Abstract
In this study, we describe the structure of bismuth vanadate (BiVO4)/graphene oxide/polyaniline (PANI) compound with exploited interfacial coupling, their use as visible-light photocatalysts and safety property. Thin graphene oxide sheets could completely cover BiVO4 polyhedrons with vastly conductive polymer PANI through an evaporation-induced hydrothermal process. The enhanced surface adsorption outcome of GO, a huge improvement in the photoactivity of BiVO4, has been proved through the degradation of methylene blue (MB) and safranin O (SO) upon the covering of polyaniline. The improved photocatalytic activity is recognized for the development of well-defined BiVO4/GO/PANI interfaces which considerably increases the charge separation efficacy. Conversely, safety aspects are investigated for identifying the toxicity of samples on the different kinds of bacteria. Our study found that in the existence of every sample the bacteria could not be killed throughout the culture medium. Considering its ease of preparation and excellent performance, BiVO4/GO/PANI could be a promising, competitive and safe visible-light-driven photocatalyst in the field of environmental remediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the start of the twenty-first century, water pollution has been a big problem which exerts serious effects on the ecological atmosphere and human survival [1, 2]. Organic dyes [3, 4], benzene-based organics [5, 6] and phenol [7, 8] are the key pollutants of concern in wastewater owing to their high toxicity and almost non-degradable characteristics. Therefore, seeking efficient, green and non-toxic purification technology and material that can efficiently eliminate organic toxins from wastewater is of great importance for environmental safety. Compared with the conventional methods, photocatalysis technology shows greater promise in environmental remediation because of its low cost, lack of harmful by-products and durable oxidative capability for contaminants under facile conditions [9,10,11,12].
In contrast, materials with delocalized conjugated forms have been broadly studied, owing to their quick photoinduced charge separation and relatively slow-moving charge recombination [13]. Polyaniline (PANI), a conducting polymer with an unmitigated π-conjugated electron system, has recently shown immense assure because of its good absorption coefficients in the visible-light range and extraordinary mobility of charge carriers [14].
Furthermore, PANI, in its undoped or partially doped states, acts as an electron contributor upon photoexcitation and is recognized as a respectable hole conductor which can carry a current of several milliamperes [15]. Moreover, compared to doped materials such as noble metal, PANI is more in practice for its relative ease of commercial-scale production. Considering the well-organized carrier transfer character of PANI, it is projected that the mixture of photocatalyst and PANI seems ideal for enhancing the photoactivity under visible light. There are only a few studies published on the amalgamation of PANI and simple metal oxide catalysts to grow their photocatalytic performance [12, 16,17,18,19]. Therefore, further research is desired to extend its uses. Bismuth vanadate (BiVO4) has been recognized as a photocatalyst for water splitting [20,21,22] and the destruction of dye pollutants with extraordinary mineralization [23,24,25,26,27,28,29] under visible-light irradiation. Moreover, the discoloration of the dye and photocatalytic degradation of phenol on BiVO4 was also reported [30, 31]. Phenol is widely recognized as a carbon-based chemical that is present in a variety of wastewaters from different industries and is relatively poisonous and sluggish to decompose in the environment [32]. The need to find a good photocatalyst for phenol removal is a trouble of extraordinary environmental concern [33]. The action of pure BiVO4 is stumpy, because of its reduced adsorptive performance and complex movement of electron–hole pairs [34]. Graphene, a two-dimensional (2D) single atomic sheet of sp2-hybridized carbon atoms, has attracted great interest over the past decade owing to its unexpected electrical properties, optical transparency and biocompatibility [35, 36]. Graphene oxide (GO) is a water-dispersible graphene sheet with carboxylic, phenol hydroxyl and epoxide groups on its edges and basal planes, which can be produced by the chemical oxidation of graphite and consequent exfoliation [37, 38]. Based on its aqueous stability, low production cost and amphiphilic behavior [39,40,41], GO is a favorable material as a building block for graphene-based nanomaterials and their different uses such as conductive thin film, biosensors and biomedical devices [42, 43]. Additionally, it is value noticing that there has been no report on the synthesis and photoactivity in the BiVO4/GO/PANI system. It is important to twitch complete lessons on the pathway and mechanism of photoinduced electron–hole pairs under visible-light irradiation, to project more efficient visible-light-focused photocatalytic combined materials that will come across the prerequisite of the applied environmental application. Herein, for the first time, spherical-like BiVO4/GO/PANI photocatalyst was prepared by the hydrothermal method. The work is mostly focused on the visible-light-induced photodegradation of the broadly used organic pollutants, methylene blue (MB) and safranin O. The existence of free electron was also observed, by the measurement of the electrical properties, further demonstrating the photocatalytic performance of BiVO4/GO/PANI. The photodegraded mechanism of BiVO4/GO/PANI photocatalyst was discussed, and the improved photoactivity came from the upgrade of charge separation efficacy caused by the synergy among PANI and BiVO4/GO.
