Abstract
A novel bismuth vanadate–silicon dioxide–graphene oxide (BiVO4/SiO2/GO) nanocomposite was synthesized successfully by using hydrothermal method. The as-prepared nanocomposite was characterized by various techniques, including X-ray diffraction (XRD), field emission scanning electron microscope (SEM), and Brunauer–Emmett–Teller (BET). The photocatalytic activity of nanoparticles was evaluated by measuring the removal efficiency of methylene blue (MB) in aqueous solution under visible light irradiation. The results indicated that BiVO4, BiVO4/SiO2, and BiVO4/SiO2/GO exhibited the same diffraction peaks of monoclinic scheelite structure due to the higher content of BiVO4 in the nanocomposite system. The BiVO4 and BiVO4/SiO2 were uniformly agglomerated from irregular particles with the different level of aggregation and the size of several micrometers. In the meantime, BiVO4 and SiO2 particles were adhered firmly on the GO sheets in BiVO4/SiO2/GO nanocomposite. The BiVO4/SiO2/GO nanocomposite showed a much higher efficiency than the pure BiVO4 and the BiVO4/SiO2 hybrid material for MB removal efficiency due to the considerably increased specific surface area and pore size by the adhesion of BiVO4 and SiO2 on GO sheets.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The unceasing development of industries has been leading to the serious pollution of the environment. The contaminated water source has become an urgent issue because various industrial processes and human activities have been releasing organic pollutants which can generate toxicity and carcinogenicity for aqueous environment [1, 2]. Therefore, water treatment has been considered as an effective way to mitigate the water crisis and pollution. Many conventional water treatments such as ion exchange [3], adsorption [4], membrane [5], and biological processes [6] have been used over the past years. However, these conventional treatments can only transfer organic pollutants into other phases or secondary toxic byproducts; hence, it is difficult to meet the increasing criteria of water quality [7]. As a result, the development of alternative technologies has been acquired to solve these issues. By far, photocatalytic technology using semiconductor has drawn much attention of scientists due to its advantages such as complete mineralization, no further waste disposal, low cost, mild temperature, and pressure conditions [8]. Among photocatalytic semiconductors, titanium dioxide (TiO2) is considered as a benchmark catalyst with a wide band gap (3.2 eV), which has been used extensively for years [9,10,11]. Due to its wide band gap, TiO2 is only activated under ultraviolet (UV) light which occupies 3–5% of the whole solar light [12]. Therefore, this is a huge limitation and is economically inefficient for a photocatalytic system.
Recently, bismuth vanadate (BiVO4) has emerged as an ideal visible light-driven photocatalyst which can adsorb effectively the visible light due to the narrow band gap of 2.4 to 2.8 eV. Besides, it also has outstanding properties such as environmentally friendly, resistance to corrosion, high stability, good dispersibility, and inexpensiveness [2, 13, 14]. However, the photocatalytic applications of BiVO4 are limited by the poor charge transportation and the weak surface adsorption [15]. Due to these drawbacks, various strategies have been developed, such as constructing heterostructure system [16, 17], adding bias energy [18, 19] and doping with other materials [20] for enhancing the photocatalytic activity of BiVO4.
The combination of BiVO4 with metal oxide or porous materials has demonstrated the effective improvement of the specific surface area, the crystalline structure, and the photocatalytic activity in recent years [21]. Among these hybrid materials, mesoporous silica (SiO2) has been considered as a promising porous material due to its good properties such as well biocompatibility, large specific surface area, and easy functionalization [22, 23]. Thus, doping SiO2 with BiVO4 fabricates the p–n junction which enhances the separation of electron–hole pairs. Besides, the slow photons generated by SiO2 also enhance the light utilization [24]. As a result, it is expected that the immobilization of SiO2 on the surface of BiVO4 can strengthen effectively the separation of electron–hole pairs and the absorption ability leading to the enhanced photocatalytic activity. In addition to SiO2, graphene oxide (GO) has also been used as an effectively supporting material to disperse and stabilize photocatalyst in photocatalysis. It has many marvelous properties, such as high conductivity, high surface area, high transport mobility, and greater mechanical elasticity, due to the dense atoms and the honeycomb structure composed of sp2-bonded carbon atoms [25]. Furthermore, GO has oxygen containing functional groups which can act as favorable anchoring centers for active species in photocatalytic process. As a result, it helps to prevent the recombination of electron–hole pairs and further improve the photocatalytic activity [26]. However, the researches on the BiVO4/SiO2/GO nanocomposites have been rarely reported. Besides, the conventional methods use multistep approach and strong reducing chemical for the fabrication of nanocomposite.
