Abstract
High boiling point solvent additive, employed during the solution processing of active layer fabrications, impact the efficiency of bulk heterojunction polymer solar cells (PSC) by influencing the morphological of the active layer. The photovoltaic performances of the PSCs based on the donor of poly{4,8-bis[(2-ethylhexyl)oxy]benzo[1,2-b:4,5-b′]-dithiophene-2,6-diyl-alt-3-fluoro-2-[(2 ethylhexyl)carbonyl] thieno[3,4-b]thiophene-4,6-diy (PTB7) and the acceptor of [6, 6]-phenyl-C71-butyric-acidmethyl-ester (PC71BM) was optimized using 5 vol% high-boiling-point solvent additive of 2-Bromonaphthalene (BN). The optimized air-processed PSC based on PTB7:PC71BM (1:1.5 w/w) with 5 vol% BN exhibited a power conversion efficiency of 7.01% with open-circuit voltage (V oc) of 0.731 V, short-circuit current density (J sc) of 13.79 mA cm−2, and fill factor (FF) of 69.46%. The effects of the additive on photovoltaic performances were illustrated with atomic force microscopy and transmission electron microscope measurements. Our results indicate that the improved efficiency is due to the optimized PTB7/PC71BM interpenetrating network and the enhanced absorption of the active layer using the BN as solvent additive.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, polymer solar cells (PSCs) have been widely studied by promising research groups due to their effective advantages such as light weight, low cost, high flexibility and simple fabrication processes [1–9]. Thanks to the development of new low bandgap donor polymers and better control of the nanoscale morphology of the interpenetrating electron donor–acceptor networks, great progress has been made in this field, and the power conversion efficiencies (PCEs) of solution-processed PSCs have reached 10–11% [10–13]. Various processing techniques have been developed to optimize the morphology of the bulk heterojunction (BHJ) material and to improve the PCE, including thermal annealing [14, 15], solvent annealing [16, 17], solvent vapor [18–20] and spontaneous interdiffusion of bilayer heterojunction [21, 22]. In addition, the use of additives has been recently demonstrated as an easy and efficient approach to modify and control the morphology of the active layer [23–25]. This technique is particularly interesting as it is compatible with extensive polymer systems and does not require extra processing steps. The addition of a small volume percent of solvent additive is able to significantly improve the PSCs performances in several polymeric systems by altering the morphological length scales of the donor and acceptor phases. A selective solubility to fullerene and a higher boiling point than the host solvent have been identified as two criteria for effective processing additive. The solvent additives effectively dissolve the [6]-phenyl-C61-butyric acid methyl ester (PCBM) aggregate and promote the formation of smaller acceptor domains within the active layer [26–30]. High-boiling-point solvent additives, which employed during the solution processing of active-layer components, impact the efficiency of BHJ organic solar cells by influencing the morphological features of the multicomponent thin film [31–41]. The work carried out by Liang et al. showed that poly{4,8-bis[(2-ethylhexyl)oxy]benzo[1,2-b:4,5-b′]-dithiophene-2,6-diyl-alt-3-fluoro-2-[(2 ethylhexyl)carbonyl] thieno[3,4-b]thiophene-4,6-diy (PTB7): [6]-phenyl-C71-butyric acid methyl ester (PC71BM) (1:1.5) films prepared from dichlorobenzene (DCB) and 1,8-diiodoctane (DIO) (97%:3% in volume) increased the PCE from 6.22 to 7.18% comparison with the devices without DIO [42]. Guo et al. reported a PCE increment from 4.12 to 7.40% when 1-chloronaphthalene (CN) was used as the solvent additive based on poly(3-hexylthiophene) (P3HT): indene-C70 bisadduct (IC70BA) (1:1.5) films [43]. They observed the changes in the film morphology when additives were added in solvent. In this paper, a high boiling point solvent additive of 2-Bromonaphthalene (BN) was used to optimize the BHJ morphology and improved the device performances. As solvent additive, we used BN, which has a higher boiling-point (281 °C) than that of chlorobenzene (CB) (131.7 °C) [44]. The mixed CB/BN solvent may have a suitable volatilization speed for further PTB7 self-organization during solution-to-film transition stage. We have comprehensively studied the effects of various contents solvent additives BN on the BHJ PSCs based on PTB7:PC71BM. Upon adding 5 vol% BN to the BHJ active layer, a PCE as high as 7.01% with a short-circuit current density (J sc) of 13.79 mA cm−2, fill factor (FF) of 69.46% and open-circuit voltage (V oc) of 0.731 V is achieved. The techniques of atomic force microscopy (AFM) and transmission electron microscope (TEM) have been applied to study the influence of BN on the morphology of the PTB7:PC71BM blend. The results indicate that the optimum phase separation in BHJ film balances the charge transport between electron and hole and reduces bimolecular recombination, leading to an increase in PCE of the devices.