Experimental section
PANI (molecular weight ∼ 105) was purchased from Jilin Zhengji Corp. All the other reagents used in our experiments were of investigative purity and were used as received from Dejone Chemical Co., Korea.
Bismuth vanadate (BiVO4) synthesis
In a typical synthesis of bismuth vanadate, 0.1896 g of Bi(NO3)3·5H2O and 0.0630 g of NH4VO3 were individually added to two solutions of absolute ethanol (10 mL), while stirring for over 30 min at room temperature. The above two systems were then combined, adjusted to a pH of 8.0 with an ammonia solution and stirred for 40 min to yield a stable yellow-colored slurry. The resultant combination was then transferred to a 40-mL Teflon-lined stainless steel autoclave and heated to 180 °C for 8 h under autogenous pressure. The reaction mixture is then allowed to cool to room temperature, and the precipitate was sieved, washed with DI water five times and then dehydrated in a vacuum oven at 60 °C for 12 h. A yellowish-colored powder-type sample was finally obtained and labeled as BiVO4.
Synthesis of BiVO4–graphene oxide composite photocatalyst
The representative preparation of BiVO4/GO was as follows: Graphene oxide (GO) was manufactured rendering to the method described by Hummers and Offeman [43] from cleaned natural graphite with a mean particle size of 40 nm, which was bought from the Qingdao Zhongtian Company. BiVO4/GO was fashioned by the use of the facile hydrothermal method.
A characteristic experiment for the synthesis of BiVO4–graphene composite is as follows. First, 50 mg of GO (4% mass ratio) was dispersed into 20 mL of absolute ethanol and 15 mL of deionized (DI) water, with sonication for 2 h. Then, 0.1896 g of Bi(NO3)3·5H2O and 0.0630 g of NH4VO3 were individually added to two solutions of absolute ethanol (10 mL), while stirring for over 30 min at room temperature. The above three systems were then combined, adjusted to a pH of 8.0 with an ammonia solution and stirred for 40 min, to yield a stable bottle-green-colored slurry. The resultant combination was then moved to a 40-mL Teflon-lined stainless steel autoclave and heated to 180 °C for 8 h under autogenous pressure. The reaction mixture was then allowed to cool to room temperature, and the precipitate was sieved, washed with DI water five times and then dehydrated in a vacuum oven at 60 °C for 12 h. A greenish-colored powder-type sample was finally obtained and labeled as BiVO4/GO.
Preparation of composite catalysts
The classic preparation of BiVO4/GO/PANI photocatalyst is as follows: PANI was liquefied in tetrahydrofuran (THF) to acquire a concentration of 0.081 g L−1 solutions; then, a certain amount of BiVO4/GO powder was added to 100 mL of the above solution. The suspension was then ultrasonicated for a minimum of 2 h, stirred for 4 h and then filtered. The as-produced precipitate was then washed with water thrice and then dried at 70 °C for 13 h. BiVO4/GO/PANI photocatalyst with 3% mass ratio of PANI was produced by this method.
Characterization
The X-ray diffraction (XRD) decorations of the samples were precise by a D/MAX 2250 V diffractometer (Rigaku) using monochromatized Cu Kα (λ = 0.15418 nm) radiation beneath 40 kV and 100 mA and scanning above the range of (10 ≤ 2θ ≤ 70 degrees). The morphologies and microstructures of the as-equipped samples were investigated by scanning electron microscope (SEM; JEOL JSM-6700F) and transmission electron microscope (TEM; JEOL JEM-2100F; accelerating voltage, 200 kV). Fourier transforms infrared (FTIR) spectra were recorded by a Bruker VECTOR 22 spectrometer by means of the KBr pellet technique. Raman spectra were acquired on a Renishaw inVia Reflex Raman Microprobe. UV–Vis diffuse reflectance spectra (DRS) of the as-organized samples were obtained by a Shimadzu UV-2550 spectrophotometer outfitted with an integrating sphere using BaSO4 as the reflectance standard. X-ray photoelectron spectroscopy (XPS) analysis of the sample was passed out on a Physical Electronics PHI 1600 ESCA system operating at pass energy of 187.85 eV with an Al Kα X-ray source (E = 1486.6 eV). All binding energies of the composing elements were referenced to the C1s peak at 284.6 eV. UV–Vis diffuse reflectance spectra of the samples were conquered by an UV–Vis spectrophotometer (Hitachi U-3010), using H2O as the reference.