Therefore, the present work aims to improve the photocatalytic activity of BiVO4 by the addition of SiO2 and GO using a one-step hydrothermal method. Various analytical techniques, including X-ray diffraction (XRD), scanning electron microscopy (SEM), and Brunauer–Emmett–Teller (BET), were used to clarify the morphology and crystalline structure of BiVO4/SiO2/GO nanocomposites. Furthermore, the dye removal efficiency of nanoparticles was evaluated by measuring the removal percentage of methylene blue (MB) in the batch test under the visible light irradiation.
Methodology
Materials
All chemicals were obtained from Sigma-Aldrich and were used as received without any further purification, including bismuth (III) nitrate pentahydrate [Bi(NO3)3.5H2O], ammonium metavanadate (NH4VO3), graphite flake, tetraethyl orthosilicate (TEOS), potassium permanganate (KMnO4), sulfuric acid (H2SO4), sodium nitrate (NaNO3), hydrochloric acid (HCl), hydrogen peroxide (H2O2), sodium hydroxide (NaOH), methylene blue (C16H18ClN3S), benzoquinone (C6H4O2), terephthalic acid (C8H6O4), and ammonium oxalate (C2H8N2O4).
Preparation of graphene oxide
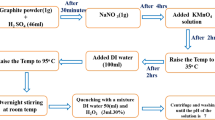
In the present work, GO was synthesized by using Hummers’ method [27] with a modification from graphite flakes. In this method, 10 g of graphite flake and 5 g NaNO3 was mixed in 600 mL of H2SO4 solution under an ice bath. Then, 60 g of KMnO4 was added slowly into the solution and stirred for 2 h. Subsequently, the mixture was heated at 35 °C for 24 h. After cooling down to room temperature, 1 L of DI water was poured slowly into this mixture and kept at temperature not exceeding 90 °C. In order to eliminate the excess of KMnO4, 5 mL of H2O2 was dropped into the mixture by which the color changes to bright yellow. The resulting mixture was washed repeatedly by the centrifugation with 5% HCl, ethanol, and DI water until the pH was neutral. Finally, the resultant was dried at 80 °C for 24 h to get GO powder.
Preparation of nanocomposites
Typically, 5 mmol of Bi(NO3)3.5H2O and 5 mmol of NH4VO3 were stirred separately in 50 mL of ethanol, while an amount of GO was sonicated in 20 mL ethanol for 30 min. Then, these solutions were mixed together, and followed by adding 1 mL of TEOS and 5 mL of DI water. This mixture was stirred for 3 h at room temperature and adjusted to pH 5 by NaOH 2M before heated at 180 °C for 10 h in a Teflon-lined stainless steel autoclave. Finally, the resultant was washed with ethanol and DI water for few times, and then dried at 80 °C for 24 h.
In comparison, pure BiVO4 and BiVO4/SiO2 were also synthesized in the same procedure without the addition of GO sheets.
Characterization
The morphological structure and crystal phase of the as-prepared nanocomposites were characterized by X-ray powder diffraction (XRD, Philips X’Pert MPD) using Cu Kα (λ = 1.54056 Å) radiation and field emission scanning electron microscopy (SEM, LEO1455VP), and Brunauer–Emmett–Teller (BET) measurements (Adtosorb 1 MP, Quantachrome) were conducted to investigate the surface properties of sample.
