Abstract
Fatty acids (FAs) participate in extensive physiological activities such as energy metabolism, transcriptional control, and cell signaling. In bacteria, FAs are degraded and utilized through various metabolic pathways, including β-oxidation. Over the past ten years, significant progress has been made in studying FA oxidation in bacteria, particularly in E. coli, where the processes and roles of FA β-oxidation have been comprehensively elucidated. Here, we provide an update on the new research achievements in FAs β-oxidation in bacteria. Using Xanthomonas as an example, we introduce the oxidation process and regulation mechanism of the DSF-family quorum sensing signal. Based on current findings, we propose the specific enzymes required for β-oxidation of several specific FAs. Finally, we discuss the future outlook on scientific issues that remain to be addressed. This paper supplies theoretical guidance for further study of the FA β-oxidation pathway with particular emphasis on its connection to the pathogenicity mechanisms of bacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fatty acids (FAs) are essential components of cell structure and play a crucial role in energy production. Common types of FAs include saturated/ unsaturated, cis/ trans, and straight-chain/ branched-chain FAs based on different configurations [1]; while according to the molecular chain lengths, they are usually divided into short-chain FAs (SCFAs), middle-chain, and long-chain FAs (MCFAs & LCFAs) [2]. Besides their functions regulating cell signals, FAs are essential substrates for energy and anabolic metabolisms [3,4,5].
Organisms can synthesize, decompose and utilize FAs through different oxidation pathways. Three known pathways of FAs degradation are β-, α-, and ω-oxidation. Among these, α- and ω-oxidation are minor ways primarily occurring in eukaryotes, whereas β-oxidation is the main pathway to degrade bacterial FAs [6]. Since the synthesis process of FAs is highly energy-consuming, organisms can also absorb and use exogenous FAs to participate in their own physiological activities [7].
FAs and their derivatives are necessary in extensive cellular activities, such as bacterial pathogenesis, membrane permeability, and phospholipid biosynthesis [8,9,10,11]. With the rapid enrichment of microbial genome sequence databases, most sequenced bacteria have been confirmed to possess β-oxidation-related genes. However, the mechanisms of bacterial β-oxidation need to be further studied. Here, we first introduce some new achievements in bacterial β-oxidation; then, using Xanthomonas as an example, we discuss the research progress on the degradation mechanism of the DSF-family quorum signal molecule; based on these findings, we predict the degradation mechanism of FAs with a specific structure. Finally, we outline some future follow-up researches directions.
Full Overview of FAs β-Oxidation in Escherichia coli
The metabolism of LCFAs (C12—C18) and MCFAs (C7—C11) in E. coli requires the fad regulon genes involved in the transportation (fadL), activation (fadD), and oxidation (fadA, B, E, H, and M) of FAs (Fig. 1). These fad genes are located at different positions on the E. coli chromosome and form a regulon consisting of a few operons [12]. In addition, fadR regulates the transcription of FA degradation-related genes by binding downstream of fad genes (fadL, fadD, fadE, and fadBA) and inhibiting their transcription [13, 14]. The transportation and utilization of SCFAs (C4—C6) are conducted by the atoDAEB operon, which is controlled by AtoC [12, 15].
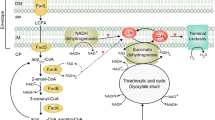
The β-oxidation pathway of fatty acid in bacteria. Schematic representation of degradation of fatty acids. The common even-carbon saturated FAs are metabolized via β-oxidation (A). While to allow the degradation of FAs with various steric variants such as VLCFAs (B), branched chain fatty acids (C) or unsaturated fatty acids (D & E & F) to proceed, different strategies have been developed. These strategies include evolution of enzymes showing specificity with respect to either chain length or modified acyl-chain, or other special conformation, to catalyze a parallel set of reactions with different substrate specificities. The β-oxidation cycles are shown in grey. FadB and FadA play dual roles in β-oxidation pathway. FadB, the α-subunit of the multienzyme complex FadBA, has dodecenoyl-CoA delta-isomerase, enoyl-CoA hydratase, 3-hydroxybutyryl-CoA epimerase and 3-hydroxyacyl-CoA dehydrogenase activity (enzymatic step shown in orange). FadA has 3-ketoacyl-CoA thiolase activity, catalyzes the last step of a β-oxidation cycle, and this small β-subunit of FadBA is responsible for the release of two carbon atoms to form acetyl-CoA in thiolysis reactions in the fatty acid β-oxidation cycle (enzymatic step shown in blue)
Fatty Acid Transmembrane Transporter (FadL)
FadL (48.5 kDa) is localized in the outer cell envelope and can bind exogenous LCFAs with high affinity [16]. Since FAs have low cell membrane permeability, FadL functions as a substrate-specific diffusion channel to achieve transmembrane [17]. The exogenous FAs transportation process is ATP- and CoA-required [12]. van den Berg firstly reported FadL’s crystal structures and found that FadL constitutes a presumed β-barrel with 14 transmembrane β-strands and 7 extracellular loops [18]. Thus, a lateral-diffusion transport mode of FadL-uptook LCFAs has been built, the FA transport activity is regulated by the C-terminal of FadL, and the FadL expression can largely control the whole-cell biotransformation rates of FAs [18,19,20]. FadL works together with fatty acyl-CoA ligase (FadD) to vectorial esterification-induced concomitant transport and activation of CoA thioester [21, 22], thereby rendering this process unidirectional.
Fatty Acyl-CoA Ligase (FadD)
FadD participates E. coli β-oxidation by catalyzing free FAs to acyl-CoA thioesters. Besides the catalyzation of endogenous FAs released from membrane lipids, FadD plays a pivotal role in the catalyzation of exogenous LCFAs [23]. The molecular weight of FadD is about 62 kDa [24]. FadD contains two structural binding domains for ATP and FA binding separately [25]. It can activate FAs with C6-C20 chain lengths, showing the best specificity for C14-C18 [21]. FadD catalyzes the conversion of LCFAs for their later reduction into intermediates, which are involved in serving as substrates for β-oxidation and phospholipid biosynthesis [26, 27]. Knocking out of the fadD gene leads to loss of transport function and degradation of LCFAs [21, 26], and resulting in significantly decreased E. coli viability in its stationary growth period [28], suggesting a critical function of FA oxidation for survival capacity. The FA transport system in microbes is partially shock-sensitive, suggesting the requirement of precise chemical composition (pH, periplasmic protein, etc.) [21]. It is interesting that loss-of-function of FadD decreased the expression of fadL via FadR [29], and the overexpression of fadD in E. coli strongly up-regulated the expression of fadBA and fadE [30]. A 3D model for the E. coli FadD has been proposed, and the crystal structure demonstrates that the N-terminal domain is the main part, composed by a twisted antiparallel β-barrel flanked by two β-sheets flanked by α-helices [31].
Fatty Acyl-CoA Ligase (FadK)
The molecular weight of FadK is about 62.77 kDa. Functional and expression identification of FadK indicate that it functions similarly to ligases FadD by producing an acyl-AMP intermediate before forming the final acyl-CoA, demonstrating that it is a second E. coli acyl-CoA ligase. With SCFAs as preferred substrates, FadK displays a stronger relative activity when compared with LCFAs for MCFAs but weaker absolute activity than FadD [32]. The fadK expression is suppressed during aerobic growth but promoted in the presence of terminal electron acceptor such as fumarate in an anaerobic environment [33].
Acyl-CoA Dehydrogenase (FadE)
As a flavinase, the flavoprotein of FadE (75 kDa) can transfer electrons from the cofactor of FAD/FMN to the electron-transport chain [12]. FadE catalyzes the first step of the activated FAs’ degradation, except for Δ2-unsaturated FAs. The fact that fadE encoding the sole acyl-CoA dehydrogenase is genetically confirmed in E. coli. FAs with various chain lengths cannot be metabolized in E. coli fadE mutants [25]. The substrates of FadE are fatty acyl-CoA esters, which limits the experiments because of their complicated chemical synthesis, especially for LCFAs [34].