Photocatalytic activity measurements
The photocatalytic activities of the samples were evaluated by the photocatalytic degradation of methylene blue (MB) and safranin O under visible light. A 500 W Xe lamp with a 420-nm cut-off filter was utilized as the light source to give visible-light irradiation. For the degradation of MB and SO, 0.1 g of photocatalyst was added to 100 mL of MB or safranin O solution (1 × 10−5 to 1 × 10−4 M). Dye solutions were made under visible light (λ ≥ 420 nm) in a home-grown reactor with a cooling water circulator to maintain the reactor at a steady temperature. Before illumination, the solution was agitated for 120 min in the dark to achieve adsorption–desorption equilibrium among the photocatalyst MB or safranin O dye. At 20-min intervals, a 7-mL solution was sampled. Then, the UV–visible absorption spectrum of the centrifuged solution was recorded by a Hitachi U-3010 UV–visible spectrophotometer.
Microbial safety test
Enterococcus aeruginosa, Pseudomonas aeruginosa and E. coli bacteria were screened for the safety test. E. coli K-12 strain of DH5a cells (Enzynomics) was pre-cultured and prepared as shown elsewhere, except for the number of bacterial cells used. Sixty-six E. coli cells were diluted with phosphate-buffered saline (PBS; Sigma-Aldrich) to 0.5 × 10 5 CFU/mL and dispensed (0.2 mL) on the film containing BiVO4, BiVO4/GO and BiVO4/GO/PANI nanosheets. Bacterial cells dispensed on an empty well served as a control in the experiment. To avoid the vaporization of the bacterial cell solution on the surface, wet conditions were used during the microbial viability test. The bacterial cell suspension was harvested after 3-h incubation at 30 °C and the outward of the wafer washed thrice by pipetting with 1 ml of PBS. This was collected to harvest any remaining bacterial cells on the exterior. All the harvested bacterial cells were centrifuged and resuspended in 1 ml of PBS. Next, 150 µL of the suspension was spread on LB agar plates, and the colonies were counted after overnight incubation at 37 °C to estimate the loss of viability of E. coli cells on the BiVO4, BiVO4/GO and BiVO4/GO/PANI nanosheet surfaces. All experiments were carried out in triplicate and repeated at least twice. Loss of viability was calculated using the following formula:
Loss of viability (%) = (counts of control − counts of samples incubated with 2D nanosheet surfaces)/counts of control × 100 (%).
Results and discussion
Characterizations of BiVO4/GO/PANI sample
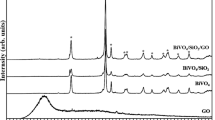
Figure 1 presents the XRD patterns of the prepared photocatalysts. Ten characteristic peaks of BiVO4 can be found at 2θ = 18.99°, 28.95°, 30.55°, 34.49°, 35.22°, 42.46°, 46.71°,47.30°, 50.31° and 53.31° corresponding to the (110), (121), (040), (200), (002), (211), (060), (240), (222) and (161) crystal faces of the clinobisvanite structure (PDF 14-0688) [18, 19]. The characteristic peaks are matched with the value of clinobisvanite BiVO4 reported in JCPDS 14-0688. The characteristic clinobisvanite BiVO4 diffraction peaks occur clearly and simultaneously in the XRD pattern of BiVO4/GO and BiVO4/GO/PANI, which prove the successful synthesis of BiVO4 in nanocomposites. In the XRD pattern of the BiVO4/GO/PANI nanocomposites, all the peaks corresponding to BiVO4 were also present, suggesting that the state of BiVO4 did not change during the synthesis process. On the other hand, a slight reduction in the peak intensity was observed for BiVO4 peaks in the BiVO4/GO/PANI [20]. This might be due to the surface coating of PANI on GO and BiVO4 during the synthesis process and the interactions between the GO, BiVO4 nanoparticles and the PANI chain [21]. The presence of very sharp peaks in the XRD pattern of BiVO4 indicates the well-defined crystalline structure. However, the diffraction peaks of GO and PANI are too weak to be found relative to the strong diffraction peaks of BiVO4, perhaps because the diffraction peaks of GO become wider and less intense because of GO’s lower crystallinity. In addition, the content of PANI is very low, so the XRD signal is not easily observed.
Furthermore, to that, no deflection peaks assigned to PANI were detected, which also recommended that the PANI layer was very thin [14].