Experimental
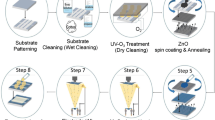
All devices were fabricated and characterized in air, without protecting environment. The PSCs were fabricated with inverted structure of ITO/ZnO/active layer/WO3/Ag, as shown in Fig. 1a. The chemical structure of PTB7, PC71BM and solvent additive BN is shown in Fig. 1b. The patterned indium tin oxide (ITO) glass substrate was cleaned in detergent, water, acetone, and isopropyl alcohol under ultrasonication for 20 min. After ultraviolet/ozone treatment for 20 min, a thin layer (~50 nm) of ZnO ETL was prepared through spin coating at 2000 rpm from a ZnO precursor solution [45]. Then the ZnO substrate was annealed at 200 °C for 1 h in air. The PTB7 and PC71BM (1:1.5 w/w) were co-dissolved in the mixed solvent of CB and BN (the content was varied from 0 to 7 vol%). The polymer concentration was 10 mg ml−1 and the solution was stirred at 60 °C for 12 h under an atmosphere environment. Then the active layer (~100 nm) was spin-coated on the ZnO layer using the as-prepared solutions at 1500 rpm for 30 s in air. Finally, a 7-nm thick WO3 layer and a 100-nm thick Ag layer were subsequently evaporated through a shadow mask (active area 6 mm2) under the pressure of 7.0 × 10−4 Pa.
The current density–voltage (J–V) characteristics of the devices were measured under 1 sun (AM 1.5G, 100 mW cm−2) simulator radiation. The surface morphologies were observed by AFM (Seiko SPA-400 SPM UNIT). TEM images were obtained using a Tecnai G2 F20, the active layer on a PEDOT:PSS substrate was removed by dipping in DI water and then a holey carbon-coated copper grid was used to hold the film, which was dried in an oven at 50 °C. The absorption spectra of active layers were measured by spectrophotometer (Cary 5000 UV–VIS).
Results and discussion
The J–V characteristics of the PSCs prepared from adding various BN concentrations (0, 1, 3, 5, and 7 vol%) in CB solvent of BHJ solution are shown in Fig. 2a and the parameters of the devices are shown in Table 1. Without using BN, the V oc is 0.752 V, but decreased as BN concentration increased. V oc of PSCs decreased down to 0.730 V when 7 vol% BN is used as the processing additive [46]. However, in contrast to V oc, the FF increased from 50.77 to 69.46% and then decreased to 66.69%. The J sc of the devices increased from 10.46 mA cm−2 (0 vol% BN) to 13.79 mA cm−2 (5 vol% BN), but decreased to 12.57 mA cm−2 when 7 vol% BN concentration is used. Table 1 shows the series resistances (R S) and shunt resistances (R Sh) of the PSCs controlled the amount of BN. The R S decreased from 16.3 Ω cm2 (0 vol% BN) to 3.5 Ω cm2 (5 vol%) and 7 vol% BN concentration (4.0 Ω cm2). On the other hand, the R Sh of the PSCs processed with BN increased from 0 vol% BN concentration (385 Ω cm2) to 3 vol% BN concentration (2633 Ω cm2) and 7 vol% BN concentration (749 Ω cm2). As the amount of BN added to the BHJ film increased, the R S of PSCs decreased, and the R Sh of PSCs increased and became saturated. As a result, therefore, the PCE of the device without using BN is only 3.99%, and increased to 4.55% (with BN 1 vol%), and increased to 6.22% (with BN 3 vol%) and 7.01% (with BN 5 vol%), but decreased to 6.18% when 7 vol% BN was used. The variation of PCE is bound up with the V oc, J sc, and FF of devices, as seen in Fig. 3. The value of J sc has been cross-checked with the integral of external quantum efficiency (EQE) spectrum and the results are within 5% error shown in Fig. 2b. In Fig. 2b, the EQE shows similar variation to J sc of the PSCs with various BN concentrations. As the BN contents in the solvent were increased, the EQE of PSCs