Photocatalytic activity
The photocatalytic activity of the as-prepared samples was evaluated by the removal MB in solution. The photocatalytic experiment was carried out as follows: 0.05 g of nanoparticle was added to 50 mL of 10−5 M of MB solution. Initially, the solution was stirred and kept in dark condition for 30 min to reach the adsorption–desorption equilibrium. Afterwards, it was irradiated by two 18-W halogen lamps (Essential MR, Philips, Thailand) for 30 min. The sample was collected every 10 min and measured by UV–Vis spectrophotometer (UV–6100 Double beam spectrophotometer, Mapada) at 664 nm of wavelength.
Results and discussions
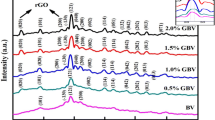
Figure 1 presents the phase compositions of GO, pure BiVO4, BiVO4/SiO2, and BiVO4/SiO2/GO nanocomposites, which was analyzed by XRD technique. Generally, it can be seen that pure BiVO4, BiVO4/SiO2, and BiVO4/SiO2/GO nanocomposites show almost the same diffraction peaks corresponding to the monoclinic scheelite of BiVO4 (JCPDS no. 14–0688) which exhibits the highest photocatalytic activity [28]. In the meantime, GO sample is observed mainly at around 11° of 2θ. However, the peak of GO disappeared in the XRD pattern of BiVO4/SiO2/GO nanocomposites, this is because GO is probably reduced to graphene during hydrothermal process and the content of GO is low in the nanocomposite system [13].
The morphology of as-prepared samples was investigated by SEM analysis as shown in Fig. 2. From Fig. 2a, it can be seen that the GO sheets are an aggregation of thin sheets with distinct edges and wrinkled surface. In the meantime, BiVO4 and BiVO4/SiO2 are formed by irregular particles with the different aggregation and the size of several micrometers. Figure 2d shows the morphology of BiVO4/SiO2/GO nanocomposite where BiVO4 and SiO2 are supported uniformly on the GO sheets, this is because the reduction of GO to graphene during hydrothermal reaction and the functional groups of GO facilitates to the adhesion of BiVO4 and SiO2 on the sheets.
Figure 3 presents the removal efficiency of MB over nanoparticles under visible light irradiation. For pure BiVO4, 25% of MB was adsorbed under the dark adsorption, and 47% of MB was degraded after 30 min under visible light irradiation.
In the meantime, 50% of MB was adsorbed under the dark adsorption in the case of BiVO4/SiO2 due to the increase of the specific surface area and the pore size as shown in Table 1. The data indicates that the BiVO4/SiO2 has a higher specific surface area than pure BiVO4. Besides, the pore size is also increased from 12.11 of BiVO4 to 12.29 of BiVO4/SiO2; thus, BiVO4/SiO2 has a better adsorption for the degradation of MB than pure BiVO4. This increases the contact of photocatalyst and dye molecules leading to approximately 77% of MB photodegraded over BiVO4/SiO2 under visible light irradiation. Similarly, the adsorptive capacity is significantly increased by the addition of GO nanosheets in the BiVO4/SiO2/GO nanocomposite, this is because the specific surface area of BiVO4/SiO2/GO nanocomposite is 3.5 and 1.7 times higher than that of the pure BiVO4 and the BiVO4/SiO2 respectively, leading to 88% of MB adsorption after only 30 min of dark condition. Moreover, the migration and the separation of photogenerated carriers are also improved by the high conductivity and the mobility of GO nanosheets. Therefore, BiVO4/SiO2/GO nanocomposite exhibited the highest efficiency for MB removal efficiency, where 95% of concentration is removed under 30 min of visible light irradiation. This proves that the photocatalytic activity of BiVO4/SiO2/GO nanocomposite has been improved greatly for the MB dye removal and it can be considered as a promising photocatalyst for further applications.