Figure 2 shows typical SEM images of the as-prepared GO, BiVO4/GO and BiVO4/GO/PANI photocatalyst. The BiVO4 has a significantly greater volume than PANI. Figure 2 shows that the product showed a layered structure with platelet-like morphology. A close-up view of the spherical (Fig. 2) shows that the majority of the crystals possess a non-uniform disordered shape with a center diameter of about 200 nm and length 400 nm. The GO particle intercalated homogenously with polymer; however, the BiVO4 is staggered on the surface [12, 15]. In addition, such an amorphous structure was also shown by TEM investigation as displayed in Fig. 3. Closer inspection revealed that BiVO4/GO/PANI contained many smaller nanoparticles with a size of about 100 nm. Figure 3 shows the polycrystalline rings resulting from the accumulation of the nanoparticles by a favored orientation. The surfaces of the graphene are coated by PANI nanoparticle. Small BiVO4 particles are distributed on the surface of PANI. Figure 3d reveals that PANI is surrounded by a few BiVO4 particles. Compared to Fig. 3b, the pure BiVO4, the surface of the prepared BiVO4/GO/PANI nanocomposites was not smooth anymore, but with a rough surface instead due to the coating of PANI nanoparticles, which could increase the reaction surface of the samples and provide more active sites, resulting in enhanced photocatalytic activity and performance [11, 12].
It has also been informed that smaller grain size and high crystallinity endowed higher photocatalytic action for the improved reactive sites and stimulated electron–hole departure efficiency [34, 35]. Thus, the as-prepared nano-BiVO4/GO/PANI was estimated to show improved photocatalytic performance.
To confirm the effective transmission of GO and to characterize the carbon species, FTIR was used to get extra perception into the arrangement of GO, BiVO4 and PANI. Figure 4 shows the FTIR spectra of BiVO4, BiVO4/GO and BiVO4/GO/PANI, respectively. We estimated to detect a durable absorption band of GO at 3010 cm−1, due to the O–H stretching vibration. However, after GO was joined with BiVO4 and PANI, the peak strength reduced. The typical peaks at 1567 cm−1 can be credited to unoxidized carbon backbone [31, 32]. It was noted that the numerous oxygen-holding groups ((800–1900) cm−1) in BiVO4/GO and BiVO4/GO/PANI significantly reduced or even extinct, showing that the hydrothermal synthesis is an effective technique for the synthesis of BiVO4/GO/PANI. In the event of the BiVO4/GO, the characteristic absorption peaks of GO are radically decreased or even vanished, as related to those of the unpolluted GO [36]. The extensive absorption cases at a short frequency (below 1000 cm−1) are associated with (VO4) and (VO4). All the outcomes explain that in our study work, we can effectively organize a compound catalyst that incorporates GO as a platform.
Optical absorption of the as-prepared BiVO4, BiVO4/GO and BiVO4/GO/PANI samples was measured by UV–visible DRS spectrometry. Figure 5 displays that the BiVO4 sample has photoabsorption from UV light to visible light and the wavelength of the absorption edge is 525 nm [38]. The absorption of the PANI-modified BiVO4/GO sample upturns over the complete range of the spectrum. On the basis of the equation \((ahv)^{2} = A\;(hv - Eg)\) [39], the band gaps of the samples were estimated to be 2.42, 2.3 and 2.21 eV from the creation of the absorption edges, corresponding to the BiVO4, BiVO4/GO and BiVO4/GO/PANI samples. This shows that the bandgap energy of the BiVO4/GO/PANI sample is lesser than that of the BiVO4. Therefore, the PANI-modified BiVO4/GO sample can be able to energize generation of more electron–hole pairs under a similar visible-light illumination, which might result in upper photocatalytic action.
It is detected that BiVO4 has a monoclinic phase, established on the attribute stretching vibrations and bending vibrations of the VO43− tetrahedron (Fig. 6). The Raman spectrum of BiVO4/GO/PANI shows two characteristic bands at 367 and 823 cm−1 which exhibit that BiVO4 has a monoclinic phase, based on the attribute stretching vibrations and twisting vibrations of the VO43− tetrahedron. The Raman spectrum of BiVO4/GO/PANI illustrates two characteristic bands at 1355 and 1600 cm−1, consistent to the D band and G band of GO, respectively [37]. Comparatively, in the BiVO4, BiVO4/GO and BiVO4/GO/PANI composites, the D band and G band are slightly blue- and redshifted to 1346 and 1606 cm−1, respectively, which may be produced by the altered surface strain, due to the interaction between GO and BiVO4.
This occurrence is reliable with that detected in the hydrothermal in situ preparation of the BiVO4/GO/PANI (BiVO4, BiVO4/GO and BiVO4/GO/PANI) composites [32], where the D/G ratio (close to zero) proposes the effective combination of BiVO4/GO with PANI polymer.
