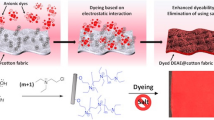
Abstract
The preparation and characterization of modified cotton fabrics with responsive pH was studied. The objective was to prepare the modified cotton fabric with pH sensitivity and evaluate the color change of the dyed cotton fabrics in acetic acid and urea solutions. The cotton fabric was cationized with diallyldimethylammonium chloride at room temperature by using potassium persulfate as an initiator, N,N′-methylenebisacrylamide as a cross-linker and N,N,N′,N′-tetramethylethylenediamine as an accelerator. The unmodified and modified cotton fabrics were dyed with a bromothymol blue at room temperature for 1 h and the color in various solutions measured. It was found that the color on the dyed cotton fabrics responsed with the pH as the smart cotton fabrics. In addition, the addition of the N,N′-methylenebisacrylamide in the polymerization reaction caused the deep shade in comparison with no N,N′-methylenebisacrylamide. Factors affecting the color change of the dyed cotton fabric with the bromothymol blue were concentration of diallyldimethylammonium chloride, ionic capacity, concentration of N,N,N′,N′-tetramethylethylenediamine and type of solutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Smart materials have properties which response to stimuli from the external environment. Also, they reverse to the original state as the stimulus is removed [1]. The change in properties of the smart materials may be from temperature, electric field, magnetic field, pH and chemical agents. Smart materials are used in the textile industry and make smart textiles. The examples of the researches about the smart textile were shape memory of the thermo-sensitive polyurethane fibers [2] and the development of a smart slimming textile fabric by coating with a smart nanocomposite [3] and the study of smart cotton fabric using encapsulating lime oil [4].
Modification of a cotton fabric might be done by physical method [5, 6] or chemical method by using different types of chemicals [7, 8]. Researches have found that the cotton fabrics were improved in various areas [9,10,11,12] and included the cationization with a quaternary ammonium compound for improvement in the properties [13, 14].
In the field of dyeing, the cotton fabric is usually dyed with reactive dye due to the good fast washing property and bright colors [15, 16]. The quaternary ammonium ion was used to modify the cellulose [17]. Thus, the cationic cotton could be dyed with anionic dyes [18]. The textile materials were, also, dyed with colorants that changed color when they were stimulated by ultraviolet light (photochromic colorant) or heat (thermochromic colorant) [19]. Furthermore, the dyeing of the textile materials with the dyes sensitive to pH is very interesting.
Acid–base indicators (also known as pH indicators) are substances which change color with pH. They are usually weak acids or bases. Bromothymol blue is one of the pH indicator and changes color in the pH range 6.0–7.6 appearing in color ranges from yellow to blue. Many research papers reported about the usage of the bromothymol blue. Cellulose/polypyrrole mixed with the bromophenol blue and bromothymol blue for optical sensors was studied [20]. Cao et al. [21] prepared the pH-sensitive cellulose via the anchoring of bromothymol blue on cellulose grafted with hydroxypropyltriethylamine. The adsorption of the bromothymol blue by chitosan was reported by Pacheco [22].
The change in color as a result of change in acidity was called the pH sensitivity of the materials [1]. The bromothymol blue is used less in textile. For preparation of the dyed fabric with the pH sensitivity, the bromothymol blue was used as the acid dye model in this study. The color change in the dyed cotton fabrics with the bromothymol blue made it act as the smart textile. It responded to change in color with the pH value. However, in the textile field, the dyed materials were usually estimated by using CIE color system especially CIELAB which measure L* (lightness), a* (red/green value) and b* (yellow/blue value) [23]. In this system, the color (or shade) of the dyed fabrics was reported in the numerical results with specific value.
The use of the bromothymol blue in the textile materials and the color changing should be investigated. Therefore, this research was studied to prepare and characterize the modified cotton fabric with responsive pH. The cotton fabric was modified with diallyldimethylammonium chloride (DADMAC) with and without N,N′-methylenebisacrylamide and dyed with the bromothymol blue. The modified cotton fabrics were characterized. Also, the color assessment of the modified cotton fabrics in the acid–base conditions was carried out.
