Abstract
Mechanochemistry as an energy-saving and highly efficient synthetic method emerges great potential in various chemical syntheses. The multi-phase compatibilizer polypropylene (PP) grafted with maleic anhydride (MAH) and styrene (St) (PP-g-(MAH-co-St)) was successfully obtained by mechanochemical method without any solvent within minutes. The PP-g-(MAH-co-St) with G MAH = 0.24–1.40 % were made by mechanochemistry successfully. The impact of monomers concentration, initiator concentrations, particle size of PP, ball-milling time and ball-milling speed on the grafting proportion of MAH onto PP (G MAH) was also investigated. The optimum conditions for PP-g-(MAH-co-St) (G MAH = 1.40 %) by ball milling were particle size of PP, 0.150–0.250 (mm); MAH/PP ratio, 6 % (m/m); St/PP ratio, 6 % (m/m); DCP/PP ratio, 0.3 % (m/m) at 400 r/min in 30 min. Infrared (IR) spectra and 1H NMR spectra confirmed the incorporation of MAH and St onto the PP backbone, which also indicated ball milling was an efficient and effective way for the graft of PP-g-(MAH-co-St).
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polypropylene (PP), one of the most available versatile polymers, faces many problems in blending and compounding owing to its hydrophobicity, especially lacking any reactive functional groups [1, 2]. The grafting technique with reactive monomers onto PP has been widely used in the modification of PP to overcome these problems [3–6]. Maleic anhydride (MAH) was extensively studied to modify PP in commercial applications due to its unique combination, low cost, high activity, and good processibility [3–9]. However, it is difficult for the copolymerized MAH to homopolymerize. This leads to a decreased trend of MAH grafted onto PP and severe degradation of the PP backbone [6, 10, 11]. Recently, for the better grafting of MAH onto PP, adding styrene (St) as a second monomer onto PP at the same time not only increases the grafting proportion of MAH onto PP (G MAH), but also reduces the PP chain scission degradation in near literature [12]. The co-monomer St can graft onto the PP backbone prior and then react with MAH. During this process, St serves as a medium to bridge the gap between the PP macroradicals and the MAH. The formed graft copolymers are able to compatibilize incompatible systems, such as blends of polar and nonpolar polymers, as well as polymer compounds containing natural fibers and among others. A remarkable work is that of Li et al. [13, 14], who used multi-monomer melt graft PP with anhydride groups and styrene segments in typical ternary blends of polypropylene (PP)/polystyrene (PS)/polyamide-6 (PA6). Lee et al. [5] evaluated performance of PP-g-MAH-St as a compatibilizer in PP/clay nanocomposites, and concluded PP-g-MAH-St enabled PP/clay nanocomposites to have the higher stiffness as well as the higher toughness.
Grafting of PP has successfully been done using solution, melts, and even a solid-state route [15–18], while there are different disadvantages of these methods. For example, xylene is usually used as a solvent in the solution method, which leads to the problems of separation, purification and even damages the environment, and the complex process made it impractical for industrial production [15, 16]. The melt grafting method, namely reactive extrusion, is carried out at above 170 °C, and the high temperature resulted in serious degradation or cross-linking of PP backbone chains [19, 20]. For the solid graft method, one important factor to modify PP is the morphology of the native powder, which strains its application [18]. Moreover, all the methods described above have some common shortcomings, such as long reaction time and low grafting proportion, and so on. Therefore, it is necessary for the researchers to pursue other highly efficient methods.
In this paper, we first reported a clean, significantly efficient and practically applicable technique for multi-phase compatibilizer polypropylene grafted with maleic anhydride (MAH) and styrene (St) (PP-g-(MAH-co-St)) under mechanochemical conditions, which reveals a clear shortcut in terms of free or limited solvent employed. As has widely been regarded, the mechanical grinding (ball milling, also known as mechanochemistry) is of great interest in inorganic and organic synthesis, co-crystals and even successful industrialization in pharmaceutical aspects [21]. Here PP-g-(MAH-co-St) synthesized by ball milling exhibits noticeable advantages in terms of ambient temperature, atmospheric pressure, graft degree, separation, and environment. Besides, effects of several factors were studied, such as monomers concentration, initiator concentrations, ball-milling time and ball-milling speed on G MAH using single-factor method, and the chemical structures were characterized by FT-IR and 1H NMR.