The configuration of the BiVO4/GO/PANI heterogeneous nanostructures has been examined by X-ray photoelectron spectroscopy (XPS). Figure 7 shows high-resolution XPS spectra of the as-prepared BiVO4/GO/PANI heterogeneous nanostructures. Figure 7a shows the binding energies located at about 160.1 and 166.5 eV that correspond to the Bi4f5/2 and Bi4f7/2 bands. Figure 7b displays the XPS spectrum of the O1s band, showing that dissimilar oxygen species exist on the surface of BiVO4/GO/PANI heterogeneous nanostructures. The binding energies located at about 532.2 and 533.1 eV are attributed to the O1s band of lattice oxygen of GO crystallites [41] and the O1s band of lattice oxygen of BiVO4 crystallites [42], respectively. Figure 7c displays the high-resolution XPS spectrum, in which the V2p bands shown in the peaks at about 527 and 518.5 eV correspond to V2p1/2 and V2p3/2 bands. The XPS spectrum of the C1s band in Fig. 7d clearly shows one peak formed at 287.3 eV, which relates to the C1s bands of GO and PANI crystallites. The C1s peak is complemented by two satellites on the high-binding-energy side denoted by peaks I and II which are set at about 287.3 and 287.5 eV, respectively. The foremost peak in the XPS spectrum of the N1s band is known to be characteristic of N2− and the shake-up satellite peaks are patent and diagnostic of an open 3p4 shell of N2− state [39], showing the existence of PANI on the surface. The fact that XRD does not display evidence of a PANI phase, while XPS displays the outward existence of N2− ions suggests that − NH2 is present only on the outward of the PANI nanocrystals and forms an exact skinny amorphous outer shell. According to the overhead experimental results of XRD, TEM and XPS, it can be realized that the N element is present in the system of NH− on the exterior of the BiVO4/GO/PANI heterogeneous nanostructures.
Visible-light-induced photocatalytic performance and mechanism on BiVO4/GO/PANI
Photocatalytic degradation of dye
MB and safranin O were selected as representative hazardous dyes to define the photocatalytic performance of the as-equipped BiVO4/GO/PANI which exhibited a major absorption band at 553 nm. Figures 8 and 9 display the respective photodegradation efficiencies of MB and safranin O that were mediated by photocatalysts under visible-light illumination (λ > 420 nm), with otherwise identical conditions. First, it was confirmed that the photolysis of MB and safranin O was slow for BiVO4 photocatalyst under visible-light illumination. The adsorption of MB and safranin O on the BiVO4/GO/PANI sample with dissimilar ratios in the dark was also checked. After 120 min, the concentrations of MB and safranin O decreased by 10% only. This suggests that the decolorizing of MB and safranin O by spherical-like BiVO4/GO/PANI is mostly produced by photodegradation, but not adsorption. In addition to that, only 73% MB and 82% safranin O can be photodegraded by BiVO4 under visible light in 180 min. However, all the BiVO4/GO/PANI samples exhibited higher photocatalytic activities than BiVO4. Among them, the BiVO4/GO/PANI photocatalyst showed the maximum activity and photodegraded 73% MB and 82% safranin O after only 180 min under a similar condition. To quantitatively understand the response kinetics of the MB and safranin O degradation in our experiments, the Langmuir–Hinshelwood model was applied as expressed by Eq. (1), which is well known for the photocatalytic experiments when the pollutant is in the millimolar concentration range [42, 43].
where C0 and C are the concentrations of dye in solution at times 0 and t, respectively, and k is the apparent first-order rate constant. Figures 10 and 11 clearly show that in the plot of C/C0 and time t, PANI had an excessive impact on the photodegraded rate (k) of the as-prepared samples. Table 1 show the list of the rate constant values. The sample with BiVO4/GO/PANI showed the maximum photodegradation efficiency, which was about threefold, compared to that of the pure BiVO4 sample. Figure 12 displays that the BiVO4/GO/PANI has an excessive impact on the photodegradation rate (k) of the as-prepared samples.