Multienzyme Complex of β-Oxidation (FadA & FadB)
As a multienzyme complex of β-oxidation, FadA and FadB are critical for the degradation of FAs in E. coli. It consists of an α subunit (FadB, 79 kDa) and β-subunit (FadA, 41 kDa), catalyzing five reactions during the degradation of various FAs [35]. The β-oxidation complex FadBA comprises 2-enoyl-CoA hydratase, 3,2-enoyl-CoA isomerase, 3-hydroxyacyl-CoA dehydrogenase, 3-hydroxyacyl-CoA epimerase and 3-oxoacyl-CoA thiolase activities. Substrate specificity studies demonstrated that this β-oxidation complex is required for the substrate metabolism [12]. The multienzyme complex of FAs β-oxidation shows features that from non-mitochondrial systems (in bacteria and peroxisomes of eukaryotes).
FadA
The β-subunit of FadBA (product of fadA) is 40.9 kDa with 387 residues. FadA conducts 3-ketoacyl-CoA thiolase activity to catalyze the last step of β-oxidation. This small β-subunit of FadBA works on FAs with different chain lengths to release two carbon atoms, formimg acetyl-CoA in thiolysis reactions.
FadB
The α subunit (79.6 kDa) of FadBA (product of fadB) contains 729 residues. FadB can acts as 3-hydroxyacyl-CoA epimerase to catalyze 2-trans-enoyl-CoA to L-3-hydroxyacyl-CoA and as enoyl-CoA hydratase to convert from D-3-hydroxy of acyl-CoA to 3-trans-enoyl-CoA [12]. Taking this configuration isomerism into consideration, it is needed to mention that a kinked steric structure of cis-unsaturated FAs (cis-UFA) leads to highly mobile membranes; Conversely, the more extended trans-UFAs reduce membrane fluidity when compared to their cis-isomers. Since the adaptation mechanism of bacteria to environmental stress is that they can change the cis-unsaturated FAs into trans ones in membrane lipids, increasing the membrane stiffness to resist stress better [9], and LCFAs are difficult to be biodegraded [36], thus, FadB may be important for bacteria adaptation and for modulating membrane permeability and fluidity.
Typically, the enzymes that catalyze 2-enoyl-CoA into S- or R-3-hydroxyacyl-CoA are named enoyl-CoA hydratases (ECHs, a domain of FadB), in which the S- and R-specific type is named ECH-1 and ECH-2, respectively, and their substrates have different specificity for chains of different lengths [37], and their geometries active sites are similar, even if in a mirror image fashion [38]. It is remarkable that only ECH-2 (R-ECH) but not ECH-1 comprises epimerase activity and can specifically catalyze 3-hydroxyacyl-CoA from R- or D- type to generate S- or L- type. Without the epimerase activity of ECH-2, all cis-UFAs are difficult to undergo the following steps of β-oxidation. ECH-2 is speculated to re required in the lipidic intermediates metabolism in bacteria.
2,4-dienoyl Reductase (FadH)
FadH assists in the β-oxidation of some unsaturated FAs [39]. FadH (72.55 kDa) is a monomer, in which N-terminus contains a FAD-binding domain and C-terminus has a NADPH-binding domain [12]. FadH catalyzes the β-oxidation reactions of specific unsaturated FAs with double bonds at contiguous even-numbered positions, producing 3-trans-enoyl-CoA, which forms 2-trans-enoyl-CoA stimulated via FadB isomerase activity [12]. Feng and Cronan [39] reported that fadH is regulated by three independent regulators, oxygen response system ArcA-ArcB, the FA degradation repressor FadR, and the complex of cyclic AMP receptor protein-cyclic AMP (CRP-cAMP). The expression level of fadH increases threefold by the deletion of arcA, and 8- to tenfold after inactivation of fadR and arcA under anaerobic conditions, suggesting that anaerobic expression is inhibited by ArcA-ArcB and FadR [39]. The binding of FadR to the fadH promoter can be reverted by the thioesters of long-chain acyl-coenzyme A, consistent with that fadH is strongly induced by LCFAs, and the activation of fadH by CRP-cAMP complex and prototype fad is similar [39].
Acyl-CoA Thioesterase (FadM)
Researchers reported a novel long-chain acyl-CoA thioesterase FadM, which can cleave the thioester bonds of acyl-CoA during β-oxidation [40, 41]. FadM is 15 kDa, composed of 132 amino acids. The expression of fadM is confirmed to be negatively regulated by CRP-cAMP complex, which is different from other fad regulon genes that are positively regulated [40, 41]. FadM is required for β-oxidation of unsaturated FAs, such as oleic acid [42].
Transcriptional Regulator of fad Genes (FadR)
FadR is 26.97 kDa, composed of 239 amino acid residues. Coexpression of FadR led to enhanced production of free FAs in E. coli [43], it is a critical transcriptional regulator which are capable of down-regulate the expression of gene involved in β-oxidation (fadBA, fadD, fadE, fadH, fadL, fadM). Interestingly, FadR can also act as an activator of transcription [44]. For example, iclR is one of the genes activated by FadR [45]. IclR is a repressor of the aceBAK operon coding for the enzymes of the glyoxylate shunt. In addition, FadR activates almost all FA biosynthesis genes (such as unsaturated FA biosynthetic genes fabA and fabB) [13]. Hence, FadR is a transcriptional factor that positively regulates anabolism and negatively regulates the catabolism of the same family of molecules [46]. This dual role of FadR in FA degradation and synthesis seems specific to E. coli, as in other bacteria, two distinct regulators are commonly present to solve the two functions [44]. During β-oxidization, the activated C12-C18 LCFAs remove the repression of FadR by binding to it and causing the protein to be released from DNA. In contrast, MCFAs (C7–C11), do not exhibit this feature [47]. Thus, E. coli utilizes LCFAs (but not MCFAs), as the only source for generating carbon and energy. However, wild-type E. coli was once reported to utilize MCFAs as a unique carbon source in anaerobic environment, illustrating that FadR is not the key determinants regulating the FA metabolism [25]. Moreover, FadR is induced to express by LCFAs, but not by MCFAs [12]. On the FadR 3D structure, a dimer and a two-domain fold, a DNA-binding domain in N-terminal, and an acyl-CoA-binding domain in C-terminal were observed [48].
Fatty Acids β-Oxidation Research in Other Bacteria
Studies aimed at better characterizing FA catabolism in bacteria other than E. coli are increasingly intriguing. As a result of high-throughput technologies, a growing number of FA β-oxidation genes have been found in various microbes. Despite many FA β-oxidation genes present in bacterial genomes with essential functions in host survival, limited information is available regarding the metabolic details and the related regulatory mechanisms of these genes. Although the mechanism of β-oxidation in bacteria is relatively conserved, the biological functions mediated by β-oxidation pathways are diverse.
Bacillus subtilis: The FA β-oxidation process in B. subtilis is similar but not identical to that in E. coli, with a major difference being that B. subtilis can break down branched chain fatty acids while E. coli cannot [49,50,51]. Many β-oxidation-related genes, such as lcfA, lcfB (yhfL), fadB (ysiB), fadN (yusL), fadA (yusK), fadE (yusJ) and fadG, have been detected in B. subtilis. The disruption of fadA, fadE, fadG or fadN significantly affects FAs utilization [51]. Simultaneously, the global transcriptional regulator FadR, which can be inactivated by long-chain acyl-CoAs, inhibits the transcription of genes regulating FA degradation in B. subtilis [50].