increased, and decreased to 7 vol% contents in solvent after reaching maximum in the BN 5 vol% condition. To analyze the optical performance of active layer, the absorption spectra of PTB7:PC71BM blend films with various BN concentrations (0, 1, 3, 5, and 7 vol%) measured are shown in Fig. 4. The absorption of the films with BN additive was significantly increased compared with that of films without BN additive, as shown in Fig. 4. Both EQE and absorption spectra of all these devices show a similar broad response covering a spectral range from 300 to 800 nm. With BN 5 vol% additive, the EQE and absorption spectra are lifted up [42].
To characterize the morphology of the PTB7:PC71BM composite BHJ films with various BN contents in the solvent, we used AFM with tapping mode. For an accurate comparison with the device characteristic, all BHJ films were prepared under the same conditions as the PSC device preparation. Figure 5 shows the AFM images of the BHJ films with various BN concentrations (0, 5 and 7 vol%) in the solvent. The root-mean-square (RMS) of the film without BN additives (Fig. 5a) is 3.9 nm, while that of the films with using additives of BN 5 and 7 vol% is 3.0 and 3.2 nm, respectively (Fig. 5b, c). Obviously, the surface roughness of the films becomes much smoother using the additives, and the surface of the film with BN 5 vol% additive shows the lower roughness. This indicates that the CB/BN solvent could adjust the miscibility between PTB7 and PC71BM leading to the more homogeneous films [11, 47].
It has been approved that AFM images alone cannot provide unambiguous information regarding the structural properties in biphasic materials unless additional data are obtained by other techniques. Thus, TEM was performed to further investigate the effect of the additives on the interpenetrating networks of the active layer. Figure 6 shows the TEM micrographs of approximately 100-nm thick active layer films under non-solvent additive and with BN solvent additives conditions. The micrograph of Fig. 6a shows large dimension clusters. These large dimensions are much greater than typical organic exciton diffusion lengths of ~10 nm, no doubt blocking the diffusion of exciton. When 5 vol% BN was added, as shown in Fig. 6b, we could observe the homogeneous distribution between PTB7 and PC71BM. In this condition, the large aggregated PC71BM could be removed fully and the PC71BM could integrate into the PTB7 aggregates. Due to the large difference in vapor pressure, the final film morphology was essentially determined by the slow evaporation of BN. Its low vapor pressure, and therefore, reduced evaporation, retards the solidification of PC71BM [48]. On the other hand, the uniform interpenetrating networks of PTB7:PC71BM is beneficial to the exciton diffusion. The interpenetrating networks with phase-separated domains in the active layer apparently provide not only interfaces for charge separation of photo-generated excitons but also percolation pathways for charge carrier transport to the respective electrodes, which can critically affect the performance of devices [49, 50]. However, when the BN was added to the active layer in a more than optimized condition (7 vol%), we could observe that the BHJ film start to coarsened again. Our results indicate that with large domains come a low interfacial area and correspondingly small J sc. In other words, the tuning ability of BN and thus the morphology of the resulting film depend on the BN percentage. With an optimal BN content, a significantly increased of PCE may be realized giving rise to an interpenetrating network of polymer and fullerene.