Experimental
Materials
Granular PP (MFR = 4 g/10 min, 230 °C/2160 g) used for the preparation of PP-g-(MAH-co-St) was purchased from Sinopec Yangzi Petrochemical Co., Ltd. (China). MAH was purchased from Shanghai Chemical Reagent Co., Ltd. (China). St, dicumyl peroxide (DCP), dibenzoyl peroxide (BPO), potassium acid phthalate were purchased from Shanghai Runjie Chemical Co., Ltd. (China). Acetic acid was purchased from Shanghai Yin Xiang Biological Technology Co., Ltd. (China). Dimethyl benzene, acetone, sodium hydroxide, alcohol and phenolphthalein were purchased from Sinopharm Chemical Reagent Co., Ltd. (China). All the reagents were of analytical grade.
Mechanochemical grafting
Mechanochemical synthesis was conducted using a planetary ball mill (Model PM; Nanjing Chishun Technology Development Co., Ltd, China). Milling experiments were conducted at ambient temperature in a stainless steel pot (volume 95 mL). Here the ball-to-powder ratio was set to approximately 85:1. We used the single-factor method to investigate the optimum conditions for mechanochemical grafting, such as initiator concentration, monomer concentration, ball-milling time, ball-milling speed and particle size of PP. The milling experiments products were first dissolved in xylene by heat refluxing, precipitated with an excess of acetone, and then washed several times with acetone for removing small molecular compounds and any unreacted MAH and St and maleic anhydride copolymer during grafting by ball milling. After keeping aside a small amount of products for further characterization, the remaining PP-g-(MAH-co-St) was precipitated, filtered and dried at 50 °C for 24 h in a vacuum oven for removing the solvent.
Measurement of grafting proportion
G MAH was determined by back titration. Briefly, approximately 0.5 g of pure PP-g-(MAH-co-St) was dissolved in xylene by heat refluxing at 120 °C for 1 h. After the compound was completely dissolved, 10 mL of a standard ethanol solution of NaOH (0.05 mol/L) was added by heat refluxing at 100 °C for 15 min. Next, two drops of phenolphthalein as an indicator were subsequently added to the solution and the mixture changed to pink. The pink solution was back titrated to colorless (at the endpoint) by the addition of a 0.05 mol/L acetic acid/xylene standard solution, and G MAH was calculated as follows:
where G MAH is the grafting proportion of MAH (%), c 1 is the concentration of the sodium hydroxide/alcohol standard solution (mol/L), c 2 is the concentration of the acetic acid/xylene standard solution (mol/L), V 1 is the volume of the sodium hydroxide/alcohol standard solution (mL) consumed, V 2 is the volume of the acetic acid/dimethyl benzene standard solution (mL) consumed, and m is the weight of pure PP-g-(MAH-co-St) (g). The molecular mass of MAH is 98.06.
Fourier transform-infrared spectroscopy (FT-IR)
The Fourier transform-infrared (FT-IR) spectra of all samples were measured on a Bruker TENSOR 27 FT-IR spectrometer (Germany) using KBr pellets under the same test conditions. The spectra were recorded at an average of 32 scans in the standard wavenumber range of 400–4000 cm−1 at a resolution of 4 cm−1.
1H nuclear magnetic resonance spectroscopy (1H NMR)
Nuclear magnetic resonance (1H NMR) spectroscopy experiments of PP-g-(MAH-co-St) samples were recorded on a Bruker AV 400 NMR spectrometer (Switzerland) at 298.0 K using deuterated benzene. The spectral width was 8223.7 Hz, pulse width was 9.6 μs, relaxation delay was 1.0 s, and the number of scans was 16.0.
Results and discussion
Effect of initiator on the G MAH of grafted products
Figure 1 shows the effect of the concentration of initiators such as DCP and BPO on G MAH. Both MAH and St concentrations were fixed at 6 wt% (based on PP), the particle size of PP was greater than 0.300 mm, ball-milling time was 30 min, and ball-milling speed was 400 r/min. G MAH exhibited a rapid increase in certain domains and reached peaks of 0.85 and 0.67 % at DCP and BPO concentrations of 0.3 and 0.5 % wt%, respectively (based on PP), and then decreased afterward. First, with an increase in the initiator concentration, the formation of free radicals increased because of the decomposition of the initiators, which led to the increase of G MAH. It is obvious that in this process, more initiators result in an increase in the transfer of chains to the polymer backbone, thereby resulting in a higher G MAH value [10]. However, when an excess of initiator was used, G MAH decreased because of extensive chain degradation of the PP backbone. Furthermore, an excess of initiator concentration increased the copolymerization between MAH and St, which in turn led to the termination of the grafting reaction. As a result, G MAH decreased [22].