Conclusions
In this work, BiVO4/SiO2/GO nanocomposites have been successfully synthesized by hydrothermal method. The analytic results showed that the as-prepared samples have the same diffraction peaks of monoclinic scheelite of BiVO4 structure. The BiVO4 and SiO2 were integrated on the GO sheets with the different levels of aggregation and the size of several micrometers. The BiVO4/SiO2/GO nanocomposites exhibited a much higher MB removal efficiency compared with BiVO4 and BiVO4/SiO2 because the specific surface area and the pore size of BiVO4/SiO2/GO were increased significantly by the addition of SiO2 and GO. As a result, nanocomposites can be considered as promising materials for aqueous organic pollutant. In addition, the results in the present work also indicated the very high significant data on the difference between dye removal and dye degradation mechanisms. The detail of this will be discussed in the further work.
References
Mubarak, N.M., Sahu, J.N., Abdullah, E.C., Jayakumar, N.S.: Removal of heavy metals from wastewater using carbon nanotubes. Sep Purif Rev. 43(4), 311–338 (2014)
Zhang, L., Wang, A., Zhu, N., Sun, B., Liang, Y., Wu, W.: Synthesis of butterfly-like BiVO4/RGO nanocomposites and their photocatalytic activities. Chin J Chem Eng. 26(3), 667–674 (2018)
Landry, K.A., Boyer, T.H.: Diclofenac removal in urine using strong-base anion exchange polymer resins. Water Res. 47(17), 6432–6444 (2013)
De Martino, A., Iorio, M., Xing, B., Capasso, R.: Removal of 4-chloro-2-methylphenoxyacetic acid from water by sorption on carbon nanotubes and metal oxide nanoparticles. RSC Adv. 2(13), 5693–5700 (2012)
Zhang, Y., Causserand, C., Aimar, P., Cravedi, J.P.: Removal of bisphenol A by a nanofiltration membrane in view of drinking water production. Water Res. 40(20), 3793–3799 (2006)
Nharingo, T., Moyo, M.: Application of Opuntia ficus-indica in bioremediation of wastewaters. A critical review. J Environ Manage. 166, 55–72 (2016)
Dang, T.T.T., Le, S.T.T., Channei, D., Khanitchaidecha, W., Nakaruk, A.: Photodegradation mechanisms of phenol in the photocatalytic process. Res Chem Intermed. 42(6), 5961–5974 (2016)
Min, O.-M., Ho, L.-N., Ong, S.-A., Wong, Y.-S.: Comparison between the photocatalytic degradation of single and binary azo dyes in TiO2 suspensions under solar light irradiation. J Water Reuse Desal. 5(4), 579–591 (2015)
Zhu, X.-D., Wang, Y.-J., Sun, R.-J., Zhou, D.-M.: Photocatalytic degradation of tetracycline in aqueous solution by nanosized TiO2. Chemosphere. 92(8), 925–932 (2013)
Zayani, G., Bousselmi, L., Pichat, P., Mhenni, F., Ghrabi, A.: Photocatalytic degradation of the Acid Blue 113 textile azo dye in aqueous suspensions of four commercialized TiO2 samples. J Environ Sci Health C. 43(2), 202–209 (2008)
Habibi, M.H., Tangestaninejad, S., Yadollahi, B.: Photocatalytic mineralisation of mercaptans as environmental pollutants in aquatic system using TiO2 suspension. Appl Catal B Environ. 33(1), 57–63 (2001)
Ahmed, T., Zhang, H.-L., Gao, Y.-Y., Xu, H.-B., Zhang, Y.: Surfactant-free synthesis of m-BiVO4 nanoribbons and enhanced visible-light photocatalytic properties. Mater Res Bull. 99, 298–305 (2018)
Dang Trung Tri, T., Duangdao, C., Willawan, K., Auppatham, N.: Photocatalytic degradation of organic contaminants by BiVO4/graphene oxide nanocomposite. Walailak J Sci Technol. 15(11), (2018)