Haemophilus parasuis, the pathogen of Glässer’s disease, can cause arthritis, fibrinous polyserositis, and meningitis [52]. The H. parasuis genes HAPS_1695 & HAPS_0217 (designated as fadD2 & fadD1) are identified as FadD and prefer LCFAs as substrate. Physiological function results show that either of these two genes is necessary for their survival, revealing that these genes are target candidates for antibiotics or other drugs to control Haemophilus pathogenicity [52]. In order to understand the virulence level among strains, Hill et al. detected the expression of virulence-related genes in H. parasuis by reverse transcription-polymerase chain reaction [53]. The expression of FadD was significantly up-regulated after acute infection, suggesting that FadD may be involved in virulence. A similar result about the relationship between FadD and virulence was reported in Pseudomonas aeruginosa [54], where FadD is implicated in bacterial pathogenicity.
Mycobacterium: Biofilms, a bacterial strategy to adapt to harsh environments, are synthesized using intermediates from the β-oxidation cycle [55, 56]. Unfortunately, the specific correlation between FA β-oxidation and mycobacterial lipid metabolism is still unclear. Xu and colleagues [57] identified a TetR-like transcription factor MmbR in M. smegmatis and found this regulator responsible for β-oxidation and biofilm formation. The genome of intracellular pathogen Mycobacterium tuberculosis (Mtb) contains approximately 100 redundant genes related to the β-oxidation process [58, 59]. To better understand the lipid metabolism in Mtb, Venkatesan and Wierenga [60] isolated and analyzed the complex of FA β-oxidation (trifunctional enzyme, TFE), which catalyzes the β-oxidation reactions. Structural analysis showed that TFE is a complex encoding for the fadA-encoded TFE-β and the fadB-encoded TFE-α. In addition, Cox et al. [58] reported the crystal structure of FadB2. They explained the functional difference between monofunctional FadB2 and the trifunctional FadA-FadB complex: FadB2 cannot dimerize or replace FadB in their complex because it doesn’t have the hydrates domain. However, this does not eliminate the effect of FadB2 on the FadB hydration product [58]. A recent investigation [61] provided evidence that the mycolic acid desaturase regulator (MadR) mutant of M. smegmatis showed a damaged cell wall and accumulation of desaturated α-mycolate, indicating that MadR controls the desaturation and biosynthesis of mycolic acid. Additionally, the transcriptomic profiling results also implicated that MadR indirectly regulates the β-oxidation pathways, as lipid metabolism genes were significantly down-regulated in madR mutant.
Myxococcus: Lipid bodies, such as triacylglycerides, are important for fruiting body formation, and lipid body catabolism by FA β-oxidation provides the major energy during Myxococcus xanthus development [62]. Bioinformatics and experimental analysis showed that M. xanthus has two gene sets, MXAN_5371 (designated as fadJ) and MXAN_5372 (designated as fadI), and their expression increased at the developmental stage when lipid body accumulation reaches its maximum, which involved in the degradation of lipid bodies and flavin adenine dinucleotide. Deletion of fadI (MXAN_5372 and MXAN_6988) and fadJ (MXAN_5371 and MXAN_6987) caused developmental defects, increased lipid body content, and slowed spore coat maturation [63, 64]. Therefore, this finding suggests that β-oxidation participates in fruiting body growth and spore coat maturation, as well as increases the ability of spores to resist UV light and/or heat [62].
Pseudomonas aeruginosa is a well-known pathogen causing lung infection in patients who have cystic fibrosis (CF) [65]. Kang et al. [65] found that the expression of P. aeruginosa fadD2 influences the production of phospholipases, rhamnolipids, lipases, proteases, and is also relevant to swimming and swarming motilities, whereas fadD1 gene only participates in swarming motility. Zhang et al. [66] illustrated that the expression levels of rhamnolipid biosynthetic genes, rhlA and rhlB, were increased by 200- to 600-fold, while fadA was only up-regulated 3- to 4- fold with the addition of octadecanoic acid. These findings demonstrated that there are indeed pathophysiological interconnections between virulence factors and FAs β-oxidation. Moreover, it was reported that psrA, a TetR-type transcriptional regulator, could control quinolone signal and β-oxidation-related genes in P. aeruginosa, such as fadE [67, 68]. Induction of the fadBA5 operon by PsrA produces a signaling response to LCFA, indicating that binding of LCFAs to PsrA relieves fadBA5 repression [67].
In Ralstonia eutropha, acetyl-CoA or FA metabolic intermediates can be used for high yield production of polyhydroxyalkanoates (PHAs) or different PHA precursors, respectively. PHA, as an energy and carbon storage compound produced by microorganisms, has the potential to replace the petroleum-based plastics because of their excellent biodegradability and biocompatibility. Besides PHAs and PHA-related polymers, R. eutropha can also produce many other polymers, making this species a model organism for PHA biosynthesis [69]. However, few studies have reported that these genes in this species are actually involved in FA degradation, even though the genomic sequence of R. eutropha H16 is known and some potential β-oxidation pathway gene homologs have been annotated [49, 70]. Microarray and mutation analyses demonstrated two operons candidates that encode the β-oxidation-related enzymes in R. eutropha, but at least one operon is needed for FA degradation [71, 72].
Salmonella enterica: Although the fatty acid metabolism pathways in S. enterica and E. coli are considered almost the same according to the sequence alignment, experiments have shown that S. enterica has greater efficiency of β-oxidation than E. coli. The difference is manifested in (1) S. enterica could grow normally with decanoic acid, while E. coli cannot; (2) FAs can be completely degraded to acetyl-CoA in S. enterica, whereas many intermediate products are produced in E. coli, which can be explained by the different functions of fadE and fadBA in different organisms [73]. Except for being involved in FA degradation, FadD also activates hilA gene expression, which is required for invasion. In S. enterica serovar Typhi HEp-2 cells, the hilA expression and invasiveness was reduced by fadD mutation [74].
Streptomyces coelicolor is commonly used to produce antibiotics for clinical use and can accumulate high-level triacylglycerol (TAG) to supply energy and carbon. TAG mobilization is correlated with high-energy production due to the high reductive status of acyl moieties [75]. S. coelicolor can efficiently take up exogenous FAs as specific carbon and energy sources for membrane phospholipids synthesis or store them in neutral lipid compounds, like TAG [75]. Menendez-Bravo et al. [76] discovered that three gene clusters (SCOfadAB_1, SCOfadAB_2 and SCOfadAB_3) are involved in the FA β-oxidation in S. coelicolor. They confirmed the correlation of SCOfadAB with TAG by flux balance and mutation analyses, suggesting that defective β-oxidation could inhibit the mobilization of TAG.
Vibrio: Vibrio cholerae is the leading cause of the diarrheal disease cholera. Bile unsaturated FAs drastically inhibit the transcript of virulence factors and enhance motility [77]. To verify the relationship between the TetR family transcriptional repressor PsrA and the pathogenicity of V. cholerae, Yang et al. [78] found a significant decreased in colonization ability, as well as reduced the expression of fadB, fadA, fadI and fadJ genes in the psrA mutant, demonstrating that PsrA is a key regulator that inhibits the expression of fad regulon genes. In addition, the fadR gene is essential for bacterial infection in many pathogens. Brown and Gulig [79] first demonstrated that fadR can cause infection in animal hosts during their study on V. vulnificus, with a mechanism closely related to FAs metabolism. The fadR mutants led to a low infection rate in localized skin damage and fatal systemic liver infections, insensitivity to envelope pressure, reduced mobility, and changes of membrane lipid structure. Furthermore, infection with oleate in vitro promoted infection in mice by fadR mutant. Simultaneously, it showed significantly decreased and increased content of unsaturated and saturated FAs, respectively, suggesting that these fadR mutants have defective ability to biosynthesize unsaturated FAs [79].
