relative humidity (RH) for at least 48 h in order to perform the analysis.
Thickness of the film
Film thickness was evaluated with a manual micrometer with a precision of 0.01 mm. Measurements were done at fifteen random places on films. The thicknesses were used for the evaluation of water vapor permeability (WVP) and mechanical attributes.
Moisture content (MC)
Small pieces of the film samples were weighted before (W1) and after (W2) drying in oven at 110 °C for 24 h, and the MC was calculated as follows:
Color analysis and opacity
The color values were evaluated by a CIE colorimeter (Minolta, Japan). L, a, and b values were measured to illustrate lightness, redness/greenness, and yellowness/blueness, respectively. The films opacity was also measured according to Adilah et al. [3]. The film samples were cut into 1 × 4 cm2 rectangular strips and placed inside the test cell. The absorbance was recorded spectrophotometrically at 600 nm. Finally, the opacity values were calculated, accordingly to the following equation:
where Abs600 is the selected absorbance and x is the thickness of the film (mm).
Scanning electron microscopy (SEM)
The surface and cross section microstructure of the films were analyzed using a scanning electron microscope (VEGA II, TESCAN, Czech Republic) with an accelerating voltage of 10 kV and a magnification of 5000 ×. For cross section, film samples prior to visualization were immersed in liquid nitrogen and fractured. The samples were fixed on an aluminum stub and spluttered with gold adhesive tape before the visualization.
Water vapor permeability
The WVP of samples was measured using the method described by Adilah and Hanani [22] with slight modifications. The films disk was mounted on test cups filled with 6 mL of distilled water and placed inside a desiccator (53 ± 2% relative humidity) containing magnesium nitrate and room temperature (23 ± 2 °C). The test cups were weighed every 1.0 h for 10 h. The water vapor transfer rate was calculated from the slope obtained of weight loss over time. Finally, the WVP was calculated with the following equation:
where the slope is the weight loss versus time plot (g s−1), l is the film thickness (m), A is the film area (m2) covering the test cup, and ΔP is the partial pressure difference of water vapor across the film (Pa).
Determination of mechanical properties
The tensile strength (TS) and elongation at break (EAB) of films were tested with a texture analyzer (Testometric Co., Ltd., UK) [10]. The preconditioned film samples (50 ± 5% RH at 25 ± 2 °C for 48 h) were cut into the rectangular strips (15 mm × 60 mm) and fixed on cardboard grips with an initial distance separation of 30 mm. The crosshead speed was set on 50 mm min−1 until breaking. Six replicates were done for each film sample.
Antioxidant properties
Total phenol content (TPC)
The TPC of film samples was determined by Folin–Ciocalteu colorimetric reaction method according to Rambabu et al. [23] with minor adjustments. The film samples (25 mg) were immersed in 3 mL distilled water at 37 °C to be entirely dissolved and then centrifuged at 1500×g for 10 min. After that, 0.3 mL of the resulting supernatant was mixed with 2.5 mL Folin–Ciocalteu reagent (10% v/v) and it was incubated for 5 min at ambient temperature, and then 2 mL sodium carbonate solution (7.5 g L−1) was added to the mixture. Subsequently, the mixture was again vortexed and incubated at 25 ± 2 °C for 1 h prior to reading the absorbance at 760 nm with the UV–Vis spectrophotometer. Gallic acid solutions (0–125 μg mL−1) were exploited as standard element to obtain the calibration curve. The result was reported as milligram gallic acid equivalent per unit gram weight of film (mg GAE/g film).
ABTS radical scavenging activity
ABTS analysis was performed according to Aguirre-Joya et al. [24]. ABTS stock solution (7.0 mM) was mixed with 2.45 mM potassium persulfate (K2S2O8) solution, and then it was allowed to react for 16 h in a dark place at room temperature. The ABTS solution was then diluted with distilled water to obtain an initial absorbance of 0.7 ± 0.02 at 734 nm. After that, 25 mg of each film sample was dissolved in 3 mL of distilled water at 37 °C for 30 min and then was centrifuged at 1500×g for 15 min. A volume of 200 μL of the resulting supernatant was added to 1.0 mL of the diluted ABTS solution. The resulting solution was incubated for 6 min at the room temperature, and its absorbance was determined at 734 nm using a UV–Vis spectrophotometer. The same volume of distilled water was mixed with working solution of ABTS as a control sample. The ABTS radical scavenging activity was calculated by the following equation:
where Acontrol is the absorbance value of the control and Asample is the absorbance value of the film sample.