Zong, L., Cui, P., Qin, F., Zhao, K., Wang, Z., Yu, R.: Heterostructured bismuth vanadate multi-shell hollow spheres with high visible-light-driven photocatalytic activity. Mater Res Bull. 86, 44–50 (2017)
Nguyen, D.T., Hong, S.-S.: Synthesis of needle-like BiVO4 with improved photocatalytic activity under visible light irradiation. J Nanosci Nanotechnol. 19(12), 7696–7701 (2019)
Ibrahim, A.A.M., Khan, I., Iqbal, N., Qurashi, A.: Facile synthesis of tungsten oxide – bismuth vanadate nanoflakes as photoanode material for solar water splitting. Int J Hydrog Energy. 42(5), 3423–3430 (2017)
Lopes, O.F., Carvalho, K.T.G., Avansi, W., Ribeiro, C.: Growth of BiVO4 nanoparticles on a Bi2O3 surface: effect of heterojunction formation on visible irradiation-driven catalytic performance. J Phys Chem C. 121(25), 13747–13756 (2017)
Xi, L., Jin, Z., Sun, Z., Liu, R., Xu, L.: Enhanced photoelectrocatalytic performance for water oxidation by polyoxometalate molecular doping in BiVO4 photoanodes. Appl Catal A Gen. 536, 67–74 (2017)
Yu, F., Li, F., Yao, T., Du, J., Liang, Y., Wang, Y., Han, H., Sun, L.: Fabrication and kinetic study of a ferrihydrite-modified BiVO4 photoanode. ACS Catal. 7(3), 1868–1874 (2017)
Chen, F., Yang, Q., Li, X., Zeng, G., Wang, D., Niu, C., Zhao, J., An, H., Xie, T., Deng, Y.: Hierarchical assembly of graphene-bridged Ag3PO4/Ag/BiVO4 (040) Z-scheme photocatalyst: an efficient, sustainable and heterogeneous catalyst with enhanced visible-light photoactivity towards tetracycline degradation under visible light irradiation. Appl Catal B Environ. 200, 330–342 (2017)
Channei, D., Nakaruk, A., Khanitchaidecha, W., Jannoey, P., Phanichphant, S.: Adsorption and photocatalytic processes of mesoporous SiO2-coated monoclinic BiVO4. Front Chem. 6, 415–415 (2018)
Liu, B., Wang, Z., Zhou, S., He, J.: Synthesis and characterization of a novel BiVO4/SiO2 nanocomposites. Mater Lett. 160, 218–221 (2015)
Zhuravlev, L.T.: Concentration of hydroxyl groups on the surface of amorphous silicas. Langmuir. 3(3), 316–318 (1987)
Liu, B., Lin, L., Yu, D., Sun, J., Zhu, Z., Gao, P., Wang, W.: Construction of fiber-based BiVO4/SiO2/reduced graphene oxide (RGO) with efficient visible light photocatalytic activity. Cellulose. 25(2), 1089–1101 (2018)
Posa, V.R., Annavaram, V., Somala, A.R.: Fabrication of graphene–TiO2 nanocomposite with improved photocatalytic degradation for acid orange 7 dye under solar light irradiation. Bull Mater Sci. 39(3), 759–767 (2016)
Sheshmani, S., Nayebi, M.: Modification of TiO2 with graphene oxide and reduced graphene oxide; enhancing photocatalytic activity of TiO2 for removal of remazol Black B. Polym Compos. 40(1), 210–216 (2019)
Hummers, W.S., Offeman, R.E.: Preparation of graphitic oxide. J Am Chem Soc. 80(6), 1339–1339 (1958)
Phiankoh, S., Munprom, R.: Effect of pH on crystal structure and morphology of hydrothermally-synthesized BiVO4. Mater Today Proc. 5(3, Part 2), 9447–9452 (2018)
Acknowledgements
This work was supported by the Thailand Research Fund (TRF) and Office of the Higher Education Commission (CHE) under grant number MRG6280017.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Trinh, D.T.T., Channei, D., Chansaenpak, K. et al. Photocatalytic degradation of organic dye over bismuth vanadate–silicon dioxide–graphene oxide nanocomposite under visible light irradiation. J Aust Ceram Soc 56, 1237–1241 (2020). https://doi.org/10.1007/s41779-020-00470-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-020-00470-4