Abstract
Wound healing is a dynamic, interactive process involving soluble mediators, blood cells, and ECM (Extra Cellular Matrix). Significant changes in ECM degradation may lead to delayed wound healing. Matrix metalloproteinase (MMPs) are a family of endopeptidases that function in the remodeling of ECM proteins, wherein over-expression of these MMPs is capable of degrading ECM and biologically active proteins at the wound sites. Regulations of these MMPs at the wound sites hasten the wound healing. The aim of this study is to release the product which inhibits MMPs and as well as to reduce the bacterial load on the wound site in a controlled manner. Siderophore, organic ion chelator was isolated from Pseudomonas aeruginosa strain S1 and purified through various chromatographic techniques, used as for dual purpose in this study. The design and development of wound dressing through a carrier system, such as microspheres, are indeed a novel approach to promote healing. The design in this study includes preparation of microspheres using gelatin and siderophore (S-GM). The morphological characteristics of the prepared microspheres were found to be rigid, highly porous, and their mean diameter of five siderophore-loaded microspheres formulations was between 7.0 ± 0.52 and 25.3 ± 0. 31 µm. The drug release of the prepared samples was fast, and entrapment efficiency was about 93% at 24 h in Batch 3. Gelatin-modified microspheres were found to be non-toxic and a good biocompatible product which was assessed using NIH 3T3 fibroblast cell lines. The overall study suggests that S-GM microspheres could be used as a potent tool for MMP inhibitor for wound healing applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wound dressing is a sterile pad applied to a wound to promote healing and also to prevent further harm. A wound dressing is designed to be in direct contact with the wound to accelerate the healing faster. An ideal wound dressing is one, which induces host cells to regenerate, prevent infection regulate MMPs, and providing an optimal environment for healing to take place quickly [1]. Wound dressing may be broadly classified into the conventional dressings and modern dressings. The conventional dressings form a natural barrier to the migrating epidermal cells, forcing them to move beneath the drying dead tissues, thus prolonging the healing time and loss of healthy tissue. Use of these types of dressings can result in dehydration followed by de-vitalization and necrosis. The coagulum which forms after these dressing eventually dries up to form a difficult scar, which is hard to remove and cause trauma when removed. Moreover, an occlusive dressing may possess a clinical benefit in the treatment of wounds, but also provide the favourable environment for microbes to proliferate at the wound sites [2, 3].
To accelerate wound healing, MMPs at the wound sites are targeted. MMPs are a family of enzymes that function in the remodeling of the ECM proteins and also play a crucial role in healing of chronic wounds [4]. They are essential for various normal physiological processes and also an ECM pathological process. Over-expression of these MMPs is capable of degrading and biologically active proteins at the wound sites [5]. Hence, it is necessary to down-regulate MMPs at the wound sites. The ultimate goal is to release a product which inhibits MMPs as well as reduce the bacterial load on the wound site in a controlled manner, without altering or modifying the pharmaceutical agent for a sustained period. Controlled release of drugs is handled today due to its several potential advantages [6]. First, drug release can be modified to the needs of specific applications. Second, controlled release system provides protection of drugs, especially proteins and finally, controlled release systems can increase patient compliance. There are various approaches in delivering therapeutic substances to the target site in a sustained, controlled release fashion [7]. One such approach is using microspheres as a carrier of drugs. Microspheres are basically defined as a free flowing powder consisting of proteins or synthetic polymers having a particle size ranging from 1 to 1000 µm [8]. Basically, natural polymers were concentrated in preparation of microspheres and one such approach is the use of gelatin. Gelatin is a natural polymer obtained by alkaline or acidic pre-treatment and thermal denaturation of collagen, the most widespread protein in the body. Gelatin does not express antigenicity in physiological conditions, and it is completely reabsorbable in vivo conditions. The physicochemical properties of the gelatin were suitably modulated, and it is much cheaper and easier to obtain in concentrate solutions [9]. It is biodegradable, biocompatible, and non-immunogenic, which makes it suitable for biomedical applications, such as sealant for a vascular prosthesis [10], and in drug delivery as hard and soft capsules, hydrogels [11] or microspheres [12], and in a wide variety of wound dressings [13]. These features of gelatin provide enormous scope to utilize it as an effective carrier material, and hence, preparation microspheres were focused. Microspheres received much attention for prolonged release and also targeting the wound sites in an effective manner. An emulsification of gelatin technique provides a safe method for mass production of microspheres [14]. Thus, it has added a new dimension to the design of biomaterial-based delivery system.