a MB photodegradation as a function of illumination time for BiVO4/graphene oxide/polyaniline (BiVO4–GO–PANI), BiVO4/graphene oxide (BiVO4–GO) and BiVO4 under visible light. b The plots of Ct/C0 versus t for BiVO4/graphene oxide/polyaniline (BiVO4–GO–PANI), BiVO4/graphene oxide (BiVO4–GO) and BiVO4. C0 is the initial concentration of MB and Ct is the concentration at irradiation time t
a SO photodegradation as a function of illumination time for BiVO4/graphene oxide/polyaniline (BiVO4–GO–PANI), BiVO4/graphene oxide (BiVO4–GO) and BiVO4 under visible light. b The plots of Ct/C0 versus t for BiVO4/graphene oxide/polyaniline (BiVO4–GO–PANI), BiVO4/graphene oxide (BiVO4–GO) and BiVO4. C0 is the initial concentration of SO and Ct is the concentration at irradiation time t
Moreover, the improved photocatalytic activity after PANI and the photostability of the photocatalyst were also retained [15]. The circulating runs in the photocatalytic degradation of the attendance of BiVO4/GO/PANI under visible light (λ > 420 nm) were checked after five reuses for the photodegradation of MB; the catalyst did not expose any vital loss of activity. This indicates that the BiVO4/GO/PANI photocatalyst has high steadiness and does not photocorrode through the photocatalytic oxidation of the model pollutant molecules. PANI is cheaper than noble metals; so, the BiVO4/GO/PANI photocatalyst is promising for practical application in water purification.
Photocatalytic mechanism
The above experiment demonstrates the tremendous photocatalytic performance of the as-prepared spherical-like BiVO4/GO/PANI on the degradation of most commercial dyes. It follows that the PANI-modified BiVO4/GO photocatalyst may have high potential applications in the conservation of the environment. Not only limited to the investigational results, but the photodegraded mechanism of dyes in the visible-light BiVO4/GO/PANI structure was also vital to discover and guide the further improvement in its photocatalytic performance. The promising photocatalytic mechanism was estimated as follows:
Owing to the relative energy level of PANI (π-orbital and π*-orbital) and BiVO4/GO (conduction band, CB, and valence band, VB) [13, 29], which leads to a synergetic effect, the photogenerated holes in VB can directly transport to the π-orbital of PANI. In this case, GO acts as an electron transferring media and helps electron–hole recombination (shown in Fig. 13).
Simultaneously, the photogenerated electrons can transport to the CB of BiVO4, which results in charge separation and stabilization, hence hindering the recombination process. PANI is a high-quality substantial for transferring holes and the grain size of the photocatalyst is also comparatively minor [12]; so, the photogenerated charges can easily travel to the exterior of photocatalysts and photodegrade the adsorbed contaminations.
Investigation of safety aspects
There are various techniques for exploring the safety characteristics of nanomaterials such as colony counting method. This generally is used to verify the viability of bacteria by a live/dead fluorescent staining method by using impermeable propidium iodide dye where the viability of a cell is determined by the green or red color under confocal laser scanning microscope. Though, these are limited in tracing the bacterial cells that came in contact with the BiVO4, BiVO4/GO and BiVO4/GO/PANI nanomaterials over time and observing the continuous change in the bacterial membrane for a detailed study on the antibacterial mechanism of BiVO4, BiVO4/GO and BiVO4/GO/PANI nanomaterials.
To measure the special effects of the BiVO4, BiVO4/GO and BiVO4/GO/PANI nanocomposite film, the volume and dry mass of individual bacteria were more calculated from the measured 3D RI tomograms. Figure 14 shows the relative dry mass and bacterial cell volume over time. The relative dry masses of live bacteria after 20 or 80 min of incubation on the BiVO4, BiVO4/GO and BiVO4/GO/PANI nanocomposite film were intact. It was detected that the dry mass of live bacteria did not change substantially. These effects noticeably specify that BiVO4, BiVO4/GO and BiVO4/GO/PANI nanocomposite film could not decrease both bacterial volume and dry mass, attended by damage of intracellular components and cell membranes.
Conclusions
For the first time, the spindle-like BiVO4/GO/PANI photocatalyst was prepared by a hydrothermal process. The lesser grain size, an intrinsic belonging of PANI and the synergistic effect of PANI and BiVO4/GO resulted in the rapid electron–hole separation and slow recombination. In consequence, the photodegradation of MB and safranin O with PANI-modified BiVO4/GO photocatalysts under visible light (λ > 420 nm) was remarkably enhanced. The photocatalytic efficacy was also enhanced by the PANI. In addition to the enhanced photoactivity, the photostability of the photocatalyst was also retained. This work not only provides a mechanism for photocatalytic degradation but also opens a new perspective and gives some vision into the design of newly improved photocatalysts with greater activity for environmental purification and other applications.