Experimental
Materials
Diallyldimethylammonium chloride (DADMAC), potassium persulphate, N,N′-methylenebisacrylamide (MBAM), N,N,N′,N′-tetramethylethylenediamine (TMEDA) and bromothymol blue (BTB) were purchased from Aldrich (Singapore). Cotton fabric was supplied by the Rajamangala University of Technology Krungthep.
Preparation of modified cotton fabrics
First, DADMAC [2.5–10% (v/v)] and water were taken in 250-mL beaker at room temperature. Next, K2S2O8 (2% by wt of monomer), MBAM (0–0.5% by wt of monomer) and TMEDA (1 mL) were added and stirred gently. And then a cotton fabric was soaked in this solution. The stirring was continued for 1 h. Finally, the treated sample was washed with distilled water and dried.
Dyeing procedure
The unmodified and modified cotton fabrics were dyed at 1% (owf) with a 40:1 liquor ratio at room temperature for 1 h. The samples were then rinsed in distilled water for three times and dried.
Characterization
Determination of percentage grafting
The percentage grafting of modified cotton was determined by weighing method. The modified cotton was weighed after washing. The percentage of graft yield was calculated from the following equation.
where Wo represents the dry weight of sample initially and W is the dry weight of modified sample after washing.
Elemental analysis
The nitrogen content of the unmodified and modified cotton fabrics was measured by Perkin-Elmer CHNS/O Analyzer 2400 Series II.
FTIR measurement
FTIR spectra of the poly(diallyldimethylammonium chloride) (poly(DADMAC)), cotton and modified cotton were recorded in KBr pellets by using an Omnic Nicolet Impact 400 D FTIR spectrophotometer.
Morphology of cotton fabrics
The surface morphologies of the cotton fabrics were observed with scanning electron microscope (SEM: JEOL JSM-5410LV).
Color assessment
The color of the dyed cotton fabrics in various solutions (1% acetic acid, 10% acetic acid, 1% urea and 10% urea) was measured using datacolor 600. When a color is expressed in CIELAB, L* denotes the lightness, a* means the green/red value and b* the yellow/blue value. In this experiment, the a* and b* values were plotted in the CIELAB color chart.
Results and discussion
Modification of cotton fabrics
The cotton fabric was modified with DADMAC at concentration of 2.5–10% (v/v). Also, it was modified with DADMAC at concentration of 5% (v/v) mixed with MBAM (0.1–0.5% by wt of monomer). Table 1 shows result of the effects of DADMAC and MBAM concentration on the graft yield obtained. Table 1 shows that the grafting of cotton fabric with DADMAC gave low % graft yield. This may be due to the fact that DADMAC is a water-soluble quaternary ammonium compound. It favors to form homopolymer in water rather than grafting onto the cotton fabrics. The homopolymer accumulated increases the reaction viscosity, which inhibits DADMAC diffusion toward grafting polymer chain. However, in the determination of nitrogen content, it was found that the % nitrogen increased with increase in the monomer concentration. Thus, grafting could occur. For grafting of the cotton fabrics with DADMAC mixed with MBAM, the % graft yield and nitrogen content increased with increase in the MBAM concentration. This resulted from the cross-linking of the poly(DADMAC) with MBAM.