Conclusions
In summary, we have investigated the morphology and device performance relation of BHJ solar cells based on the donor of PTB7 and the acceptor of PC71BM. By adding a suitable of BN additive (5 vol%) to the host solvent CB, the PCE was improved from 3.99 to 7.01%. The enhancement in PCE is due to the optimized PTB7:PC71BM interpenetrating network and the stronger absorption of the active layer caused by the solvent additive.
References
Szarko JM, Guo J, Liang Y, Lee B, Rolczynski BS, Strzalka J, Xu T, Loser S, Marks TJ, Yu L (2010) When function follows form: effects of donor copolymer side chains on film morphology and BHJ solar cell performance. Adv Mater 22:5468–5472
Lu L, Zheng T, Wu Q, Schneider AM, Zhao D, Yu L (2015) Recent advances in bulk heterojunction polymer solar cells. Chem Rev 115:12666–12731
Krebs FC, Gevorgyan SA, Alstrup J (2009) A roll-to-roll process to flexible polymer solar cells: model studies, manufacture and operational stability studies. J Mater Chem 19:5442–5451
Li C, Liu M, Pschirer NG, Baumgarten M, Müllen K (2010) Polyphenylene-based materials for organic photovoltaics. Chem Rev 110:6817–6855
Dennler G, Scharber MC, Brabec CJ (2009) Polymer-fullerene bulk-heterojunction solar cells. Adv Mater 21:1323–1338
Forrest SR (2004) The path to ubiquitous and low-cost organic electronic appliances on plastic. Nature 428:911–918
Zeng W, Song G, Li Y, Yuan C, Fan T, Tang W, Wang J, Zhao C, Lai W, Zhang H (2016) Enhanced performance of poly (3-hexylthiophene-2, 5-diyl):[6, 6]-phenyl-C61-butyric acid methyl ester solar cells by UV irradiation. Thin Solid Films 600:136–141
Zhou Y, Shim JW, Fuentes-Hernandez C, Khan TM, Kippelen B (2014) Inverted organic solar cells with polymer-modified fluorine-doped tin oxide as the electron-collecting electrode. Thin Solid Films 554:54–57
Qi L, Zhang C, Chen Q (2014) Performance improvement of inverted organic solar cells by adding ultrathin Al2O3 as an electron selective layer and a plasma enhanced chemical vapor deposition of SiO x encapsulating layer. Thin Solid Films 567:1–7
He Z, Xiao B, Liu F, Wu H, Yang Y, Xiao S, Wang C, Russell TP, Cao Y (2015) Single-junction polymer solar cells with high efficiency and photovoltage. Nat Photonics 9:174–179
Zhao J, Li Y, Yang G, Jiang K, Lin H, Ade H, Ma W, Yan H (2016) Efficient organic solar cells processed from hydrocarbon solvents. Nat Energy 1:15027
Etxebarria I, Ajuria J, Pacios R (2015) Solution-processable polymeric solar cells: a review on materials, strategies and cell architectures to overcome 10%. Org Electron 19:34–60
Vohra V, Kawashima K, Kakara T, Koganezawa T, Osaka I, Takimiya K, Murata H (2015) Efficient inverted polymer solar cells employing favourable molecular orientation. Nat Photonics 9:403–408
Ma W, Yang C, Gong X, Lee K, Heeger AJ (2005) Thermally stable, efficient polymer solar cells with nanoscale control of the interpenetrating network morphology. Adv Funct Mater 15:1617–1622
Yang X, Loos J, Veenstra SC, Verhees WJ, Wienk MM, Kroon JM, Michels MA, Janssen RA (2005) Nanoscale morphology of high-performance polymer solar cells. Nano Lett 5:579–583
Li G, Shrotriya V, Huang J, Yao Y, Moriarty T, Emery K, Yang Y (2005) High-efficiency solution processable polymer photovoltaic cells by self-organization of polymer blends. Nat Mater 4:864–868