Siderophore isolated from the microbes is encapsulated in a polymer matrix to form microspheres. Siderophore is secondary metabolites which are secreted by microbes under iron-deprived conditions for their viability [15]. These are iron chelators that play a dual role by reducing the bacterial load and also inhibit MMPs by binding to the active sites of Zn moiety at the wound sites which were proved in our previous work [16]. In the present investigation, this siderophore was encapsulated in a gelatin matrix to form microspheres and evaluated for a wound-dressing material through physiochemical parameters. Apart from its basic biochemical properties, the microspheres were assessed for its biocompatibility through its ability to support in vitro fibroblast and keratinocytes attachment and growth.
Materials and methods
All glassware used in this study was soaked in 5% v/v of RBS concentrate-20 and washed with deionized water and dried. Gelatin (Bovine source), dihydroxybenzoic acids (DBHA) a model compound of siderophore type was used as a standard. 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), Calcein AM, Dulbecco’s modified Eagle’s medium (DMEM), fetal calf serum (FCS), and supplementary antibiotics for tissue culture were purchased from Sigma Aldrich, India. The NIH 3T3 fibroblast and Human keratinocyte (HaCaT) cell lines were obtained from the National Centre for Cell Science (NCCS), Pune, India. The isolated catechol-type siderophore from Pseudomonas aeruginosa S1 (Accession No. KM881475) was used in this study [16].
Preparation of siderophore and DBHA-loaded gelatin microspheres (S-GM and DBHA-GM)
Siderophore-loaded gelatin microspheres were prepared by water-in-oil emulsion method by adding 200 mg siderophore in 10 mL of 2–8 wt% concentration of gelatin solution that was added dropwise to 50 mL of liquid paraffin pre-treated to 60 °C. The mixture was emulsified using an overhead stirrer throughout the process of microspheres preparation. In addition, the mixture 4 wt% Sodium Tetra Meta Phosphate (STMP) was added to the emulsion and continuous stirring was done for an hour to allow cross-linking. After cross-linking, the oil phase of the mixture containing siderophore-loaded gelatin microspheres (S-GM) was slowly decanted and the spheres were added quickly to 100 mL acetone. The obtained microspheres were washed twice with acetone to remove the last traces of oil. Various formulations of siderophore-loaded gelatin microspheres were prepared using the variables, as shown in Table 1. Simultaneously, DBHA-loaded gelatin microspheres (DBHA-GM) were also prepared and evaluated [17, 18].
Physiochemical characterization
The ultra-structural features for prepared microspheres (S-GM) were analyzed by VEGA3SBH TESCAN series scanning electron microscope (SEM) equipped with an electron optical system consisting of 0.5–30 kV capacity electron gun and detector. For surface imaging, the samples were fixed and coated with fine gold [19]. Fourier transform infrared spectroscopy (FTIR) (Perkin Elmer, USA) spectral measurements were carried out for the determination of their functional groups. Siderophore (S), gelatin (G), siderophore-loaded gelatin microspheres (S-GM), and DBHA-loaded gelatin microspheres (DBHA-GM) were taken and finely grounded with KBr individually, the pellets were subjected to hydraulic pressure of 600 dynes/m2, and spectra were scanned between 4000 and 400 cm−1 [20, 21]. The mean particle size was measured by photon correlation spectroscopy (PCS) (Malvern Instruments 3000SH, UK). The sample was diluted with double distilled water to an appropriate scattering intensity. The surface charge of S-GM was determined by measurement of zeta potential [22, 23].
Swelling behavior
To determine the swelling index of both S-GM and DBHA-GM, a known amount of the samples was added separately in PBS (pH 7.4) at room temperature. The change in size of the particles at appropriate time intervals was determined. The swelling ratio was measured by taking weight periodically and interpreted with dry samples [24]. The swelling ratio was calculated by
where W 0 and W 1 are the initial and the final weights of the film, respectively.
In vitro drug release studies and evaluation of the functionality of S-GM
The efficiency of drug release was evaluated by dissolving 5 mg of S-GM in PBS and stirred gently for 48 h. At every 1 h, 1 mL of medium was aspirated and simultaneously replaced with fresh medium. The aspirated medium was centrifuged at 5000 rpm for 5 min, and the supernatant was recovered and assessed spectroscopically at 267 nm [25]. The percentage of drug entrapment was determined by the following equation:
The functional integrity of prepared gelatin microspheres was evaluated by subjecting the aspirated supernatant to Arnow’s assay. The change of colour indicates the presence of secondary metabolites siderophore [16].