References
Xiao J, Xie Y, Cao H (2015) Organic pollutants removal in wastewater by heterogeneous photocatalytic ozonation. Chemosphere 121:1–17
Linsebigler AL, Lu G, Yates JT (1995) Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results. Chem Rev 95:735–758
Li H, Yu H, Quan X, Chen S, Zhao H (2015) Improved photocatalytic performance of heterojunction by controlling the contact facet: high electron transfer capacity between TiO2 and the 110 facet of BiVO4 caused by suitable energy band alignment. Adv Func Mater 25:3074–3080
Ameen S, Seo HK, Akhtar MS, Shin HS (2012) Novel graphene/polyaniline nanocomposites and its photocatalytic activity toward the degradation of rose Bengal dye. Chem Eng J 210:220–228
Yuan H, Liu J, Li J, Li Y, Wang X, Zhang Y, Jiang J, Chen S, Zhao C, Qian D (2015) Designed synthesis of a novel BiVO4–Cu2O–TiO2 as an efficient visible-light-responding photocatalyst. J Colloid Interface Sci 444:58
Liao G, Chen S, Quan X, Zhang Y, Zhao H (2011) Remarkable improvement of visible light photocatalysis with PANI modified core–shell mesoporous TiO2 microspheres. Appl Catal B 102:126–131
Nanakkal AR, Alexander LK (2017) Graphene/BiVO4/TiO2 nanocomposite: tuning band gap energies for superior photocatalytic activity under visible light. J Mater Sci 52:7997–8006
Dreyer DR, Park S, Bielawski CW, Ruoff RS (2009) The chemistry of graphene oxide. Chem Soc Rev Chem Soc Rev 39:228–240
Zhu Y, Murali S, Cai W, Li X, Suk JW, Potts JR, Ruoff RS (2010) Graphene and graphene oxide: synthesis, properties, and applications. Adv Mater 22:3906–3924
Zhu Z, Han Q, Yu D, Sun J, Liu B (2017) A novel p-n heterojunction of BiVO4/TiO2/GO composite for enhanced visible-light-driven photocatalytic activity. Mater Lett 209:379–383
Ahmad I, Koziol K, Deveci S, Kim HK, Kumar R (2018) Advancing the use of high-performance graphene-based multimodal polymer nanocomposite at scale. Nanomaterials 8(11):947
Paulchamy B, Arthi G, Lignesh BD (2015) A simple approach to stepwise synthesis of graphene oxide nanomaterial. J Nanomed Nanotechnol 6(1):1
Wang X, Zhang J, Zhang K, Zou W, Chen S (2016) Facile fabrication of reduced graphene oxide/CuI/PANI nanocomposites with enhanced visible-light photocatalytic activity. RSC Adv 6:44851–44858
Zhao J, Biswas MRUD, Oh WC (2019) A novel BiVO4–GO–TiO2–PANI composite for upgraded photocatalytic performance under visible light and its non-toxicity. Environ Sci Pollut Res 26(12):11888–11904
Pandey M, Balachandran M, Joshi GM, Ghosh NN, Vendan AS (2019) Superior charge discharge ability of reduced graphene oxide/Li-ion embedded polymer composite films. J Mater Sci: Mater Electron 30(3):2136–2145
Ventura SP, de Barros RL, Sintra T, Soares CM, Lima AS, Coutinho JA (2012) Simple screening method to identify toxic/non-toxic ionic liquids: agar diffusion test adaptation. Ecotoxicol Environ Saf 83:55–62
Kirby W, Bauer AW, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Pathol 45:493–496
Hokimura S, Moniz SA, Handoko A, Tang J (2014) Enhanced photoelectrochemical water splitting by nanostructured BiVO4–TiO2 composite electrodes. J Mater Chem A 2:3948–3953
Miao J, Xie A, Li S, Huang F, Cao J, Shen Y (2016) A novel reducing graphene/polyaniline/cuprous oxide composite hydrogel with unexpected photocatalytic activity for the degradation of Congo red. Appl Surf Sci 360:594–600
Al-Hussaini S, Eltabie RK, Rashad MEE (2016) One-pot modern fabrication and characterizati on of TiO 2 @terpoly (aniline, anthranilic acid and o-phenylenediamine) core–shell nanocomposites via polycondensation. Polymer 101:328–337
Patil PT, Anwane RS, Kondawar SB (2015) Development of electrospun polyaniline/ZnO composite nanofibers for LPG sensing. Procedia Mater Sci 10:195–204
Wu W, Liang S, Shen L, Ding Z, Zheng H, Su W, Wu L (2012) Preparation, characterization and enhanced visible light photocatalytic activities of polyaniline/Bi3 NbO7 nanocomposites. J Alloy Compd 520:213–219
Lin YC, Hsu FH, Wu TM (2013) Enhanced conductivity and thermal stability of conductive polyaniline/graphene composite synthesized by in situ chemical oxidation polymerization with sodium dodecyl sulfate. Synth Met 184:29–34