Li G, Yao Y, Yang H, Shrotriya V, Yang G, Yang Y (2007) “Solvent annealing” effect in polymer solar cells based on poly (3-hexylthiophene) and methanofullerenes. Adv Funct Mater 17:1636–1644
Fukuda T, Toda A, Takahira K, Suzuki K, Liao Y, Hirahara M, Saito M, Osaka I (2016) Molecular ordering of spin-coated and electrosprayed P3HT: PCBM thin films and their applications to photovoltaic cell. Thin Solid Films 612:373–380
Wu FC, Li YH, Tsou CJ, Tung KC, Yen CT, Chou FS, Tang FC, Chou WY, Ruan J, Cheng HL (2015) Synergistic effects of binary-solvent annealing for efficient polymer-fullerene bulk heterojunction solar cells. ACS Appl Mater Interfaces 7:18967–18976
Wang D, Zhang F, Li L, Yu J, Wang J, An Q, Tang W (2014) Tuning nanoscale morphology using mixed solvents and solvent vapor treatment for high performance polymer solar cells. RSC Adv 4:48724–48733
Moon JS, Takacs CJ, Sun Y, Heeger AJ (2011) Spontaneous formation of bulk heterojunction nanostructures: multiple routes to equivalent morphologies. Nano Lett 11:1036–1039
Wang DH, Moon JS, Seifter J, Jo J, Park JH, Park OO, Heeger AJ (2011) Sequential processing: control of nanomorphology in bulk heterojunction solar cells. Nano Lett 11:3163–3168
Peet J, Kim JY, Coates NE, Ma WL, Moses D, Heeger AJ, Bazan GC (2007) Efficiency enhancement in low-bandgap polymer solar cells by processing with alkane dithiols. Nat Mater 6:497–500
Yao Y, Hou J, Xu Z, Li G, Yang Y (2008) Effects of solvent mixtures on the nanoscale phase separation in polymer solar cells. Adv Funct Mater 18:1783–1789
Moon JS, Takacs CJ, Cho S, Coffin RC, Kim H, Bazan GC, Heeger AJ (2010) Effect of processing additive on the nanomorphology of a bulk heterojunction material†. Nano Lett 10:4005–4008
Demeshko S, Dechert S, Meyer F (2004) Anion-π interactions in a carousel copper (II)-triazine complex. J Am Chem Soc 126:4508–4509
Schottel BL, Chifotides HT, Dunbar KR (2008) Anion-π interactions. Chem Soc Rev 37:68–83
Chaban VV, Maciel C, Fileti EE (2014) Does the like dissolves like rule hold for fullerene and ionic liquids? J Solut Chem 43:1019–1031
Hedley GJ, Ward AJ, Alekseev A, Howells CT, Martins ER, Serrano LA, Cooke G, Ruseckas A, Samuel ID (2013) Determining the optimum morphology in high-performance polymer-fullerene organic photovoltaic cells. Nat Commun 4:1–10
Liu X, Huettner S, Rong Z, Rong Z, Sommer M, Friend RH (2012) Solvent additive control of morphology and crystallization in semiconducting polymer blends. Adv Mater 24:669–674
van Duren JK, Yang X, Loos J, Bulle-Lieuwma CW, Sieval AB, Hummelen JC, Janssen RA (2004) Relating the morphology of poly (p-phenylene vinylene)/methanofullerene blends to solar-cell performance. Adv Funct Mater 14:425–434
Shaheen SE, Brabec CJ, Sariciftci NS, Padinger F, Fromherz T, Hummelen JC (2001) 2.5% efficient organic plastic solar cells. Appl Phys Lett 78:841–843
Hoppe H, Glatzel T, Niggemann M, Schwinger W, Schaeffler F, Hinsch A, Lux-Steiner MC, Sariciftci N (2006) Efficiency limiting morphological factors of MDMO-PPV: PCBM plastic solar cells. Thin Solid Films 511:587–592
Padinger F, Rittberger RS, Sariciftci NS (2003) Effects of postproduction treatment on plastic solar cells. Adv Funct Mater 13:85–88
Kim Y, Cook S, Tuladhar SM, Choulis SA, Nelson J, Durrant JR, Bradley DD, Giles M, McCulloch I, Ha C-S (2006) A strong regioregularity effect in self-organizing conjugated polymer films and high-efficiency polythiophene: fullerene solar cells. Nat Mater 5:197–203