β-Oxidation of DSF-Family Quorum Sensing Signal Molecules and the Relevant Mechanisms Underlying Bacterial Pathogenicity
The Gram-negative plant pathogenic bacteria Xanthomonas comprise various species that can cause illness in more than 400 kinds of economically important crops [80]. All Xanthomonas can synthesize and secrete a type of DSF (diffusible signal factor)-family quorum sensing (QS) signal to sense population density and regulate various biological processes, such as virulence, biofilm diffusion, and ecological ability [81, 82]. The major quorum-sensing signal molecules from DSF-family identified from Xanthomonas campestris pv. campestris (hereafter referred to as Xcc) belong to a class of cis-unsaturated LCFAs, including the DSF (cis-11-methyl-2-decenoic acid) and BDSF (cis-2-decenoic acid) [83,84,85]. Barber [86] and Slater [87] demonstrated that in batch cultures of Xcc, DSF accumulated at the early stationary stage, but then decreased. Wang et al. [83] solved the structures of DSF by NMR, and further confirmed that the level of DSF remained relatively low at the early-growing stage, subsequently increased substantially, and then decreased rapidly at the post-logarithmic growth phase. For Xanthomonas oryzae pv. oryzae (Xoo hereafter) strain KACC10331 cultured in YEB medium, He et al. [88] found that BDSF production started to increase after 18 h inoculation and reached its maximum at 36 h, and then significant decreased at 42 h in the supernatants. These results indicated a naturally-occurring signal turnover phenomenon in Xanthomonas.
RpfB enzymatic activity is required for DSF and BDSF turnover. A recent study unveiled that RpfB functions as a fatty acyl-CoA ligase that plays a role in FA β-oxidation to give acyl-CoAs for membrane lipid synthesis in Xcc. It also plays a more important role in pathogenesis by counteracting the thioesterase activity of DSF synthase RpfF [89]. Deletion of rpfB (ΔrpfB hereafter) slightly increased Xcc virulence in Chinese radish, while overexpression of rpfB [ΔrpfB(rpfB) hereafter] significantly decreased virulence [85]. Zhou et al. [85] developed new methods to quantify the DSF family genes by the combined use of ultra-performance liquid chromatographic (UPLC) and time-of-flight mass spectrometry (TOF–MS). using these new methods, Zhou discovered that mutation of rpfB (ΔrpfB) increased the biological production of DSF and BDSF for ~ 10.0-fold compared to the parental strain Xcc or Xoo. Overexpression of rpfB or E. coli fadD in ΔrpfB [ΔrpfB(rpfB)] blocked the production of DSF and BDSF; ΔrpfB(rpfB) could rapidly degrade exogenously added DSF and BDSF, but ΔrpfB had no degradation ability [83]; RpfB with an E-365 mutation showed largely decreased DSF and BDSF degradation activities. Therefore, RpfB is needed for the degradation of DSF and BDSF signal molecules and represents to a naturally-occurring signal conversion system targeting at DSF-family QS signals in Xcc and Xoo (Fig. 2). RpfB-dependent signaling turnover is also widely observed in human opportunistic pathogens, like Burkholderia spp. and Stenotrophomonas maltophilia, as well as environmental bacteria including Leptospirillum, Frateuria, Lysobacter, Luteibacter, Methylobacillus flagellates, Rhodanobacter, and Thiobacillus denitrificans [90]. Thus, future studies on these microbes could also benefit from this review. Furthermore, the Xcc genome also contains homologues of other genes regulating E. coli FA β-oxidation, such as fadL(Xcc0017), fadE (Xcc2870), fadA (Xcc1978), fadB (Xcc1979, Xcc0810) and fadH (Xcc0933). Recently, preliminary results confirmed that Xcc1979 and Xcc1978 are involved in DSF degradation. Although the functions of other genes remain to be verified, one of the Xcc potential naturally occurring DSF signal turnover systems should be FAs β-oxidation pathway.
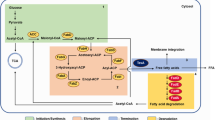
Schematic representation of DSF-mediated QS signaling network and QS signal turnover system in Xcc. Fatty acids β-oxidation pathway participates in the degradation of DSF and BDSF signal molecules and represents a naturally occurring signal turnover system that targets DSF-family QS signals in Xcc. Taking Xanthomonas as an example, the DSF-family quorum sensing signal molecular degradation mechanism is introduced. RpfF is one of the key enzymes required for DSF biosynthesis, DSF is sensed and transduced by the two-component signaling system RpfC–RpfG through a phosphorelay cascade, and the downstream second-messenger cyclic di-GMP, The global regulator Clp is the effector of cyclic di-GMP, which regulates directly and indirectly the expression of genes for virulence and adaptation [92]. Zur a transcriptional regulator involving in zinc uptake regulation, FhrR a transcriptional regulator required for flagella biosynthesis and hypersensitive response (Hrp)
Compared with E. coli β-oxidation, the DSF signaling molecule degradation pathway in Xanthomonas has at least two unique phenomena. (1) An unique epimerase is needed, and the function of the epimerase that converts (R)-3-hydroxyacyl-CoAs to (S)-3-trans-hydroxyacyl-CoAs may come from FadB (Xcc1979); otherwise DSF cannot be degraded; (2) Xcc has some predicated homologues involved in FA metabolism but lacks a FadR homologue; rpfB is under the direct regulation of a global transcription factor Clp [85, 91].
DSF-producing microbes are widely distributed [92]. Bacteria other than Xanthomonas also synthesize DSF-family quorum sensing signals. P. aeruginosa can synthesize cis-2-decenoic acid, which can trigger dispersion during microbial biofilms [93]; Xylella fastidiosa can produce cis-2-tetradecenoic acid and cis-2-hexadecenoic acid (XfDSF1 & XfDSF2) [94]; and the Gram-positive bacterium Streptococcus mutans can produce signaling molecule trans-2-decenoic acid (SDSF) to inhibit Candida albicans hyphal formation [95].
The core rpf signaling-components, including genes such as RpfB, RpfG, RpfF, and RpfC, have been functionally verified in all sequenced Xanthomonas species. Homologous gene clusters also exist in Lysobacter dokdonensis DS-58, Methylobacillus flagellatus, Pseudoxanthomonas spadix BDA-59, Stenotrophomonas maltophilia, Thiobacillus denitrificans and X. fastidiosa. RpfF encodes a key enzyme required for DSF biosynthesis, whereas RpfC and RpfG constitute a two-component system involved in signal perception and transduction in Xcc [87, 96]. The activated HD-GYP domain of RpfG has phosphodiesterase activity and can degrade cyclic di-GMP (c-di-GMP), an inhibitory ligand of the global transcription factor Clp. Consequently, derepressed Clp drives the expression of several hundred genes, including those encoding virulence factor production [97]. BDSF (cis-2-decenoic acid) and other similar structure signal molecules have been found in Burkholderia cenocepacia and P. aeruginosa, and homologous proteins of RpfB are also present in the two strains [91]. Two RpfB proteins in Lysobacter enzymogenes were identified by Li et al. [98]. RpfB was shown to have substitutional function for FadD in Xcc [89]. In vitro experiments demonstrated that RpfB could activate various FAs to the corresponding CoA esters [89], and these RpfB-activated fatty acyl-CoAs would be degraded further during the β-oxidation process. Recent researche confirmed that the mutants of rpfG, rpfC, or clp, in Xcc and Xoo all showed increased transcriptional and translational levels of rpfB [85]. The above-mentioned results demonstrated that RpfB-dependent FAs β-oxidation may be a conserved quorum sensing turnover mechanism in bacteria (Fig. 2).