Reducing power assay
Reducing power of film samples was measured by the method of Kchaou et al. [10]. The film samples (25 mg) were dissolved in 3 mL of distilled water at 37 °C, and the resulting mixtures were centrifuged at 1500×g for 15 min for removing the insoluble particulates. Subsequently, 0.5 mL of supernatant was diluted with 1.25 mL of 0.2 M phosphate buffer of pH 6.6, and then 1.25 mL of 1% aqueous potassium ferricyanide was added. After incubation at 50 °C for 20 min, 1.25 mL of trichloroacetic acid 10% (w/v) was added. Then, the mixture was centrifuged at 1500×g for 10 min and 1.25 mL of the supernatant of each sample solution was mixed with 1.25 mL of distilled water and 0.25 mL of 0.1% (w/v) ferric chloride (FeCl3). Finally, after 10 min of incubation at room temperature, their absorbance was read at 700 nm.
Antibacterial activity
The antimicrobial activity of film samples against Escherichia coli (Gram-negative) and Staphylococcus aureus (Gram-positive) was evaluated using the disc diffusion method. Briefly, bacterial strains were inoculated in nutrient broth and incubated at 36 °C for 24 h. The nutrient agar culture was then spread with broth culture containing approximately 105–106 colony-forming unit (CFU mL−1) of each tested microorganism using an aseptic cotton swap. Then, the film samples which were aseptically cut in discs with 8 mm diameter were laid on the agar culture medium. Finally, the petri dishes were incubated at 30 °C for 24 h. The results were expressed as diameters of the inhibition zones surrounding film disk in millimeter.
Statistical analysis
All data obtained from the assays were analyzed by one-way analysis of variance (ANOVA) using Minitab (version 18, Pennsylvania, USA) statistical software and the differences among means were compared using Tukey’s multiple range test of comparison at 95% confidence level.
Results and discussion
Thickness of the film
The thicknesses of different film samples are illustrated in Table 1. The increase in OPP up to 12% in the gelatin films did not give a significant difference (P > 0.05) on film thickness. Nevertheless, the observed trend in the thickness of the film after the addition of OPP was incremental. However, the incorporation of 15% OPP into the film showed a significant effect (P < 0.05) on thickness. The gelatin film with added 15% OPP created up to 21% thicker film compared to the control. This increase in the thickness can be attributed to an enhancement in the amount of solid content in the gelatin film. Since peels are composed of soluble and insoluble fibers, they may not be fully solubilized in the gelatin solution and this can increase the thickness of the films [1, 2].
Moisture content
The moisture content values of gelatin films with various concentration of OPP are represented in Table 1. As observed, in comparison with the control film, the moisture content of the gelatin films was increased as a result of the OPP addition. However, the incorporation of OPP to 12% into film had no significant change (P > 0.05) on moisture content, while a film with 15% OPP exhibited a notable increase (P < 0.05) in moisture content. The orange peel is implicated of both hydrophilic and hydrophobic ingredients. Hence, when added into the film-forming solution, the hydrophilicity and hydrophobicity of the film may have been changed and this can influence the moisture content of the film [1]. The moisture content can be an important indicator for packaging films due to the influence of water on the flexibility and stretch-ability properties of the film [25]. Therefore, higher moisture content of films can create ability to pack different types of food products.