Effect of the MAH concentration on the G MAH of grafted products
The effect of the MAH concentration on G MAH was investigated, where the DCP and St concentrations were fixed at 0.3 and 6 wt%, respectively (based on PP), the particle size of PP was greater than 0.300 mm, the ball-milling time was 30 min, and ball-milling speed was 400 r/min. Table 1 shows the effect of the MAH concentration on G MAH. G MAH significantly increased with increasing MAH concentration, reaching a maximum value at 0.66 % with an MAH concentration of 6 wt% (based on PP). In the mechanochemical method, the total energy was fixed at a certain ball-milling speed and ball-milling time. To achieve the highest G MAH, the higher concentration of the free radicals formed initially must be consistent with the increase of MAH concentration, which enhances the probability that MAH and St will react with PP macroradicals for grafting. Beyond the critical MAH concentration, more MAH results in the homopolymerization of monomers leading to low G MAH [3, 10]. As can be seen in Table 1, the PP-g-(MAH-co-St) prepared by ball milling is competitive to that synthesized in a traditional manner, as reported in recent studies, with regard to G MAH [3–6, 10, 11].
Effect of the concentration of St as a co-monomer on the G MAH of grafted products
To increase the G MAH of the grafted product, St was used as a co-monomer in the MAH grafting reaction, where the DCP and MAH concentrations were fixed at 0.3 and 6 wt%, respectively (based on PP), the particle size of PP was greater than 0.300 mm, ball-milling time was 30 min, and ball-milling speed was 400 r/min. Table 2 shows the influence of the St concentration on the G MAH of the grafted products. In the presence of St, G MAH was always higher than that in the absence of St. St improved the grafting reactivity of MAH [23]. It reached a maximum when the molar ratio of MAH and St was approximately 1:1 and then decreased when the concentration of St was higher than that of MAH, because St has the ability of providing the electron to capture radicals on the PP backbone as rapidly as possible [24]. Therefore, G MAH could be significantly improved in the presence of St. However, when the number of moles of St was higher than that of MAH, a part of the St monomer reacted with MAH to form the poly(styrene–co–maleic anhydride) (SMA) copolymer, while the remaining St monomers might preferentially react with PP macroradicals to form relatively stable styryl macroradicals. As a result, G MAH decreased.
Effect of the ball-milling speed on the G MAH of grafted products
The effect of the ball-milling speed on the G MAH of grafted products was investigated by maintaining a ball-milling time of 30 min, PP particle size of greater than 0.300 mm, DCP concentration of 0.3 wt% (based on PP), and concentrations of both MAH and St at 6 wt% (based on PP). Keeping with the minimum limit of the equipment, the ball-milling speed was maintained at 400 r/min. From Table 3, G MAH kept on decreasing with continuous increase in the ball-milling speed. The motion of the balls in the stainless steel pots was extremely complex, which was controlled by the ball-milling speed. Usually, a low ball-milling speed results in insufficient grinding whereas a very high ball-milling speed makes some of these balls attach to the bottom or inside walls of the stainless steel pots, which reduce the knock-on effect of the balls on the reactants. However, when the ball-milling speed is appropriate, the balls will tightly attach to the inside walls and then fall away from the walls of the stainless steel pots to the reactants with the greatest abrasive action. Therefore, the G MAH of grafted products proved to be the highest [25].
Effect of the ball-milling time on the G MAH of grafted products
Table 4 lists the experimental results showing the dependence of the ball-milling time on the G MAH of grafted products. The grafted conditions were a ball-milling speed of 400 r/min, PP particle size of greater than 0.300 mm, a DCP concentration of 0.3 wt% (based on PP), and concentrations of both MAH and St at 6 wt%, (based on PP). The G MAH of the grafted products first increased then decreased as the milling varied from 5 to 40 min. Hence, the peak ball-milling time is 30 min as the highest G MAH was obtained. At the first stage of ball milling, the particle size of the reactant powder was large, and the specific surface area among powders was small, which could lead to the incomplete reaction [26, 27]. In addition, the decomposition of initiators was mainly affected by temperature. At the first stage of ball milling when the size of reactants was still large, the activation period was too long to produce a local high temperature; therefore, it is difficult to induce the reaction. Moreover, as the ball-milling time increased, both powder size the impact buffer area were smaller, thereby making it easier to produce a local high temperature while accelerating the decomposition speed of initiators as well as inducing and promoting the grafting reaction [28, 29]. However, if the ball-milling time was excessively increased, the G MAH of grafted products would decrease instead because the surface energy of the reactants would significantly increase owing to their small size. The extensive particle agglomeration led to the decrease of the G MAH [30, 31].