Zhang K, Zhang LL, Zhao XS, Wu J (2010) Graphene/polyaniline nanofiber composites as supercapacitor electrodes. Chem Mater 22:1392–1401
Li H, Sun Y, Cai B, Gan S, Han D, Niu L, Wu T (2015) Hierarchically Z-scheme photocatalyst of Ag@ AgCl decorated on BiVO4 (040) with enhancing photoelectrochemical and photocatalytic performance. Appl Catal B 170:206–214
Huang CM, Pan GT, Peng PY, Yang CK (2010) In situ DRIFT study of photocatalytic degradation of gaseous isopropanol over BiVO4 under indoor illumination. J Mol Catal A: Chem 327:38–44
Yousefzadeh S, Faraji M, Moshfegh AZ (2016) Constructing BiVO4/Graphene/TiO2 nanocomposite photoanode for photoelectrochemical conversion applications. J Electroanal Chem 763:1–9
Jing L, Yang ZY, Zhao YF, Zhang YX, Guo X, Yan YM, Sun KN (2013) Ternary polyaniline-graphene-TiO2 hybrid with enhanced activity for visible-light photo-electrocatalytic water oxidation. J Mater Chem A 2:1068–1075
Domingues SH, Salvatierra RV, Oliveira MM, Zarbin AJ (2011) Transparent and conductive thin films of graphene/polyaniline nanocomposites prepared through interfacial polymerization. Chem Commun 47:2592–2594
Li X, Teng W, Zhao Q, Wang L (2011) Efficient visible light-induced photoelectrocatalytic degradation of rhodamine B by polyaniline-sensitized TiO2 nanotube arrays. J Nanopart Res 13:6813–6820
Biswas MRUD, Oh WC (2018) Synthesis of BiVO4–GO–PVDF nanocomposite: an excellent, newly designed material for high photocatalytic activity towards organic dye degradation by tuning band gap energies. Solid State Sci 80:22–30
Yu J, Xiong J, Cheng B, Liu S (2005) Fabrication and characterization of Ag–TiO2 multiphase nanocomposite thin films with enhanced photocatalytic activity. Appl Catal B 60(3–4):211–221
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6(3):183
Azam A, Ahmed AS, Oves M, Khan MS, Habib SS, Memic A (2012) Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. Int J Nanomed 7:6003
Reddy KR, Karthik KV, Prasad SBB, Soni SK, Han MJ, Raghu AV (2016) Enhanced photocatalytic activity of nanostructured titanium dioxide/polyaniline hybrid photocatalysts. Polyhedron 120:169–174
Biswas MRUD, Oh WC (2019) Comparative study on gas sensing by a Schottky diode electrode prepared with graphene–semiconductor–polymer nanocomposites. RSC Adv 9(20):11484–11492
Kim F, Cote LJ, Huang J (2010) Graphene oxide: surface activity and two-dimensional assembly. Adv Mater 22(17):1954–1958
Cote LJ, Kim J, Tung VC, Luo J, Kim F, Huang J (2010) Graphene oxide as surfactant sheets. Pure Appl Chem 83(1):95–110
Wang Y, Li Z, Wang J, Li J, Lin Y (2011) Graphene and graphene oxide: biofunctionalization and applications in biotechnology. Trends Biotechnol 29(5):205–212
Biswas MRUD, Cho KY, Jung CH, Oh WC (2019) Novel synthesis of LaNiSbWO4–G–PANI designed as quaternary type composite for high photocatalytic performance of anionic dye and trihydroxybenzoic acid under visible-light. Process Saf Environ Prot 126:348–355
Xu Y, Mo Y, Tian J, Wang P, Yu H, Yu J (2016) The synergistic effect of graphitic N and pyrrolic N for the enhanced photocatalytic performance of nitrogen-doped graphene/TiO2 nanocomposites. Appl Catal B 181:810–817
Su W, Lu X, Jia S, Wang J, Ma H, Xing Y (2015) Catalytic reduction of NOX Over TiO2–graphene oxide supported with MnOX at low temperature. Catal Lett 145(7):1446–1456
Dowla BMRU, Cho JY, Jang WK, Oh WC (2017) Synthesis of BiVO4–GO–PTFE nanocomposite photocatalysts for high efficient visible-light-induced photocatalytic performance for dyes. J Mater Sci: Mater Electron 28(20):15106–15117
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received in this study. It has been done by self-funding.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Biswas, M.R.U.D., Ho, B.S. & Oh, WC. Eco-friendly conductive polymer-based nanocomposites, BiVO4/graphene oxide/polyaniline for excellent photocatalytic performance. Polym. Bull. 77, 4381–4400 (2020). https://doi.org/10.1007/s00289-019-02973-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-02973-y