Figure 1 shows FTIR spectra of the poly(DADMAC), cotton and cotton modified with DADMAC at concentration of 10.0% (v/v). In the FTIR spectrum of poly(DADMAC), –CH3 stretching was confirmed at 1477 and 2945 cm−1, attributed to –CH2 groups, C–N stretching at 1105 cm−1 and quaternary ammonium groups at 961 cm−1. In the FTIR spectrum of the cotton, the absorption band at 3411 cm−1 corresponded to the absorbance of –OH functional groups; at 1058 cm−1, it attributed to C–O stretching. In the FTIR spectrum of the modified cotton, the absorption spectrum appeared at 961 cm−1, 1477 cm−1 and 2945 cm−1. It was due to C–H bending and stretching of methyl groups, confirming the introduction of quaternary ammonium groups in the cotton cellulose structure. The processes of modification of the cotton fabrics as well as cross-linking are shown in Schemes 1 and 2.
Figure 2 shows scanning electron micrographs of the unmodified and modified cotton fabrics. As a result of polymerization reaction of DADMAC, on the surface of the cotton fabric appeared the small cluster of the poly(DADMAC) attached to the cotton fibers. Also, the result showed that quaterinization of the cotton fabric decreased the ribbon-like structure of the fibers and the ridge appeared.
Effect of DADMAC on color change in dyed cotton fabrics
The role of DADMAC concentration on the color change in the dyed cotton fabric with BTB in the solutions of acetic and urea was observed by changing the amount of the DADMAC from 2.5 to 10%. The color measurement in L*a*b* unit was used to evaluate the change in color. The results obtained are shown in Fig. 3 with the plot of the a* versus b* on the CIELAB color chart and Fig. 4 with the lightness.
When a color is expressed in L*a*b* or CIELAB, L* defines lightness, a* is the green/red value and b* denotes the yellow/blue value. The a* axis runs from left to right. A color measurement movement in the − a to + a direction means a shift from green to red. Along the b* axis, the movement of the − b to + b represents a color change from blue to yellow.
In general, when cellulose is in the water, it is ionized to form cellulosate ion with the negative charge. While BTB goes into the water, it is solubilized giving dye anions and sodium ions. During dyeing, both the negative ions of the BTB and cellulose repelled each other. Thus, very little exhaustion of the BTB appeared in the cotton fabric.
The modification of the cotton fabric with the DADMAC increased the BTB absorption. The presence of poly(DADMAC) onto the surface of the cotton fabric increased the affinity of the BTB toward the cellulose substrate. The interaction between poly(DADMAC) and BTB was the ionic interaction. However, the BTB acts as weak acid in solution. It can be in protonated or deprotonated form, showing yellow or blue, respectively. Also, in neutral solution, it appears bluish green. The pH range of the BTB is 6.0–7.6.
The color change in the unmodified and modified cotton fabric with DADMAC showed an interesting result. In the water (pH 7.10), the dyed cotton fabrics changed color from yellowish green (a* − 11.0, b* 33.95) to reddish yellow (a* 3.08, b* 29.97) as DADMAC concentration increased (see Fig. 3a). In the 1% acetic acid (pH 6.62), the a* and b* changed from (a* − 10.54, b* 35.94) to (a* 0.47, b* 29.52) with increasing the DADMAC concentration (see Fig. 3b). Also, in the 10% acetic acid (pH 3.98), the a* value changed from − 9.62 to 3.05 (see Fig. 3c). It indicated that the color showed more red or less green with the increase in the DADMAC concentration. In the alkaline solution of the 1% urea (pH 7.57) and in the 10% urea (pH 8.14), the color of the modified cotton fabric was similar (see Fig. 3d, e). In 1% urea, the a* and b* values change from (a* − 12.38, b* 33.51) to (a* − 1.36, b* 27.39), exhibiting more red (see Fig. 3d). In addition, in the 10% urea, the color shifted from yellowish green (a* − 13.95, b* 25.58) to dark green blue (a* − 5.52, b* 23.02) (see Fig. 3e). The lightness (L*) of the samples is shown in Fig. 4. The result showed that an increase in the DADMAC concentration decreased the lightness for all conditions. This result corresponded to the a* versus b* plots for each condition. Thus, the color change in the dyed cotton fabric resulted from the change in the concentration of DADMAC and type of solutions or pH of the solutions.