Effect of the PP particle size on the G MAH of grafted products
Table 5 shows the effect of the particle size of PP on the G MAH of the grafted products. The ball-milling speed was 400 r/min, ball-milling time was 30 min, PP particle size was greater than 0.300 mm, DCP concentration was 0.3 wt% (based on PP), and concentrations of both MAH and St were fixed at 6 wt% (based on PP). The G MAH of the grafted products first increased then decreased along with an increase in the particle size of PP, and the optimal particle size of PP was 0.150–0.250 mm where the grafting reaction mostly occurred on the PP powder surface. The reactivity of the powders could be increased by reducing the particle size and increasing the specific surface areas of the particles, which highly facilitated the grafting reactions [32]. However, when the mesh size reaches a certain extent, G MAH slightly decreased, probably because PP powder exhibits strong adsorption properties, which promotes particle agglomeration; this agglomeration is unfavorable for contact with reactants, thereby affecting the grafting reaction [33].
FT-IR analysis
Figure 2 shows the FT-IR spectra of pure PP-g-(MAH-co-St) (G MAH = 1.40 %) prepared by mechanochemistry (a) and PP (b). A new absorption band for PP-g-(MAH-co-St) was observed at 1749 cm−1, which corresponds to the C=O stretching of anhydride groups; this is a characteristic peak observed for MAH grafted onto PP backbones [34]. On the other hand, an absorption band was observed at 729 cm−1, which is attributed to the off-plane bending vibration and is characteristic of aromatic C–H for St [12]. The observation of this peak implies that St is also grafted onto the main chains of PP. There was overlap between the antisymmetric bending vibration of methyl and scissor bending vibration of methylene at 1459 cm−1. Meanwhile, the absorption peak corresponding to symmetric bending vibration of the methyl group was observed at 1378 cm−1 [35]. Absorption peaks were also observed at 1167, 998, 973, and 841 cm−1, attributed to the crystalline states of isotactic PP [36]. In addition, an absorption peak was also observed at 973 cm−1, attributed to the amorphous state of isotactic PP [37]. The FT-IR spectrum of pure PP-g-(MAH-co-St) (G MAH = 1.40 %) by mechanochemistry was basically similar to that observed for pure PP-g-(MAH-co-St) prepared by a conventional solvent-based method [38].
1H NMR analysis
To further confirm that MAH and St were grafted onto the PP backbone, the 1H NMR spectrum of pure PP-g-(MAH-co-St) (G MAH = 1.40 %) was recorded, as shown in Fig. 3. The spectrum exhibited characteristic peaks in the range of 6.9–7.4 ppm attributed to the aromatic protons and C=C of MAH obtained from disproportion termination. Characteristic peaks were observed at 3.3, 4.0, and 4.9 ppm attributed to the proton of the anhydride ring with different linkages [39, 40]. Peaks were also observed in the range of 0.5–2.0 ppm attributed to the protons of saturated PP carbons. These confirmed that MAH and St are successfully grafted onto PP.
Mechanisms of the St-assisted grafting of MAH onto PP by the mechanochemical method
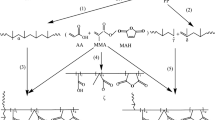
Only few studies have reported the use of mechanochemistry for the synthesis of PP-g-(MAH-co-St). This study aims at broadening this synthetic strategy and elucidating the manner in which chemical substances react. Typically, the reaction mechanism of PP-grafted MAH is considered to follow a free-radical grafting reaction. This mechanochemical method starting from materials in the solid state employs ball milling and is governed by a mechanochemical methodology. The mechanical interaction between two solids enables chemical reactions to be initiated by an activation energy lower than that required for regular thermochemical reactions [41]. During ball milling, materials experience extreme high-energy impact by ball-to-ball and ball-to-vial-wall collisions, which are considered to initiate grafting. In this case, the solid reactions occur at the interfaces of the grains and the defects formed in the process are continuously regenerated during milling. As a result, reactions that would normally require high temperatures to occur can occur under mild conditions. By assuming that the aforementioned mechanism is applicable for a system of grafting MAH and St onto PP, in combination with FT-IR and 1H NMR results, the conceivable mechanisms of MAH- and St-grafted PP under our experimental conditions are shown in Fig. 4, where possible structures of the grafted products a and b are shown.