Steric-Problems-Solving Strategies for β-Oxidizing in Different Acyl-CoA Variants
The common even-carbon saturated FAs are metabolized via β-oxidation (Fig. 1). However, FAs with special structures also exist in living organisms. β-oxidation includes four reactions that occur in repeating cycles, producing intermediates such as trans-2-enoyl-CoA, 3-hydroxyacyl-CoA, and 3-ketoacyl-CoA. In this way, β-oxidation can be easily explained for straight-chain saturated FAs, but not for all modified general FAs. Several methods, such as evolutionary analysis of enzymes’ specificity regarding chain length, modified acyl-chain or any special conformation, have been explored to ensure the degradation of FAs with various steric variants such as very long chain fatty acids (VLCFAs), unsaturated fatty acids, and branched chain fatty acids [1]. In general, FAs can be degraded via different mechanisms, including α-, β- and ω-oxidation.
α- and β-oxidation for VLCFAs. The acyl-group with more than 20 carbons (C20) can undergo β oxidation (Fig. 1B). Currently, there are three strategies to oxidize substrates of different chain lengths, such as development of the adaptable active sites, acquisition of paralogues, and functional convergence of isoforms [1]. α-oxidation shortens FAs by one carbon from the carboxyl-end. It is a peroxisome reaction that activates the 3-methyl-branched FA to a 3-methyl-branched fatty acyl-CoA, which is aerobically oxidized by fatty acyl CoA 2-hydroxylase to produce a 2-hydroxy-3-methyl-branched fatty acyl-CoA.
β-oxidation of unsaturated FAs (Fig. 1). β-oxidation allows for the complete degradation of unsaturated FAs with an even number of carbon though FadE-mediated dehydrogenation to form double bonds. However, such cases are not suitable for all FAs. Extra steps are needed for the oxidation of double-bonds FAs based on the double bond position and the configuration of FAs. Each double bond reduces the generation of one FADH2, but one less step of FadE-mediated dehydrogenation. The double bond position and the configuration ofFAs also affect the β-oxidation process. (1) The prevented double bond between C2–C3 by that of C3–C4 can be solved by a reaction that converts the double bond of the cis-Δ3 into a trans-Δ2 type via cis-Δ3-enoyl CoA isomerase (ECI1). Thus, the double bond between C2 and C3 is finally generated, sharing the following reactions with those of saturated FAs, as trans-Δ2-enoyl CoA is a regular substrate. For example, in unsaturated palmitoleate with one double bond between C9 and C10, its cis-Δ3 -enoyl CoA is converted into trans-Δ2-enoyl CoA first, allowing the oxidation pathway continue. (2) The existence of a double bond between C2 and C3 improves the hydration reaction of both trans-Δ2-enoyl CoA and cis-Δ2-enoyl CoA into 3-hydroxyacyl-CoA, whereas the product of cis-Δ2-enoyl CoA is D-3-hydroxyacyl-CoA, not L-3-hydroxyacyl-CoA. Therefore, 3-hydroxyacyl-CoA epimerase is required to convert D-3-hydroxyacyl-CoA into L-3-hydroxyacyl-CoA, resulting in the continuation of the FAs β-oxidation. Typically, 3-hydroxyacyl-CoA epimerase participates in the metabolism of cis double bonds at even-numbered positions. The mechanism of epimerization indicates that the epimerization of 3-hydroxyacyl-CoA is accomplished by the combined functions of ECH-1 and ECH-2. (3) Polyunsaturated fatty acids (PUFAs) are precursors of signaling molecules. The β-oxidation of PUFAs is a little different because the 2,4-dienoyl intermediate produced by PUFAs β-oxidation cannot be used as a substrate for the next step’s enzymes. Fortunately, this issue is avoided by 2,4-dienoyl-CoA reductase and cis-Δ3-enoyl CoA isomerase, which transform the 2,4-dienoyl intermediate into a customary trans-Δ2.
β-oxidation of methyl-branched fatty acids (Fig. 1C) needs the joint collaboration of β- and α-oxidation pathways, as well as α-methylacyl-CoA racemases [1]. β-oxidation would be halted if the methyl groups cause steric problems and inhibit the second dehydrogenation step. Fortunately, this issue will be solved by the α-oxidation pathways. α-oxidation usually degrades FAs with special structure. For example, phytanic acids attached to CoA and form phytanoyl-CoA, which is then oxidized to 2-hydroxyphytanoyl-CoA. This is followed by the cleavage of 2-hydroxyphytanoyl-CoA to form pristanal and formyl-CoA, and then the oxidization of pristanal to form pristanic acid, which conducts β-oxidation.
Fatty acid ω-oxidation. In ω-oxidation, the methyl group is firstly hydroxylated at the ω-end under the canalization of cytochrome P450, followed by ω-end oxidation to produce 1, ω-dicarboxylic acid, which can be shortened by β-oxidation. It has been reported that the α-chlorinated FAs can be degraded by ω- and β-oxidation successively from the ω-end [99]. Some microbes can convert alkanes into FAs through ω-oxidation, which can be used for environmental protection, such as cleaning up oil spills. It has been also demonstrated that VLCFA can undergo ω-oxidation [100].
Concluding Remarks and Future Perspectives
Regarding the even-carbon saturated-FAs in E. coli, their primary metabolic pathways have been well-studied, and the metabolic mechanism has been basically clarified. Fatty acid oxidation pathways and mechanisms in other bacteria are also being studied. The specific roles of fatty acid β-oxidation in Xanthomonas, particularly its connection to DSF-mediated QS and bacterial pathogenicity, are being explored. However, several areas require further investigation to fully elucidate the complexities of fatty acid β-oxidation in bacteria, particularly in pathogenic contexts: (1) The selectivity of substrates, enzyme activity and molecular mechanism of the key enzymes within β-oxidation pathway, such as FadBA complex, need further elucidation, to shed light on how bacteria utilize β-oxidation to regulate virulence through QS signaling etc. (2) The degradation enzymes of many specialized structures of FAs (such as DSF-family QS signals, which comprises cis-2-unsaturated FAs of different chain lengths and branching) and their mechanisms require additional study. (3) The correlation and crossover between β-oxidation and other FAs metabolic pathways deserve further investigation. (4) An important future research direction should be the use of synthetic biology methods to modify the β-oxidation pathway and synthesize products with application value. (5) The function of FA degradation pathways in the symbiotic environment of animals, plants, humans and microorganisms needs further study. As a factor of the global regulatory network, QS controls many microbial traits, such as symbiosis, virulence, development and maintenance of population, and community structures. Addressing the above issues will be key to gaining a more detailed understanding of the bacterial utilization, modification or prevention strategies.