Effect of MBAM on color change in dyed cotton fabrics
Figure 5 shows the color change in various solutions of the modified cotton fabric with DADMAC with or without the addition of MBAM and dyed with the BTB. It can be seen that all the modified fabrics showed similar a* versus b* plots. The addition of the MBAM in polymerization reaction changed the color of the dyed cotton fabrics from − a (more green) to + a (less green). In the acid solution of 1% acetic acid (pH 6.62; weak acidity) (see Fig. 5b), it showed more red (less green) in comparison with the 10% acetic acid (pH 3.98; strong acidity) (see Fig. 5c). However, in the 1% urea (pH 7.57) (see Fig. 5d) and 10% urea (pH 8.14) (see Fig. 5e), the color of the dyed cotton fabrics was similar. The color shifted from bluish green (in 0% MBAM) to dark green (in 0.5% MBAM). Also, the lightness decreased with the increase in the MBAM concentration (see Fig. 6). Therefore, the MBAM had an effect on the color of the dyed cotton fabrics. Also, the modification of the cotton fabrics with the DADMAC mixed with MBAM exhibited intense color in comparison with no MBAM.
pH response of modified cotton fabrics
The pH sensitivity of the cationized cotton fabrics dyed with BTB was summarized in Table 2. In the case of modified cotton fabrics with DADMAC, the increase in the ionic capacity caused the a* value to moved from − a* to + a* in the direction of a* axis in all pH values (see Fig. 3). It meant the color changed from green to red. Also, in all pH values the b* value tended to decrease as the pH and ionic capacity increased (see Fig. 3). In the acid condition, the dyed cationization of cotton fabrics exhibited yellowish green shade (see Fig. 3b, c). However, in the neutral condition, it showed reddish yellow (see Fig. 3a). Also, in the alkaline condition, the blue shade appeared (see Fig. 3d, e).
For modification of the cotton fabrics with DADMAC mixed with MBAM, the a* value shifted from − a* to + a*(see Fig. 5). It indicated that the color moved from green to red which was similar to the modified cotton fabrics with DADMAC. In addition, the b* value increased for each type of solution. It meant the color showed more yellow as the MBAM increased. The color of the modified cotton fabrics was intense color of dark green, dark reddish brown and dark blue in acid, neutral and alkaline solutions, respectively (see Fig. 5). Therefore, the pH response established that the modified cotton fabric was sensitive to pH change from acid to alkaline and related to ionic capacity and concentration of MBAM.
Conclusions
The pH-sensitive fabric could be prepared by polymerization of the diallyldimethylammonium chloride onto the cotton fabric and dyed with the bromothymol blue. The dyeing of the cotton fabric with bromothymol blue induced the color change in the acetic acid and urea solutions. Modification of the cotton fabric with the diallyldimethylammonium chloride exhibited more dark color than unmodified one in all solutions. Also, the using of the diallyldimethylammonium chloride with N,N′-methylenebisacrylamide increased the intensity of color. The color change in the dyed cotton fabrics depended on the concentration of diallyldimethylammonium chloride, ionic capacity, N,N′-methylenebisacrylamide and pH of the solutions.