Conclusions
High-purity PP-g-(MAH-co-St) with a higher G MAH (1.40 %) was successfully prepared by a mechanochemical method in the absence of any chemical solvent, which exhibits advantages of energy efficiency, low cost, as well as being a facial process. The choice of starting materials and grinding parameters probably has a dramatic impact on the mechanochemical reaction. Grafting was confirmed by FT-IR and 1H NMR results, and the possible structures of the grafted products and the possible mechanisms of grafting were proposed. There is clear potential for the application of the mechanochemical method for polymer modification.
References
Gawish SM, Kantouch A, El-Naggar AM et al (1992) Grafting of 2-(dimethylamino) ethyl methacrylate onto gamma irradiated polypropylene fabric. J Appl Polym Sci 44:1671–1677
Shi D, Chen HB, Li RKY (2007) Preparation of PP-g-PA6 copolymers through reactive blending. J Mater Sci 42:9495–9497
Ye YQ, Qian J, Xu YS (2011) Ultrasonic induced grafting of maleic anhydride onto polypropylene in melt state. J Polym Res 18:2023–2031
Qian J, Huang ZJ, Dang SY, Xu YS (2011) Improvements of polypropylene grafted maleic anhydride with ultrasonication, pre-irradiation and co-irradiation methods. J Polym Res 18:1557–1565
Lee J, Kim J-K, Son Y (2012) Evaluation of polypropylene grafted with maleic anhydride and styrene as a compatibilizer for polypropylene/clay nanocomposites. Polym Bull 68:541–551
Zheng YY, Zhao SF, Zeng AR, Guo Y (2012) The application of response surface methodology on the synthesis of grafted polypropylene through the solvothermal route. Adv Polym Technol 31:109–117
López-Quintanilla ML, Sánchez-Valdés S, Ramos de Valle LF, Medellín-Rodríguez FJ (2006) Effect of some compatibilizing agents on clay dispersion of polypropylene-clay nanocomposites. J Appl Polym Sci 100:4748–4756
Lin WT, Shao Z, Dong JY, Mike Chung TC (2009) Cross-linked polypropylene prepared by PP copolymers containing. Macromolecules 42:3750–3754
Burkinshaw SM, Froehling PE, Mignanelli M (2002) The effect of hyperbranched polymers on the dyeing of polypropylene fibres. Dyes Pigm 53:229–235
Zheng YY, Zhao SF, Cheng L, Li BM (2010) Synthesis and reaction kinetics model of suspension phase grafting polypropylene with dual monomers. Polym Bull 64:771–782
Bettini Sívia H P, de Mello Lara C, Muñoz Pablo AR, Ruvolo-Filho Adhemar (2013) Grafting of maleic anhydride onto polypropylene, in the presence and absence of styrene, for compatibilization of poly(ethylene terephthalate)/(ethylene–propylene) blends. J Appl Polym Sci 127:1001–1009
Li Y, Xie XM, Guo BH (2001) Study on styrene-assisted melt free-radical grafting of maleic anhydride onto polypropylene. Polymer 42:3419–3425
Li Y, Wang D, Zhang JM, Xie XM (2011) Influences of component ratio of minor phases and charge sequence on the morphology and mechanical properties of PP/PS/PA6 ternary blends. Polym Bull 66:841–852
Wang D, Li Y, Xie XM, Guo BH (2010) Compatibilization and morphology development of immiscible ternary polymer blends. Polymer 52:191–200
Qiu WL, Takashi Endo, Takahiro Hirotsu (2005) A novel technique for preparing of maleic anhydride grafted polyolefins. Eur Polym J 41:1979–1984
Russell KE (2002) Free radical graft polymerization and copolymerization at higher temperatures. Prog Polym Sci 27:1007–1038
Moad G (1999) The synthesis of polyolefin graft copolymers by reactive extrusion. Prog Polym Sci 24:81–142
Ratzcsh M, Arnold M, Borsig E, Bucka H, Reichelt N (2002) Radical reactions on polypropylene in the solid state. Prog Polym Sci 27:1195–1282
Galia A, Gregorio RD, Spadaro G, Scialdone O, Filardo G (2004) Grafting of maleic anhydride onto isotactic polypropylene in the presence of supercritical carbon dioxide as a solvent and swelling fluid. Macromolecules 37:4580–4589