Optical properties
Color and opacity are two important properties for film appearance thanks to their effective role on the product request and consumer acceptation degree [16]. The values of L, a, b, and opacity are presented in Table 1. All the parameters were influenced by the incorporation of OPP into the film. After the incorporation of OPP, the L* was markedly (P < 0.05) decreased, while a* increased, indicating a trend to redness and darkness of the film. A similar result was also obtained by Hanani et al. [2] who stated that the incorporation of pomegranate peel powder that contained a high amount of polyphenol compounds reduced the lightness of films. The significant (P < 0.05) increase in the b* of gelatin film compared to the control film indicated that the films colors became more yellowish with increasing the concentration of OPP. This observation was in accordance with the results of Sucheta et al. [26] who reported that the commercial pectin-based edible packaging films containing orange peel powder exhibited a yellow color due to the presence of pigments such as carotenoid in the orange peel. In addition, the enriching of gelatin films with OPP significantly changed their opacity, higher opacity for higher concentration of OPP. The changes in the transparency can be attributed to the light scattering effects resulted by pigments and phenolic compounds added within the gelatin films and also could be due to the increase in films thickness. This is in agreement with those of Adilah et al. [3] who observed that the addition of mango peel extract increased the opacity of gelatin films. Therefore, although food packaging materials are mostly clear and colorless, these colored films due to protection effect toward UV and visible light might help to prevent the lipid oxidation, nutrient losses, and off-flavor of food product [27].
Scanning electron microscopy
SEM analysis of both film surface and cross section was performed to identify the morphology characteristics of films, which can help to explain the mechanical and WVP property of the film. It can be observed from Fig. 1 that the surface microstructure of gelatin film showed smoothness and homogeneous morphology without any discontinuous. The results demonstrated that the incorporation of OPP from 3 to 12% had no significant effect on the microstructures of films and the surface was uniform without any cracks, breaks, or pores, whereas for the gelatin film with 15% OPP, some powder particles were observed. However, after the addition of OPP, some white spots dispersing on the surface of films were present, that the extent of these small white dots increased with an increase in the OPP concentration. These white spots may be related to the insoluble particles embedded in the film forming solution. This observation is in harmony with that reported by Riaz et al. [15] who incorporated apple peel polyphenols to chitosan-based film. Meanwhile, the cross-sectional images of control and films with OPP up to 6% exhibited a continuous, compact, and uniform microstructure without pores and roughness. These compact and dense microstructures of film can increase the water vapor barrier property and enhance the shelf life of the packaged materials. However, some layers and micro-void appeared in cross section for gelatin–OPP films with increasing the concentration of OPP from 9 to 15%; more layers and porosity were observed at higher concentrations of the OPP. This can be probably due to the network disorganization of gelatin matrix as a result of enriching with higher concentrations of the OPP. In addition, these pores and layers can be due to the presence of insoluble compounds in the OPP, which might have unfavorable influence on the barrier properties. Similar observations have been reported by Theerawitayaart et al. [28] in which the effect of oxidized linoleic acid on fish gelatin-based films was studied.
Water vapor permeability
Permeability of water vapor is a main index to evaluate the application of biodegradable packaging film, and this property has important effects on the shelf life and quality of packed food [29]. As presented in Table 1, the WVP value of the gelatin film was 4.28 × 10–10 (g s−1 m−1 Pa−1). The addition of 3% OPP had no significant effect on the WVP. In contrast, the incorporation of OPP from 6 to 15% significantly increased the WVP values, ranging from 4.62 to 5.04 × 10–10 (g s−1 m−1 Pa−1). These results are in accordance with the microstructure of the films obtained from the morphological analysis, which the films matrix was influenced by the increase in the OPP concentration. Similar result was also observed by Hanani et al. [2] on fish gelatin films when incorporated with pomegranate peel powder. They reported that the increase in WVP could be due to the presence of soluble compounds in pomegranate peel which can influence the WVP values. Salazar et al. [30] also suggested that the pectin present in orange peel is the hydrophilic material that is able to interact with water via hydrogen bond formations and thus increase the water vapor permeability. On the other hand, orange peel has insoluble compounds which may increase the transmission of water vapor from gelatin film. Therefore, the films containing hydrophilic and heterogeneous materials were more penetrable to water vapor [29].