References:
Hiltunen JK, Qin YM (2000) Beta-oxidation-strategies for the metabolism of a wide variety of acyl-CoA esters. BBA-Mol Cell Biol L 1484(2–3):117–128
Sawant N, Singh H, Appukuttan D (2022) Overview of the cellular stress responses involved in fatty acid overproduction in E. coli. Mol Biotechnol 64(4):373–387
Schönfeld P, Wojtczak L (2016) Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res 57(6):943–954
Kumar P, Lee JH, Beyenal H, Lee J (2020) Fatty acids as antibiofilm and antivirulence agents. Trends Microbiol 28(9):753–768
Kralj T, Nuske M, Hofferek V (2022) Multi-omic analysis to characterize metabolic adaptation of the E. coli lipidome in response to environmental stress. Metabolites 12(2):171
Cintolesi A, Rodriguez-Moya M, Gonzalez R (2013) Fatty acid oxidation: systems analysis and applications. Wiley Interdisciplinary Rev-Sys Biol Med 5(5):575–585
Yao J, Rock CO (2015) How bacterial pathogens eat host lipids: Implications for the development of fatty acid synthesis therapeutics. J Biol Chem 290(10):5940–5946
Velázquez-Sánchez C, Vences-Guzmán M, Moreno S, Tinoco-Valencia R, Espín G, Guzmán J, Sahonero-Canavesi DX, Sohlenkamp C, Segura D (2021) PsrA positively regulates the unsaturated fatty acid synthesis operon fabAB in Azotobacter vinelandii. Microbiol Res 249:126775
Kondakova T, Kumar S, Cronan JE (2019) A novel synthesis of trans-unsaturated fatty acids by the Gram-positive commensal bacterium Enterococcus faecalis FA2-2. Chem Phys Lipids 222:23–35
Karlinsey JE, Fung AM, Johnston N, Goldfine H, Libby SJ, Fang FC (2022) Cyclopropane fatty acids are important for Salmonella enterica serovar Typhimurium virulence. Infect Immun 90(1):e0047921
Hosmer J, McEwan AG, Kappler U (2024) Bacterial acetate metabolism and its influence on human epithelia. Emerg Top Life Sci 8(1):1–13
Kunau W-H, Dommes V, Schulz H (1995) Beta-oxidation of fatty acids in mitochondria, peroxisomes, and bacteria: A century of continued progress. Prog Lipid Res 34(4):267–342
Campbell JW, Cronan JE (2001) Escherichia coli FadR positively regulates transcription of the fabB fatty acid biosynthetic gene. J Bacteriol 183(20):5982–5990
Jaswal K, Shrivastava M (2021) Revisiting long-chain fatty acid metabolism in Escherichia coli: integration with stress responses. Curr Genet 67(4):573–582
Matta MK, Lioliou EE, Panagiotidis CH, Kyriakidis DA, Panagiotidis CA (2007) Interactions of the antizyme AtoC with regulatory elements of the Escherichia coli atoDAEB Operon(del). J Bacteriol 189(17):6324–6332
van den Berg B (2005) The FadL family: unusual transporters for unusual substrates. Curr Opin Struct Biol 15(4):401–407
Jeon EY, Song JW, Cha HJ, Lee SM, Lee J, Park JB (2018) Intracellular transformation rates of fatty acids are influenced by expression of the fatty acid transporter FadL in Escherichia coli cell membrane. J Biotechnol 281:161–167
van den Berg B, Black PN, Clemons WM, Rapoport TA (2004) Crystal structure of the long-chain fatty acid transporter FadL. Science 304(5676):1506–1509
Hearn EM, Patel DR, Lepore BW, Indic M, van den Berg B (2009) Transmembrane passage of hydrophobic compounds through a protein channel wall. Nature 458(7236):367–370
Somboon K, Doble A, Bulmer D, Basle A, Khalid S, van den Berg B (2020) Uptake of monoaromatic hydrocarbons during biodegradation by FadL channel-mediated lateral diffusion. Nat Commun 11(1):6331
Black PN, DiRusso CC (2003) Transmembrane movement of exogenous long-chain fatty acids: Proteins, enzymes, and vectorial esterification. Microbiol Mol Biol Rev 67(3):454–472
Schmelter T, Trigatti BL, Gerber GE, Mangroo D (2004) Biochemical demonstration of the involvement of fatty acyl-CoA synthetase in fatty acid translocation across the plasma membrane. J Biol Chem 279(23):24163–24170
Pech-Canul Á, Nogales J, Miranda-Molina A, Álvarez L, Geiger O, Soto MJ, López-Lara IM (2011) FadD is required for utilization of endogenous fatty acids released from membrane lipids. J Bacteriol 193(22):6295–6304
Black PN, DiRusso CC, Metzger AK, Heimert TL (1992) Cloning, sequencing, and expression of the fadD gene of Escherichia coli encoding acyl coenzyme A synthetase. J Biol Chem 267(35):25513–25520
Campbell JW, Morgan-Kiss RM, Cronan JE (2003) A new Escherichia coli metabolic competency: growth on fatty acids by a novel anaerobic beta-oxidation pathway. Mol Microbiol 47(3):793–805
Weimar JD, DiRusso CC, Delio R, Black PN (2002) Functional role of fatty acyl-coenzyme A synthetase in the transmembrane movement and activation of exogenous long-chain fatty acids - Amino acid residues within the ATP/AMP signature motif of Escherichia coli FadD are required for enzyme activity and fatty acid transport. J Biol Chem 277(33):29369–29376
Rock CO (2008) CHAPTER 3 - Fatty acid and phospholipid metabolism in prokaryotes. In: Vance DE, Vance JE (eds) Biochemistry of Lipids, Lipoproteins and Membranes (Fifth Edition). Elsevier, San Diego, pp 59–96
Pech-Canul ÁC, Rivera-Hernández G, Nogales J, Geiger O, Soto MJ, López-Lara IM (2020) Role of Sinorhizobium meliloti and Escherichia coli long-chain Acyl-CoA synthetase FadD in long-term survival. Microorganisms 8(4):470
Bae JH, Park BG, Jung E, Lee PG, Kim BG (2014) fadD deletion and fadL overexpression in Escherichia coli increase hydroxy long-chain fatty acid productivity. Appl Microbiol Biotechnol 98(21):8917–8925
Zhang H, Wang P, Qi Q (2006) Molecular effect of FadD on the regulation and metabolism of fatty acid in Escherichia coli. FEMS Microbiol Lett 259(2):249–253
Black PN, DiRusso CC, Sherin D, MacColl R, Knudsen J, Weimar JD (2000) Affinity labeling fatty acyl-CoA synthetase with 9-p-azidophenoxy nonanoic acid and the identification of the fatty acid-binding site. J Biol Chem 275(49):38547–38553
Ford TJ, Way JC (2015) Enhancement of E. coli acyl-CoA synthetase FadD activity on medium chain fatty acids. PeerJ 3:1040
Morgan-Kiss RM, Cronan JE (2004) The Escherichia coli fadK (ydiD) gene encodes an anerobically regulated short chain acyl-CoA synthetase. J Biol Chem 279(36):37324–37333
Wheeler PR (2009) Analyzing lipid metabolism: activation and beta-oxidation of fatty acids. Methods Mol Biol 465:47–59
He XY, Deng HN, Yang SY (1997) Importance of the gamma-carboxyl group of glutamate-462 of the large alpha-subunit for the catalytic function and the stability of the multienzyme complex of fatty acid oxidation from Escherichia coli. Biochemistry (Mosc) 36(1):261–268
Sun M, Shi Z, Zhang C, Zhang Y, Zhang S, Luo G (2022) Novel long-chain fatty acid (LCFA)-degrading bacteria and pathways in anaerobic digestion promoted by hydrochar as revealed by genome-centric metatranscriptomics analysis. Appl Environ Microbiol 88(16):e0104222
Abdel-Mawgoud AM, Lepine F, Deziel E (2013) A chiral high-performance liquid chromatography-tandem mass spectrometry method for the stereospecific analysis of enoyl-coenzyme A hydratases/isomerases. J Chromatogr A 1306:37–43