Henry Gaëtan RP, Xavier Drooghaag, Rousseaux Dimitri DJ et al (2008) A practical way of grafting maleic anhydride onto polypropylene providing high anhydride contents without sacrificing excessive molar mass. J Polym Sci Pol Chem 46:2936–2947
James SL, Adams CJ, Bolm C et al (2012) Mechanochemistry: opportunities for new and cleaner synthesis. Chem Soc Rev 41:413–447
Jia DM, Luo YF, Li YM, Lu H et al (2000) Synthesis and characterization of solid-phase graft copolymer of polypropylene with styrene and maleic anhydride. J Appl Polym Sci 78:2482–2487
Xie XM, Chen NH, Li S (1999) The effect of comonomer in the melt grafting PP system. Acta Polym Sin 3:351–354
Qi R, Chen Z, Zhou C (2005) Solvothermal preparation of maleic anhydride grafted onto acrylonitrile-butadiene-styrene terpolymer (ABS). Polymer 46:4098–4104
Lou BZ (2008) Preparation of nano-WO3 ceramic powder by mechanical ball milling method and influence of ball milling parameters. Mater Heat Treat 37:33–35
Xiao X, Yin T, Tao Y et al (2001) Investigation on reactive milling of NiAl–TiC composite. Chin J Mater Res 15:330–444
Zhang LN (2012) Preparation of nano-TiC by ball-milling technique and its formation mechanism. Spec Cast Nonferr Alloys 32:71–73
Li Y, Lu QH (2000) Study on CB-g-An of solid phase by grinding. Ind Miner Process 10:10–12
Wang XL, Wang XJ, Chen XD et al (2004) Influence of milling parameters on Mg–Cu amorphous alloy prepared by ball milling. Nonferr Met (Extr Metall) 5:43–45
Liu Y, Wang J, Zhang MX, Qin XY (2003) Research and development of mechanical attrition method in nano-structural materials. Mater Rev 17:20–29
Liu XQ, Li JY, Min H, Shi H (2011) Preparation of ultrafine rice husk silica by ball milling method. Chem Bioeng 28:66–69
Ruan JM, Pan YK, Chen Q, Zhou DF (1999) Studies on solid-phase grafting of GMA onto PP. Polym Mater Sci Eng 15:80–83
Yao Y, Zhang J, Wang XL (2004) Research review on solid phase graft modification of polypropylene. China Elastom 14:64–71
Xu JY, Ban HZ, Ye CQ, Yang J, Hao Y (2010) The long-term stress aging on the structure and properties of nylon 6. Polym Mater Sci Eng 26:79–85
Wu J, Han WX (2005) using infrared spectroscopy to identify polymer. Rubber Resour 35(1):38–44
Chen ZL (1977) The iso-regularity of polypropylene by an infrared spectrometry. Chemistry 160:47–49
Li C, Liu LW, Jiang XX et al (2013) The application of infrared spectroscopy in the analysis of polypropylene isotacticity. Guangzhou Chem Ind 41(4):135–137
Tian Z, Pan LS, Xiong YL, Xu N et al (2012) Study on the properties of polypropylene grafted with maleic anhydride and styrene and its preparation. Plast Sci Technol 40:84–89
Vlad-Bubulac T, Hamciuc C (2009) Aliphatic-aromatic copolyesters containing phosphorous cyclic bulky groups. Polymer 50:2220–2227
Henry GRP, Drooghaag X, Vandeuren M et al (2009) Controlled reduction of polypropylene isotacticity and crystallinity by epimerization during reactive processing. J Polym Sci Polym Chem 47:4505–4518
Kajdas C (2015) Mechanical activation of chemical process. Mater Sci Appl 6:60–67
Acknowledgments
The authors would like to thank the Production and Research of Hainan province (No. CXY20140041; No. CXY20130047) and the Natural Science Foundation of Hainan Province (No. 512109) for their financial supports. This study was also supported by the Hainan Provincial Fine Chemical Engineering Research Center, the Analytical and Testing Center of Hainan University, and Hainan University Excellent Engineers Project.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yilu, Z., Zhifang, G., Liming, Z. et al. Mechanochemistry: a novel approach to graft polypropylene with dual monomers (PP-g-(MAH-co-St)). Polym. Bull. 72, 1949–1960 (2015). https://doi.org/10.1007/s00289-015-1382-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-015-1382-8