Mechanical properties
Mechanical attributes such as TS and EAB of packaging films are important characteristics to protect physical completeness of food during storage and operation [11]. The effects of enrichment with OPP on the TS and EAB of the gelatin films are illustrated in Table 1. The initial value of TS for gelatin film was 20.92 MPa. The addition of 3% of OPP to the film resulted in a significant (P < 0.05) reinforcement in the TS value. However, an insignificant increase in TS was observed when 6%, 9%, or 12% of OPP were added to the samples. The maximum TS value for the films was 27.22 ± 0.65 MPa when the OPP concentration was 15%. This observation was in accordance with the result of Adilah et al. [3]. The orange peel is a good source of complex polysaccharides, which has the ability to form intramolecular interactions with gelatin, which can increase the stretch resistance of the resulting films [31, 32]. Al-Hasan and Norziah [31] reported that the mechanical properties (TS) increased by relatively long-chain polysaccharides through cross-linkage with gelatin. Furthermore, hydroxyl groups of phenolic compounds in the orange peel [20] could form hydrogen bonds with hydrogen acceptor molecules in gelatin improving the TS [33]. This is in good accordance with the results of Nilsuwan et al. [12] who stated that the addition of epigallocatechin gallate into the fish gelatin-based films increased the TS and decreased the EAB due to the generation of intermolecular hydrogen bonds between phenolic compounds and protein. On the contrary, control film without OPP exhibited the highest value of EAB, 64.48%. Addition of 3% OPP in gelatin films had no significant (P > 0.05) effect on the EAB compared to the control film, whereas addition of 6–15% OPP into gelatin films significantly (P < 0.05) decreased the EAB value, as indicated in Table 2. Similar results were found by Zhang et al. [34] where a decrease in EAB of rabbit skin gelatin film was observed after the incorporation of rosemary acid. They also reported that greater phenolic–gelatin molecule interactions may reduce the influences of the plasticizer within the film and cause a relatively more compact network structure, leading to a decrease in film flexibility. Furthermore, a decrease in EAB could be related to the increase in structural heterogeneity resulted by the insolubilized part of the orange peel powder in the gelatin film, as shown by SEM images.
Antioxidant properties
Antioxidant properties of film samples were studied in terms of total phenol content, ABTS radical scavenging activity, and reducing power. Results for the TPC are shown in Table 2. The TPC analysis can be useful to assess the amounts of phenolic compounds in film samples, which affect the antioxidant activity by delocalizing the unpaired electron and donating hydrogens from hydroxyl groups to prevent oxidation [35]. The TPC of gelatin film was significantly increased by enriching with the OPP, due to the presence of phenolic ingredients such as hesperidin, narirutin, tangeretin, and naringenin in the orange peel [36]. This consequence was in accordance with those of Rambabu et al. [23] who stated that the TPC of chitosan films was enhanced after the incorporation of mango leaf extract. In addition, other studies showed that the orange peel contains phytochemicals and vitamin C which can contribute in the donation of protons or electrons for stabilizing the free radicals, therefore improving the antioxidant activity [20].
The results of the ABTS radical scavenging activity and reducing power (Fig. 2) revealed that the gelatin without OPP had antioxidant potential. This ability can be related to the presence of amino acids like tyrosine in the gelatin, which can react with free radicals [37]. The radical scavenging activity and reducing ability of gelatin film increased when the OPP content increased from 3 to 15%. The higher antioxidant activity in the presence of OPP can be due to the biologically active compounds of the OPP such as phenolic compounds, vitamin C, and carotenoids. Kalaycioglu and Erim [38] also reported that the phenolic compounds in fruit peels could scavenge radicals and chelate cations. Afonso et al. [39] reported that the carotenoids and vitamin C present in the annatto seeds had high antioxidant activity and were able to neutralize the singlet oxygen cascade in the chitosan film. Therefore, the OPP-enriched gelatin films can be used to improve the oxidative stability and delay the quality deterioration of food stuffs thanks to their excellent antioxidant activity.