In vitro enzymatic degradation
To determine the biological stability and degradation of products, a known weight of S-GM in triplicates was taken and they were air dried at room temperature. For the known sample, collagenase enzyme (100 units/mL) was added and incubated at 37 °C for 24 h at pH 7.4. The percentage of weight loss was calculated by simple ratio, and the extent of biomaterial degradation was determined by weight loss of the sample [26].
In vitro biocompatibility, cell adhesion, and proliferation studies
Research on novel product for effective wound healing would not be fulfilled without the biocompatibility studies. The biocompatibility of siderophore was already reported in our previous work [16], and siderophore-loaded gelatin microspheres (S-GM) were evaluated separately in this study.
The cytotoxicity of microspheres (S-GM) was evaluated by performing MTT assay [27] using NIH 3T3 and HaCaT cells that were seeded in 24 well plates (5 × 104 cells/mL) containing DMEM with 10% FCS and allowed to attach and maintained for 16 h. About 10 mg of microspheres S-GM was immersed in 1 mL of absolute alcohol for 2 h for sterilization, and then, the alcohol is replaced with 1 mL DMEM medium. After 24 h, 72 h, and 7th day, the culture medium was replaced with a serum-free medium containing 10 μL of 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) and incubated at 37 °C for 4 h in a humidified atmosphere of 5% CO2. The medium was aspirated, and then, 500 μL/well of dimethylsulfoxide (DMSO) was added to dissolve the formazan needles with slow agitation for 10 min to yield a bluish purple solution. The absorbance of the dissolved solution was measured at 570 nm using Universal Microplate Reader. The proliferation of the cells was quantified for live cell assay at 12, 24, and 48 h. Then, the cells are stained with Calcein AM solution (2 µM; 400 µL) and viewed at fluorescence microscope (EVOS FLoid Cell Imaging Station, Thermo Fisher Scientific, USA) [26, 27].
Statistical analysis
All quantitative data were expressed as a mean ± standard error. Statistical analysis was performed using ANOVA (analysis of variance) and student’s test. The observed differences were considered as statistically significant (p < 0.05).
Results and discussion
In this present study, the prepared spherical microspheres were able to prolong the release of siderophore by a typical emulsifying technique using gelatin as a carrier. The results of various formulation variables were carried out to optimize the formulation. Throughout the investigation, it was found that the prepared microspheres would make it an ideal carrier for delivery of drugs.
Scanning electron microscopy and particle size of the S-GM
The microspheres were found to be spherical, and their mean diameters of all five batches of S-GM microsphere formulation were between 7.0 ± 0.52 and 25.3 ± 0. 31 µm. It was observed that uniform spherical microspheres were obtained in Batch 3 (B3) with 6% w/v of gelatin with 600 rpm when compared to other batches. SEM micrograph of siderophore-loaded gelatin microspheres (S-GM) from B3 showed smooth and spherical shapes is shown in Table 2.
The siderophore microspheres were rigid, highly porous, and non-interactive with organic solvents, such as acetone and isopropyl alcohol. The obtained microspheres from B3 range from 10 to 100 µm, and these microspheres are suitable for intramuscular administration, as reported in [28]. The surface view of the gelatin microspheres impregnated with S and DBHA was depicted in Fig. 1c and d. This provides an evidence for the smooth surface in S-GM than the DBHA-GM. SEM image adds further evidence for its, high porosity, structural integrity of microspheres. Both S-GM and DBHA-GM microspheres did not lose their morphology and shape, which can be visualized from the magnification. However, the DBHA-GM microspheres appeared to have some roughness over the surface of the particle. Hence, these microspheres found to be more efficient in drug delivery and wound healing application [29–31]. The hydrodynamic diameter of the S-GM was measured in the presence of the liquid medium. From Fig. 2a, it was observed that the size of the microsphere was slightly decreased in the particle size due to the hydrophilic nature of the microspheres. However, the size of the microspheres can be correlated from SEM micrograph [22]. The zeta potential of the S-GM was given in Fig. 2b. The zeta potential was measured in positive charge 13.9 ± 2.89 mV due to the ionic neutralization of the positive charge with the cross-linking agent STMP and the siderophore. Moreover, this makes the microspheres more stable during the sustained drug release behavior of the S-GM [22, 23].