Wu L, Lin S, Li D (2008) Comparative inhibition studies of enoyl-CoA hydratase 1 and enoyl-CoA hydratase 2 in long-chain fatty acid oxidation. Org Lett 10(15):3355–3358
Feng Y, Cronan JE (2010) Overlapping repressor binding sites result in additive regulation of Escherichia coli FadH by FadR and ArcA. J Bacteriol 192(17):4289–4299
Ren Y, Aguirre J, Ntamack AG, Chu CH, Schulz H (2004) An alternative pathway of oleate beta-oxidation in Escherichia coli involving the hydrolysis of a dead end intermediate by a thioesterase. J Biol Chem 279(12):11042–11050
Feng Y, Cronan JE (2009) A new member of the Escherichia coli fad regulon: transcriptional regulation of fadM (ybaW). J Bacteriol 191(20):6320–6328
Nie L, Ren Y, Schulz H (2008) Identification and characterization of Escherichia coli thioesterase III that functions in fatty acid beta-oxidation. Biochemistry (Mosc) 47(29):7744–7751
Park WS, Shin KS, Jung HW, Lee Y, Sathesh-Prabu C, Lee SK (2022) Combinatorial metabolic engineering strategies for the enhanced production of free fatty acids in Escherichia coli. J Agric Food Chem 70:13913
Pavoncello V, Barras F, Bouveret E (2022) Degradation of exogenous fatty acids in Escherichia coli. Biomolecules 12(8):1019
Gui L, Sunnarborg A, LaPorte DC (1996) Regulated expression of a repressor protein: FadR activates iclR. J Bacteriol 178(15):4704–4709
Collins CM, D’Orazio SE (1993) Bacterial ureases: structure, regulation of expression and role in pathogenesis. Mol Microbiol 9(5):907–913
DiRusso CC, Nunn WD (1985) Cloning and characterization of a gene (fadR) involved in regulation of fatty acid metabolism in Escherichia coli. J Bacteriol 161(2):583–588
van Aalten DMF, DiRusso CC, Knudsen J, Wierenga RK (2000) Crystal structure of FadR, a fatty acid-responsive transcription factor with a novel acyl coenzyme A-binding fold. EMBO J 19(19):5167–5177
Brigham CJ, Budde CF, Holder JW, Zeng Q, Mahan AE, Rha C, Sinskey AJ (2010) Elucidation of beta-oxidation pathways in Ralstonia eutropha H16 by examination of global gene expression. J Bacteriol 192(20):5454–5464
Fujita Y, Matsuoka H, Hirooka K (2007) Regulation of fatty acid metabolism in bacteria. Mol Microbiol 66(4):829–839
Matsuoka H, Hirooka K, Fujita Y (2007) Organization and function of the YsiA regulon of Bacillus subtilis involved in fatty acid degradation. J Biol Chem 282(8):5180–5194
Feng S, Xu C, Yang K, Wang H, Fan H, Liao M (2017) Either fadD1 or fadD2, which encode acyl-CoA synthetase, is essential for the survival of Haemophilus parasuis SC096. Front Cellular Infect Microbiol 7:72
Hill CE, Metcalf DS, MacInnes JI (2003) A search for virulence genes of Haemophilus parasuis using differential display RT-PCR. Vet Microbiol 96(2):189–202
Martínez-Alcantar L, Orozco G, Díaz-Pérez AL, Villegas HJ, Garcia-Pineda E, Campos-García J (2021) Participation of Acyl-Coenzyme A synthetase FadD4 of Pseudomonas aeruginosa PAO1 in acyclic terpene/fatty acid assimilation and virulence by lipid a modification. Front Microbiol 12:785112
Yamada H, Yamaguchi M, Igarashi Y, Chikamatsu K, Aono A, Murase Y, Morishige Y, Takaki A, Chibana H, Mitarai S (2018) Mycolicibacterium smegmatis, Basonym Mycobacterium smegmatis, Expresses morphological phenotypes much more similar to Escherichia coli than Mycobacterium tuberculosis in quantitative structome analysis and CryoTEM examination. Front Microbiol 9:01992
Raghunandanan S, Jose L, Gopinath V, Kumar RA (2019) Comparative label-free lipidomic analysis of Mycobacterium tuberculosis during dormancy and reactivation. Sci Rep 9(1):3660
Xu H, Su Z, Li W, Deng Y, He Z-G (2021) MmbR, a master transcription regulator that controls fatty acid beta-oxidation genes in Mycolicibacterium smegmatis. Environ Microbiol 23(2):1096–1114
Cox JAG, Taylor RC, Brown AK, Attoe S, Besra GS, Fuetterer K (2019) Crystal structure of Mycobacterium tuberculosis FadB2 implicated in mycobacterial β-oxidation. Acta Crystallographica Section D-Structural Biol 75(Pt 1):101–108
Williams KJ, Boshoff HI, Krishnan N, Gonzales J, Schnappinger D, Robertson BD (2011) The Mycobacterium tuberculosis β-oxidation genes echA5 and fadB3 are dispensable for growth in vitro and in vivo. Tuberculosis (Edinb) 91(6):549–555
Venkatesan R, Wierenga RK (2013) Structure of Mycobacterial beta-oxidation trifunctional enzyme reveals its altered assembly and putative substrate channeling pathway. ACS Chem Biol 8(5):1063–1073
Cooper C, Peterson EJR (2022) MadR mediates acyl CoA-dependent regulation of mycolic acid desaturation in mycobacteria. Proc Natl Acad Sci U S A 119(8):e2111059119
Bullock HA, Shen H, Boynton TO, Shimkets LJ (2018) Fatty acid oxidation is required for Myxococcus xanthus development. J Bacteriol 200(10):e00572
Shi X, Wegener-Feldbruegge S, Huntley S, Hamann N, Hedderich R, Sogaard-Andersen L (2008) Bioinformatics and experimental analysis of proteins of two-component systems in Myxococcus xanthus. J Bacteriol 190(2):613–624
Bhat S, Boynton TO, Pham D, Shimkets LJ (2014) Fatty acids from membrane lipids become incorporated into lipid bodies during Myxococcus xanthus differentiation. PLoS ONE 9(6):e99622
Kang Y, Zarzycki-Siek J, Walton CB, Norris MH, Hoang TT (2010) Multiple fadD acyl-CoA synthetases contribute to differential fatty acid degradation and virulence in Pseudomonas aeruginosa. PLoS ONE 5(10):e13557
Zhang L, Veres-Schalnat TA, Somogyi A, Pemberton JE, Maier RM (2012) Fatty acid cosubstrates provide β-oxidation precursors for rhamnolipid biosynthesis in Pseudomonas aeruginosa, as evidenced by isotope tracing and gene expression assays. Appl Environ Microbiol 78(24):8611–8622
Kang Y, Nguyen DT, Son MS, Hoang TT (2008) The Pseudomonas aeruginosa PsrA responds to long-chain fatty acid signals to regulate the fadBA5 beta-oxidation operon. Microbiol-Sgm 154:1584–1598
Wells G, Palethorpe S, Pesci EC (2017) PsrA controls the synthesis of the Pseudomonas aeruginosa quinolone signal via repression of the FadE homolog, PA0506. PLoS ONE 12(12):e0189331
Riedel SL, Lu J, Stahl U, Brigham CJ (2014) Lipid and fatty acid metabolism in Ralstonia eutropha: relevance for the biotechnological production of value-added products. Appl Microbiol Biotechnol 98(4):1469–1483
Pohlmann A, Fricke WF, Reinecke F, Kusian B, Liesegang H, Cramm R, Eitinger T, Ewering C, Poetter M, Schwartz E et al (2006) Genome sequence of the bioplastic-producing “Knallgas” bacterium Ralstonia eutropha H16. Nat Biotechnol 24(10):1257–1262
Chen JS, Colon B, Dusel B, Ziesack M, Way JC, Torella JP (2015) Production of fatty acids in Ralstonia eutropha H16 by engineering beta-oxidation and carbon storage. PeerJ 3:e1468
Raberg M, Volodina E, Lin K, Steinbuechel A (2018) Ralstonia eutropha H16 in progress: Applications beside PHAs and establishment as production platform by advanced genetic tools. Crit Rev Biotechnol 38(4):494–510