Antimicrobial activity
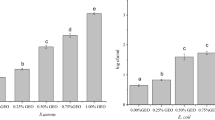
The antimicrobial activities of gelatin films without or with OPP against E. coli and S. aureus are presented in Table 2. As expected, no inhibitory zone was detected for the gelatin film (without OPP), suggesting that gelatin films did not have any antimicrobial activities. However, films containing different concentration of OPP exhibited distinctive antibacterial activity against E. coli and S. aureus, which significantly increased with increasing the concentration of OPP in the film samples. The OPP-enriched films showed stronger antibacterial activity against Gram-positive bacteria (S. aureus) than E. coli as a Gram-negative. These results are supported by Casquete et al. [40] and Kanmani and Rhim [41] who stated, respectively, that orange peel extract and grapefruit extract showed higher antimicrobial effects on Gram-positives in comparison with the Gram-negatives. Moghadam et al. [42] also reported that the antibacterial activity of mung bean protein-based edible films was significantly improved by enriching with pomegranate peel powder. Moreover, they also investigated that the enriched films were more effective against the Gram-positive bacteria compared to the Gram-negative bacteria. Wu et al. [16] also indicated that Gram-positive organisms are more susceptible to phenolic compounds. Therefore, the addition of orange peel powders into the edible films can be considered as a simple strategy for improving the antimicrobial activity of films and coatings.
Conclusion
Orange peel powder was successfully incorporated into the gelatin films. SEM results showed that the addition of OPP can reduce the surface smoothness and increase pores and cracks in the cross section. Incorporation of OPP into gelatin films also drastically augmented the tensile strengths and decreased the percentage of EAB due to the formation of intermolecular interactions between gelatin and OPP. The WVP also increased due to hydrophilic compounds and insoluble particles in the film. The antioxidant and antimicrobial activities of gelatin films were also considerably improved. Therefore, these results suggested that the OPP has a good potential to be used as a low-cost ingredient for developing biodegradable and bio-functional packaging films with enhanced antioxidant and antibacterial properties to ensure food safety.
References
Hanani ZN, Husna AA, Syahida SN, Khaizura MN, Jamilah B (2018) Effect of different fruit peels on the functional properties of gelatin/polyethylene bilayer films for active packaging. Food Packag Shelf Life 18:201–211
Hanani ZN, Yee FC, Nor-Khaizura MAR (2019) Effect of pomegranate (Punica granatum L.) peel powder on the antioxidant and antimicrobial properties of fish gelatin films as active packaging. Food Hydrocoll 89:253–259
Adilah AN, Jamilah B, Noranizan MA, Hanani ZN (2018) Utilization of mango peel extracts on the biodegradable films for active packaging. Food Packag Shelf Life 16:1–7
Mushtaq M, Gani A, Gani A, Punoo HA, Masoodi FA (2018) Use of pomegranate peel extract incorporated zein film with improved properties for prolonged shelf life of fresh Himalayan cheese (Kalari/kradi). Innov Food Sci Emerg Technol 48:25–32
Suderman N, Isa MIN, Sarbon NM (2018) The effect of plasticizers on the functional properties of biodegradable gelatin-based film: a review. Food Biosci 24:111–119
Hsissou R, Berradi M, El Bouchti M, El Bachiri A, El Harfi A (2019) Synthesis characterization rheological and morphological study of a new epoxy resin pentaglycidyl ether pentaphenoxy of phosphorus and their composite (PGEPPP/MDA/PN). Polym Bull 76:4859–4878