Fourier transform infrared spectra
FTIR spectra were studied to confirm the chemical functional groups of prepared microspheres, as shown in Fig. 3. FTIR measurements of both the products displayed the characteristic bands of amide I peak (C=O stretch) at 1600–1640 cm−1, amide II peak (N–H bends and C–H stretch) at 1500–1550 cm−1, and amide III peak (C–N stretch) at ~3000 cm−1 indicating the presence of gelatin all prepared microspheres [32, 33]. IR spectra of catecholate siderophore showed a peak at 3453 cm−1, indicating the presence of a primary alcohol group. A strong peak at 1080 cm−1 shows the presence of aromatic groups in the purified compound. The FTIR results supported the fact that the siderophore was encapsulated in the gelatin microspheres.
Swelling behavior
The swelling ratio of the both S-GM and DBHA-GM was exhibited in Fig. 4. The S-GM microspheres showed increasing swelling behavior than DBHA-GM microspheres. However, the plot of the swelling ratio with respect to time showed that microspheres were able to swell one fold than its original size in 1 h and remained in equilibrium for 6 h. The spheres retained their morphology throughout the study, without any disruptions. Moreover, S-GM exhibited about 40% swelling than the DBHA-GM, and this was attributed to the high porosity of the spheres. The prepared microspheres observed to attain equilibrium only after 28 h [19].
In vitro drug release studies and evaluation of the functionality of S-GM
The drug release experiments were carried out to obtain a definite dose of therapeutic agent which is applied to the injured skin [34]. From Fig. 5, it was observed that the release of siderophore from gelatin microspheres (S-GM) was mild up to 3 h. Subsequently, the gelatin microspheres showed around 94 and 74% of drug release of siderophore and DBHA at the end of 48 h, respectively. The therapeutic efficacy of a deliver system solely depends on the drug release mechanism on the target site. Moreover, siderophore and DBHA-loaded microspheres should sustain release behavior over a period of 48 h, which would be an appropriate release to reduce the MMPs at the wound sites to hasten healing. This would reduce the invasion of microbes and able to keep the wound environment free from infection [35].
Although the results predict the good impregnation of siderophore encapsulation, the functionality of the product is important. The isolated siderophore was a catechol type, and therefore, the prepared samples were checked for Arnow’s positive [36]. Evidence of their activity was ascertained by the presence of siderophore on S-GM, which indicates that it is functionally active (Fig 6).
In vitro enzymatic degradation
Measurement of degradation and biostability of both, S-GM and DBHA-GM, was obtained using collagenase (100 units/mL). It has been observed that STMP cross-linked microspheres (S-GM and DBHA-GM) resulted in a decrease in weight loss at 69 and 63%, respectively, which indicates that the developed microspheres were biodegradable and proved to be biologically stable material (Fig. 7). DBHA-GM exhibited with fast degradation than S-GM, which thereby decreases the biostability and affects the drug delivery process [26, 37].
In vitro biocompatibility, cell adhesion, and proliferation studies
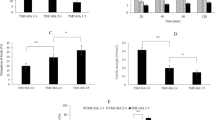
The in vitro biocompatibilities of prepared microspheres were evaluated using both NIH 3T3 fibroblast and Human keratinocyte (HaCaT) cell lines. From Figs. 8 and 9, it is evident that S-GM observed to have more than 92% cell viability. Thus, cells were well attached over the microspheres surface which clearly indicates that the developed microspheres were cell friendly and thus biocompatible. Figures 10 and 11 clearly depict that S-GM microsphere provided with good cell adhesion and proliferation with uniform growth of both cells for the easy healing and better clinical outcome. [16, 38].
Conclusions
Research investigations on the controlled release of drug on the target sites are indeed very extensive. The present work describes the designing of siderophore-loaded gelatin microspheres for an effective wound-care product. This was an attempt to inhibit the MMPs by siderophore, a secondary metabolites from microbes in a positive manner. Evidences of SEM images clearly indicate sizes of the microspheres which would be ideal for external wound therapy. The swelling property and the drug release study exhibited a controlled release of the drug and maintained its equilibrium until its next level. The microspheres were proved to possess morphological characteristics confirming the presence cell viability through in vitro studies. Moreover, it exhibits the excellent attachment and proliferation of cells which in turn support the potential application in wound-care products.