Iram SH, Cronan JE (2006) The beta-oxidation systems of Escherichia coli and Salmonella enterica are not functionally equivalent. J Bacteriol 188(2):599–608
Lucas RL, Lostroh CP, DiRusso CC, Spector MP, Wanner BL, Lee CA (2000) Multiple factors independently regulate hilA and invasion gene expression in Salmonella enterica serovar typhimurium. J Bacteriol 182(7):1872–1882
Alvarez HM, Steinbuchel A (2002) Triacylglycerols in prokaryotic microorganisms. Appl Microbiol Biotechnol 60(4):367–376
Menendez-Bravo S, Paganini J, Avignone-Rossa C, Gramajo H, Arabolaza A (2017) Identification of FadAB complexes involved in fatty acid β-oxidation in Streptomyces coelicolor and construction of a triacylglycerol overproducing strain. Front Microbiol 8:1428
Chatterjee A, Dutta PK, Chowdhury R (2007) Effect of fatty acids and cholesterol present in bile on expression of virulence factors and motility of Vibrio cholerae. Infect Immun 75(4):1946–1953
Yang S, Xi D, Wang X, Li Y, Li Y, Yan J, Cao B (2020) Vibrio cholerae VC1741 (PsrA) enhances the colonization of the pathogen in infant mice intestines in the presence of the long-chain fatty acid, oleic acid. Microb Pathog 147:104443
Brown RN, Gulig PA (2008) Regulation of fatty acid metabolism by FadR is essential for Vibrio vulnificus to cause infection of mice. J Bacteriol 190(23):7633–7644
Ferreira-Tonin M, Rodrigues-Neto J, Harakava R, Lanza Destefano SA (2012) Phylogenetic analysis of Xanthomonas based on partial rpoB gene sequences and species differentiation by PCR-RFLP. Int J Syst Evol Microbiol 62:1419–1424
He YW, Zhang LH (2008) Quorum sensing and virulence regulation in Xanthomonas campestris. FEMS Microbiol Rev 32(5):842–857
Sarnyai F, Donko MB, Matyasi J, Gor-Nagy Z, Marczi I, Simon-Szabo L, Zambo V, Somogyi A, Csizmadia T, Low P et al (2019) Cellular toxicity of dietary trans fatty acids and its correlation with ceramide and diglyceride accumulation. Food Chem Toxicol 124:324–335
Wang LH, He YW, Gao YF, Wu JE, Dong YH, He CZ, Wang SX, Weng LX, Xu JL, Tay L et al (2004) A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol Microbiol 51(3):903–912
Deng Y, Wu Je, Tao F, Zhang L-H (2011) Listening to a new language: DSF-based quorum sensing in gram-negative bacteria. Chem Rev 111(1):160–173
Zhou L, Wang X-Y, Sun S, Yang L-C, Jiang B-L, He Y-W (2015) Identification and characterization of naturally occurring DSF-family quorum sensing signal turnover system in the phytopathogen Xanthomonas. Environ Microbiol 17(11):4646–4658
Barber CE, Tang JL, Feng JX, Pan MQ, Wilson TJG, Slater H, Dow JM, Williams P, Daniels MJ (1997) A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol Microbiol 24(3):555–566
Slater H, Alvarez-Morales A, Barber CE, Daniels MJ, Dow JM (2000) A two-component system involving an HD-GYP domain protein links cell-cell signalling to pathogenicity gene expression in Xanthomonas campestris. Mol Microbiol 38(5):986–1003
He YW, Wu JE, Cha J-S, Zhang LH (2010) Rice bacterial blight pathogen Xanthomonas oryzae pv oryzae produces multiple DSF-family signals in regulation of virulence factor production. BMC Microbiol 10:187
Bi H, Yu Y, Dong H, Wang H, Cronan JE (2014) Xanthomonas campestris RpfB is a fatty Acyl-CoA ligase required to counteract the thioesterase activity of the RpfF diffusible signal factor (DSF) synthase. Mol Microbiol 93(2):262–275
Song K, Chen B, Cui Y, Zhou L, Chan KG (2022) The plant defense signal salicylic acid activates the RpfB-dependent quorum sensing signal turnover via altering the culture and cytoplasmic pH in the phytopathogen Xanthomonas campestris. MBio 13(2):e0364421
Zhou L, Zhang L-H, Camara M, He Y-W (2017) The DSF family of quorum sensing signals: diversity, biosynthesis, and turnover. Trends Microbiol 25(4):293–303
He YW, Deng Y, Miao Y, Chatterjee S, Tran TM, Tian J, Lindow S (2022) DSF-family quorum sensing signal-mediated intraspecies, interspecies, and inter-kingdom communication. Trends Microbiol 31:36
Davies DG, Marques CNH (2009) A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J Bacteriol 191(5):1393–1403
Ionescu M, Yokota K, Antonova E, Garcia A, Beaulieu E, Hayes T, Iavarone AT, Lindow SE (2016) Promiscuous diffusible signal factor production and responsiveness of the Xylella fastidiosa Rpf system. MBio 7(4):e01054-16
Vilchez R, Lemme A, Ballhausen B, Thiel V, Schulz S, Jansen R, Sztajer H, Wagner-Doebler I (2010) Streptococcus mutans inhibits Candida albicans hyphal formation by the fatty acid signaling molecule trans-2-decenoic acid (SDSF). ChemBioChem 11(11):1552–1562
He YW, Xu M, Lin K, Ng YJA, Wen CM, Wang LH, Liu ZD, Zhang HB, Dong YH, Dow JM et al (2006) Genome scale analysis of diffusible signal factor regulon in Xanthomonas campestris pv. campestris: identification of novel cell-cell communication-dependent genes and functions. Mol Microbiol 59(2):610–622
Xu H, Wang R, Zhao Y, Fu ZQ, Qian G, Liu F (2017) LesR is a novel upstream regulator that controls downstream Clp expression to modulate antibiotic HSAF biosynthesis and cell aggregation in Lysobacter enzymogenes OH11. Microb Cell Fact 16(1):202
Li K, Hou R, Xu H, Wu G, Qian G, Wang H, Liu F (2020) Two functional fatty acyl coenzyme A ligases affect free fatty acid metabolism to block biosynthesis of an antifungal antibiotic in Lysobacter enzymogenes. Appl Environ Microbiol 86(10):e00309-00320
Brahmbhatt VV, Albert CJ, Anbukumar DS, Cunningham BA, Neumann WL, Ford DA (2010) Omega-oxidation of alpha-chlorinated fatty acids identification of alpha-chlorinated dicarboxylic acids. J Biol Chem 285(53):41255–41269
Sanders RJ, Ofman R, Valianpour F, Kemp S, Wanders RJA (2005) Evidence for two enzymatic pathways for omega-oxidation of docosanoic acid in rat liver microsomes. J Lipid Res 46(5):1001–1008
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 32200094), the Natural Science Foundation of Hubei Province (2023AFB484), the Enshi Science and Technology Plan Project (Young Talent Project) (No. D20220071), and the Open Research Fund of Hubei Engineering Research Center of selenium food nutrition and health intelligent technology (Hubei Minzu University) (No. PT082301).
Funding
This article was funded by National Natural Science Foundation of China, No. 32200094, Mu Peng, Natural Science Foundation of Hubei Province, 2023AFB484, Zhiyong Wang.
Author information
Authors and Affiliations
Contributions
Z.W. primarily wrote the initial draft of the manuscript. X.H., G.D. and M.P. contributed to the manuscript writing and generated the figures. K.L., G.S. and M.P. conceived the idea and made final edits. All of the authors discussed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Z., Hou, X., Shang, G. et al. Exploring Fatty Acid β-Oxidation Pathways in Bacteria: From General Mechanisms to DSF Signaling and Pathogenicity in Xanthomonas. Curr Microbiol 81, 336 (2024). https://doi.org/10.1007/s00284-024-03866-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-024-03866-8