Bekhta A, Hsissou R, Elharfi A (2020) Evaluation of mechanical compressive strength of cementitious matrix with 12% of IER formulated by modified polymer (NEPS) at different percentages. Sci Rep 10:1–8
Hsissou R, Elharfi A (2020) Rheological behavior of three polymers and their hybrid composites (TGEEBA/MDA/PN),(HGEMDA/MDA/PN) and (NGHPBAE/MDA/PN). J King Saud Univ Sci 32:235–244
Reyes-Avalos MC, Femenia A, Minjares-Fuentes R, Contreras-Esquivel JC, Aguilar-González CN, Esparza-Rivera JR, Meza-Velázquez JA (2016) Improvement of the quality and the shelf life of figs (Ficus carica) using an alginate–chitosan edible film. Food Bioprocess Technol 9:2114–2124
Kchaou H, Benbettaïeb N, Jridi M, Abdelhedi O, Karbowiak T, Brachais CH, Léonard ML, Debeaufort F, Nasri M (2018) Enhancement of structural, functional and antioxidant properties of fish gelatin films using Maillard reactions. Food Hydrocoll 83:326–339
Ghaderi J, Hosseini SF, Keyvani N, Gómez-Guillén MC (2019) Polymer blending effects on the physicochemical and structural features of the chitosan/poly (vinyl alcohol)/fish gelatin ternary biodegradable films. Food Hydrocoll 95:122–132
Nilsuwan K, Benjakul S, Prodpran T (2018) Properties and antioxidative activity of fish gelatin-based film incorporated with epigallocatechin gallate. Food Hydrocoll 80:212–221
Bakry NF, Isa MI, Sarbon NM (2017) Effect of sorbitol at different concentrations on the functional properties of gelatin/carboxymethyl cellulose (CMC)/chitosan composite films. Int Food Res J 24:1753–1762
Avena-Bustillos RJ, Olsen CW, Olson DA, Chiou BS, Yee E, Bechtel PJ, McHugh TH (2006) Water vapor permeability of mammalian and fish gelatin films. J Food Sci 71:202–207
Riaz A, Lei S, Akhtar HM, Wan P, Chen D, Jabbar S, Abid M, Hashim MM, Zeng X (2018) Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int J Biol Macromol 114:547–555
Wu H, Lei Y, Zhu R, Zhao M, Lu J, Xiao D, Jiao C, Zhang Z, Shen G, Li S (2019) Preparation and characterization of bioactive edible packaging films based on pomelo peel flours incorporating tea polyphenol. Food Hydrocoll 90:41–49
Ozturk B, Parkinson C, Gonzalez-Miquel M (2018) Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Sep Purif Technol 206:1–3
Chen SY, Chyau CC, Chu CC, Chen YH, Chen TH, Duh PD (2013) Hepatoprotection using sweet orange peel and its bioactive compound, hesperidin, for CCl4-induced liver injury in vivo. J Funct Foods 5:1591–1600
Adiamo OQ, Ghafoor K, Al-Juhaimi F, Babiker EE, Ahmed IA (2018) Thermosonication process for optimal functional properties in carrot juice containing orange peel and pulp extracts. Food Chem 245:79–88
Chen XM, Tait AR, Kitts DD (2017) Flavonoid composition of orange peel and its association with antioxidant and anti-inflammatory activities. Food Chem 218:15–21
Geraci A, Di Stefano V, Di Martino E, Schillaci D, Schicchi R (2017) Essential oil components of orange peels and antimicrobial activity. Nat Prod Res 31:653–659
Adilah ZM, Hanani ZN (2016) Active packaging of fish gelatin films with Morinda citrifolia oil. Food Biosci 16:66–71
Rambabu K, Bharath G, Banat F, Show PL, Cocoletzi HH (2019) Mango leaf extract incorporated chitosan antioxidant film for active food packaging. Int J Biol Macromol 126:1234–1243
Aguirre-Joya JA, Pastrana-Castro L, Nieto-Oropeza D, Ventura-Sobrevilla J, Rojas-Molina R, Aguilar CN (2018) The physicochemical, antifungal and antioxidant properties of a mixed polyphenol based bioactive film. Heliyon 4:e00942
Aguirre-Loredo RY, Rodríguez-Hernández AI, Morales-Sánchez E, Gómez-Aldapa CA, Velazquez G (2016) Effect of equilibrium moisture content on barrier, mechanical and thermal properties of chitosan films. Food Chem 196:560–566
Rai SK, Chaturvedi K, Yadav SK (2019) Evaluation of structural integrity and functionality of commercial pectin based edible films incorporated with corn flour, beetroot, orange peel, muesli and rice flour. Food Hydrocoll 91:127–135