References
Kamila S (2013) Introduction, classification and applications of smart materials: an overview. Am J Appl Sci 10:876–880
Aslan S, Kaplan S (2018) Thermomechanical and shape memory performances of thermo-sensitive polyurethane fibers. Fiber Polym 19:272–280
Attia NF, Mousa M (2018) Facile route for development of smart slimming textile fabrics based on nanocomposites. Fiber Polym 19:471–475
Wijesirigunawardana BP, Perera BGK (2018) Development of a cotton smart textile with medicinal properties using lime oil. Acta Chim Slov 65:150–159
Molina R, Teixido JM, Kan CW, Jovancic P (2017) Hydrophobic coatings on cotton obtained by in situ plasma polymerization of a fluorinated monomer in ethanol solutions. ACS Appl Mater Interfaces 9:5513–5521
Rani KV, Prakash NH, Solomon I, Sarma B, Sarma A (2016) Surface modifications of natural Kanchipuram silk (pattu) fibers using glow discharge air plasma. Fiber Polym 17:52–58
Corteses B, Caschera D, Padeletti G, Ingo GM, Gigli G (2013) A brief review of surface-functionalized cotton fabrics. Surf Innov 1:140–156
Dong X, Bao H, Ou K, Yao J, Zhang W, He J (2015) Polymer-grafted modification of cotton fabrics by SI-ARGET ATRP. Fiber Polym 16:1478–1486
Yu D, Xu L, Hu Y, Li Y, Wang W (2017) Durable antibacterial finishing of cotton fabric based on thiol- epoxy click chemistry. RSC Adv 7:18838–18843
Gorjanc M, Jazbec K, Mozetič M, Kert M (2014) UV protective properties of cotton fabric treated with plasma, UV absorber, and reactive dye. Fiber Polym 15:2095–2104
Li S, Zhu T, Huang J, Guo Q, Chen G, Lai Y (2017) Durable antibacterial and UV-protective Ag/TiO2@ fabrics for sustainable biomedical application. Int J Nanomed 12:2593–2606
Yun C, Islam MI, Hew ML, Kim J (2016) Assessment of environmental and economic impacts made by the reduced laundering of self-cleaning fabrics. Fiber Polym 17:1296–1304
Chen C, Jia L, Shan G, Liu R, Xiao Y, Zhang H, He Y (2017) Application of triazole-containing gemini cationic darkening agent in cold pad batch dyeing for cotton knits. Fiber Polym 18:110–115
Feng X, Zheng K, Wang C, Chu F, Chen Y (2016) Durable antibacterial cotton fabrics with chitosan based quaternary ammonium salt. Fiber Polym 17:371–379
Khatri A, Peerzada MH, Mohsin M, White M (2015) A review on developments in dyeing cotton fabrics with reactive dyes for reducing effluent pollution. J Clean Prod 87:50–57
Yi S, Deng Y, Sun S (2014) Adsorption and dyeing characteristics of reactive dyes onto cotton fiber in nonionic Triton X-100 reverse micelles. Fiber Polym 15:2131–2138
Yin Y, Hong Z, Tian X et al (2018) Cellulose nanocrystals modified with quaternary ammonium salts and its reinforcement of polystyrene. Polym Bull 75:2151–2166
Fang K, Zhao H, Li J, Chen W, Cal Y, Hao L (2017) Salt-free dyeing of cotton fabrics modified with cationic copolymer nanospheres using an acid dye. Fiber Polym 18:400–406
Chowdhury MA, Joshi M, Butola BS (2014) Photochromic and thermochromic colorants in textile applications. J Eng Fiber Fabr 9:107
Goncalves D (2016) Preparation and characterization of cellulos paper/polypyrrole/bromophenol bluecomposites for disposable optical sensors. Open Chem 14:404–411
Cao L, Liang T, Zhang X, Liu W, Li J, Zhan X, Wang L (2018) In-Situ pH-sensitive fibers via the anchoring of bromothymol blue on cellulose grafted with hydroxypropyltriethylamine groups via adsorption. Polymers 10:709. https://doi.org/10.3390/polym10070709
Francisco JE, Caje JCM, Semaan FS, Pacheco WF (2016) Chemistry in sustainability and chemistry of sustainability: waste of use of fishing industry for removal of waste textile industry. Anal Bioanal Tech 1:1005
Becerir B (2011) Assessment of the results of different color difference formulae under different illuminants by wash fastness tests. Fiber Polym 12:946–956
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jareansin, S., Sukaam, P. & Kusuktham, B. Preparation and characterization of modified cotton fabrics with responsive pH. Polym. Bull. 76, 4507–4520 (2019). https://doi.org/10.1007/s00289-018-2603-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2603-8