Zhang F, Jespersen KG, Björström C, Svensson M, Andersson MR, Sundström V, Magnusson K, Moons E, Yartsev A, Inganäs O (2006) Influence of solvent mixing on the morphology and performance of solar cells based on polyfluorene copolymer/fullerene blends. Adv Funct Mater 16:667–674
Chen LM, Hong Z, Li G, Yang Y (2009) Recent progress in polymer solar cells: manipulation of polymer: fullerene morphology and the formation of efficient inverted polymer solar cells. Adv Mater 21:1434–1449
Hoven CV, Dang XD, Coffin RC, Peet J, Nguyen TQ, Bazan GC (2010) Improved performance of polymer bulk heterojunction solar cells through the reduction of phase separation via solvent additives. Adv Mater 22:E63–E66
Lee JK, Ma WL, Brabec CJ, Yuen J, Moon JS, Kim JY, Lee K, Bazan GC, Heeger AJ (2008) Processing additives for improved efficiency from bulk heterojunction solar cells. J Am Chem Soc 130:3619–3623
Pivrikas A, Stadler P, Neugebauer H, Sariciftci NS (2008) Substituting the postproduction treatment for bulk-heterojunction solar cells using chemical additives. Org Electron 9:775–782
Moulé AJ, Meerholz K (2008) Controlling morphology in polymer–fullerene mixtures. Adv Mater 20:240–245
Liang Y, Xu Z, Xia J, Tsai ST, Wu Y, Li G, Ray C, Yu L (2010) For the bright future—bulk heterojunction polymer solar cells with power conversion efficiency of 7.4%. Adv Mater 22:135–138
Guo X, Cui C, Zhang M, Huo L, Huang Y, Hou J, Li Y (2012) High efficiency polymer solar cells based on poly (3-hexylthiophene)/indene-C70 bisadduct with solvent additive. Energy Environ Sci 5:7943–7949
Turro NJ, Bolt JD, Kuroda Y, Tabushi I (1982) A study of the kinetics of inclusion of halonaphthalenes with ß-cyclodextrin via time correlated phosphorescence. Photochem Photobiol 35:69–72
Sun X, Ni J, Li C, Huang L, Xu R, Li Z, Cai H, Li J, Zhang J (2016) Air-processed high performance ternary blend solar cell based on PTB7-Th: PCDTBT: PC70BM. Org Electron 37:222–227
Kim W, Kim JK, Kim E, Ahn TK, Wang DH, Park JH (2015) Conflicted effects of a solvent additive on PTB7: PC71BM bulk heterojunction solar cells. J Phys Chem C 119:5954–5961
Huo L, Liu T, Sun X, Cai Y, Heeger AJ, Sun Y (2015) Single-junction organic solar cells based on a novel wide-bandgap polymer with efficiency of 9.7%. Adv Mater 27:2938–2944
Liu F, Zhao W, Tumbleston JR, Wang C, Gu Y, Wang D, Briseno AL, Ade H, Russell TP (2014) Understanding the morphology of PTB7: PCBM blends in organic photovoltaics. Adv Energy Mater 4:1301377
Hendriks KH, Heintges GH, Gevaerts VS, Wienk MM, Janssen RA (2013) High-molecular-weight regular alternating diketopyrrolopyrrole-based terpolymers for efficient organic solar cells. Angew Chem Int Ed 52:8341–8344
Li W, Hendriks KH, Furlan A, Roelofs WC, Wienk MM, Janssen RA (2013) Universal correlation between fibril width and quantum efficiency in diketopyrrolopyrrole-based polymer solar cells. J Am Chem Soc 135:18942–18948
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 61377031) and the National Natural Science Foundation of China (No. 61404073).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sun, X., Li, C., Huang, L. et al. Effects of high-boiling-point additive 2-Bromonaphthalene on polymer solar cells fabricated in ambient air. Polym. Bull. 74, 4515–4524 (2017). https://doi.org/10.1007/s00289-017-1971-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-017-1971-9