Rubilar JF, Cruz RM, Silva HD, Vicente AA, Khmelinskii I, Vieira MC (2013) Physico-mechanical properties of chitosan films with carvacrol and grape seed extract. J Food Eng 115:466–547
Theerawitayaart W, Prodpran T, Benjakul S, Sookchoo P (2019) Properties of films from fish gelatin prepared by molecular modification and direct addition of oxidized linoleic acid. Food Hydrocoll 88:291–300
Oymaci P, Altinkaya SA (2016) Improvement of barrier and mechanical properties of whey protein isolate based food packaging films by incorporation of zein nanoparticles as a novel bionanocomposite. Food Hydrocoll 54:1–9
Salazar AS, Cavazos PA, Paz HM, Fragoso AV (2019) External factors and nanoparticles effect on water vapor permeability of pectin-based films. J Food Eng 245:73–79
Al-Hassan AA, Norziah MH (2012) Starch–gelatin edible films: water vapor permeability and mechanical properties as affected by plasticizers. Food Hydrocoll 26:108–117
Ren JN, Hou YY, Fan G, Zhang LL, Li X, Yin K, Pan SY (2019) Extraction of orange pectin based on the interaction between sodium caseinate and pectin. Food Chem 283:265–274
Rasid NA, Nazmi NN, Isa MI, Sarbon NM (2018) Rheological, functional and antioxidant properties of films forming solution and active gelatin films incorporated with Centella asiatica (L.) urban extract. Food Packag Shelf Life 18:115–124
Zhang X, Ma L, Yu Y, Zhou H, Guo T, Dai H, Zhang Y (2019) Physico-mechanical and antioxidant properties of gelatin film from rabbit skin incorporated with rosemary acid. Food Packag Shelf Life 19:121–130
Yuan G, Lv H, Yang B, Chen X, Sun H (2015) Physical properties, antioxidant and antimicrobial activity of chitosan films containing carvacrol and pomegranate peel extract. Molecules 20:11034–11045
Barrales FM, Silveira P, Barbosa PD, Ruviaro AR, Paulino BN, Pastore GM, Macedo GA, Martinez J (2018) Recovery of phenolic compounds from citrus by-products using pressurized liquids—an application to orange peel. Food Bioprod Process 112:9–21
Gómez-Estaca J, Bravo L, Gómez-Guillén MC, Alemán A, Montero P (2009) Antioxidant properties of tuna-skin and bovine-hide gelatin films induced by the addition of oregano and rosemary extracts. Food Chem 112:18–25
Kalaycıoğlu Z, Erim FB (2017) Total phenolic contents, antioxidant activities, and bioactive ingredients of juices from pomegranate cultivars worldwide. Food Chem 221:496–507
Afonso CR, Hirano RS, Gaspar AL, Chagas EG, Carvalho RA, Silva FV, Leonardi GR, Lopes PS, Silva CF, Yoshida CM (2019) Biodegradable antioxidant chitosan films useful as an anti-aging skin mask. Int J Biol Macromol 132:1262–1273
Casquete R, Castro SM, Martín A, Ruiz-Moyano S, Saraiva JA, Córdoba MG, Teixeira P (2015) Evaluation of the effect of high pressure on total phenolic content, antioxidant and antimicrobial activity of citrus peels. Innov Food Sci Emerg Technol 31:37–44
Kanmani P, Rhim JW (2014) Antimicrobial and physical–mechanical properties of agar-based films incorporated with grapefruit seed extract. Carbohydr Polym 102:708–716
Moghadam M, Salami M, Mohammadian M, Khodadadi M, Emam-Djomeh Z (2020) Development of antioxidant edible films based on mung bean protein enriched with pomegranate peel. Food Hydrocoll 104:105735
Acknowledgements
The support of the University of Tehran is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Taghavi Kevij, H., Salami, M., Mohammadian, M. et al. Mechanical, physical, and bio-functional properties of biopolymer films based on gelatin as affected by enriching with orange peel powder. Polym. Bull. 78, 4387–4402 (2021). https://doi.org/10.1007/s00289-020-03319-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03319-9