References
Varghese MC, Balin AK, Carter DM, Caldwell D (1986) Local environment of chronic wounds under synthetic dressings. Arch Dermatol 122:52–57
Friedman SJ, Su WP (1984) Management of leg ulcers with hydrocolloid occlusive dressing. Arch Dermatol 120:1329–1336
Eaglstein WH (1993) Occlusive wound dressing. J Dermatol Surg Oncol 19:716–720
Albrecht-Gary AM, Blane S, Rochel N, Ocaktan AZ, Abdallah MA (1994) Bacterial Iron Transport: coordination Properties of Pyoverdin Paa, a Peptidic Siderophore of Pseudomonas aeruginosa. Inorg Chem 33:6391–6402
Agren MS, Mirastschijski U, Karlsmark T, Saarialho-Kere UK (2001) Topical synthetic inhibitor of matrix metalloproteinases delays epidermal regeneration of human wounds. Exp Dermatol 10:337–348
Shu XZ, Zhu KJ (2002) Controlled drug release properties of ionically cross-linked chitosan beads: the influence of anion structure. Int J Pharm 233:217–225
Cho NH, Seong SY, Chun KH, Kim YH, Kwon IC, Ahn BY, Jeong SY (1998) Novel mucosal immunization with polysaccharide-protein conjugates entrapped in alginate microspheres. J Control Rel 53:215–224
Gholap SB, Banarjee SK, Gaikwad DD, Jadhav SL, Thorat RM (2010) Hollow microsphere: a review. Int J Pharm Sci Rev Res 1:10–15
Tanioka A, Miyasaka K, Ishikawa K (1976) Reconstitution of collagen fold structure with stretching gelatin film. Biopolymers 15:1505–1511
Jonas RA, Ziemer G, Schoen FJ, Britton L, Castaneda AR (1988) A new sealant for knitted dacron prostheses: minimally cross-linked gelatin. J Vasc Surg 7:414–419
Tabata Y, Ikada Y (1989) Synthesis of gelatin microspheres containing interferon. Pharm Res 6:422–427
Narayani R, Rao KP (1994) Controlled release of anticancer drug methotrexate from biodegradable gelatin microspheres. J Microencapsul 11:69–77
Ulubayram K, Hasirci N (1998) Polymeric materials in wound healing. In: Biomedical Science and Technology. (ed) Hincal K, New York
Chen L, Subirade M (2006) Alginate-whey protein granular microspheres as oral delivery vehicles for bioactive compounds. Biomaterials 27:4646–4654
Baysse C, De Vos D, Naudet Y, Vandermode A, Ochsner U, Meyer J-M, Budzikiewicz H, Schäfer M, Fuchs R, Cornelis P (2000) Vanadium interferes with siderophore mediated iron uptake in Pseudomonas aeruginosa. Microbiol 146:2425–2434
Lakshmi Thyagarajan S, Kandhasamy S, Ramanathan G, Sivagnanam UT, Perumal PT (2016) Evaluation of stress induced microbial siderophore from Pseudomonas aeruginosa strain s1 as a potential matrix metalloproteinase inhibitor in wound healing applications. Curr Microbiol 72:583–588
Shanmugasundaram N, Sundaraseelan J, Uma S, Selvaraj D, Babu Mary (2006) Design and delivery of silver sulfadiazine from alginate microspheres-impregnated collagen scaffold. J Biomed Mater Res B Appl Biomater 77:378–388
Dandagi M, Mastiholimath VS, Gadad AP, Iliger SR (2007) Mucoadhesive microspheres of propranolol hydrochloride for nasal delivery Indian. J Pharm Sci 69(3):402–407
Ramanathan G, Singaravelu S, Raja MD, Sobhana SSL, Uma TS (2014) Extraction and characterization of collagen from the skin of arothron stellatus fish—a novel source of collagen for tissue engineerin. J Biomater Tissue Eng 4:203–209
Singaravelu S, Ramanathan G, Raja MD, Sagar B, Uma TS (2015) Preparation and characterization of keratin-based biosheet from bovine horn waste as wound dressing material. Mater Lett 152:90–93
Ashok kumar R, Ramaswamy M (2014) Phytochemical screening by FT-IR spectroscopic analysis of leaf extract of selected Indian medicinal plants. Int J Curr Sci Appl Sci 3:395–406
Devendiran RM, Kumar Chinnaiyan S, Yadav NK, Ramanathan G, Singaravelu S, Perumal PT, Sivagnanam UT (2016) Facile synthesis and evaluation of quercetin reduced and dextran sulphate stabilized gold nanoparticles decorated with folic acid for active targeting against breast cancer. RSC Adv 6:32560
Dhawan S, Singla AK, Sinha VR (2004) Evaluation of mucoadhesive properties of chitosan microspheres prepared by different methods. AAPS PharmSciTech 5(4):122–128
Zhang Y, Cheng X, Wang J, Wang Y, Shi B, Huang C, Yang X, Liu T (2006) Novel chitosan/collagen scaffold containing transforming growth factor-beta1 DNA for periodontal tissue engineering. Biochem Biophys Res Commun 344:362–369
Arifin DY, Lee LY, Wang CH (2006) Mathematical modeling and simulation of drug release from microspheres: implications to drug delivery systems. Adv Drug Deliv Rev 58(12–13):1274–1325
Kandhasamy S, Ramanathan G, Kamalraja J, Balaji R, Mathivanan N, Uma TS, Perumal PT (2015) Synthesis, characterization and biological evaluation of chromen and pyrano chromen-5-one derivatives impregnated into a novel collagen based scaffold for tissue engineering applications. RSC Adv 5:55075
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Esposito E, Cortesi R, Nastruzzi C (1996) Gelatin microspheres: influence of preparation parameters and thermal treatment on chemico-physical and biopharmaceutical properties. Biomaterials 17:2009–2020
He P, Davis SS, Illum L (1998) In vitro evaluation of the mucoadhersive properties of chitosan microspheres. Int J Pharm 166:75–88
Martin A, Bustamante P, Chun AH (1996) In: Physical Pharmacy: Physical and chemical principles in the Pharmaceuticalsciences, 4th (ed) New Delhi, BI Waverly Pvt Ltd
Naveen N, Ramadhar K, Balaji S, Uma TS, Natarajan TS, Praveen KS (2010) Synthesis of nonwoven nanofibers by electrospinning—a promising biomaterial for tissue engineering and drug delivery. Adv Eng Mater 12:B380
Kong J, Yu S (2007) Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim Biophys Sin 39(8):549–559
Ahmad M, Benjakul (2011) Characteristics of gelatin from the skin of unicorn leather jacket (Aluterus monoceros) as influenced by acid pre treatment and extraction time. Food Hydrocolloids 25:381–388
Genta I, Conti B, Perugini P, Pavanetto F, Spadaro A, Uglis G (1997) Bioadhesive microspheres for ophthalmic administration of acyclovir. J Pharm Pharmacol 49:739–742
Nagiah N, Ramanathan G, Sobhana L, Sivagnanam UT, Srinivasan NT (2014) Poly (vinyl alcohol) Microspheres Sandwiched Poly (3-hydroxybutyric acid) electrospun fibrous scaffold for tissue engineering and drug delivery. Int J Polym Mater 63:583–585
Payne SM (1994) Detection, isolation and characterization of siderophores. Method Enzymol 235:329–344
Muthukumar T, Prabu P, Thotapalli Ghosh K, Parvathaleswar S (2014) Effect of growth factors and pro-inflammatory cytokines by thecollagen biocomposite dressing material containing Macrotylomauniflorum plant extract—In vivo wound healing. Colloids Surf B 113:20
Ramanathan G, Singaravelu S, Raja MD, Nagiah N, Padmapriya P, Ruban K, Kaveri T, Natarajan S, Uma TS, Perumal PT (2016) Fabrication and characterization of a collagen coated electrospun poly(3-hydroxybutyric acid)—gelatin nanofibrous scaffold as a soft bio-mimetic material for skin tissue engineering applications. RSC Adv 6:7914
Acknowledgements
The author gratefully acknowledges financial support for this work through the grants awarded by the Department of Science and Technology (DST) New Delhi, India, (SR/WOS-A/LS-375). Financial support from CSIR under Translational Project OLP-09/TRP is acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Thyagarajan, S.L., Ramanathan, G., Singaravelu, S. et al. Characterization and evaluation of siderophore-loaded gelatin microspheres: a potent tool for wound-dressing material. Polym. Bull. 74, 2349–2363 (2017). https://doi.org/10.1007/s00289-016-1840-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-016-1840-y