Abstract
Although mucormycosis may have reached an epidemic situation during the COVID-19 pandemic, the term was much more familiar even before the COVID-19 period. The year 2020 showed an outbreak of novel coronavirus (SARS-CoV-2) which affected millions of people all over the world. One of the noticeable complications observed to be associated with this disease is mucormycosis. It is an opportunistic infection caused by members of the Order Mucorales existing worldwide and has been commonly reported as a laboratory contaminant for a long time. However, nowadays due to the changes in the host environment, they have been emerging as potent opportunistic pathogens responsible for causing primary infections or coinfections with other diseases eventually resulting in morbidity and even mortality in severe cases. Although immunocompromised patients are more susceptible to this infection, few cases have been reported in immunocompetent individuals. Various risk factors which are responsible for the acquisition of mucormycosis include diabetes mellitus type 2, ketoacidosis, hematological malignancies, organ transplants, and chemotherapy recipients. Among the various etiological agents, Rhizopus is found to be the most common, and rhino-cerebral to be the most frequent clinical presentation. As far as pathogenesis is concerned, host cell invasion, thrombosis, and necrosis are the main events in the progression of this disease. The aim of the present review is to address a complete spectrum of mucormycosis and COVID-19-associated mucormycosis (CAM) in a single article. Both global and Indian scenarios of mucormycosis are taken into account while framing this review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The novel coronavirus 2019 commonly known as COVID-19 showed its first instance in China (Wuhan, Hubei) in December 2019, later spreading all over the world, making it a global pandemic [1]. This infection is caused by a novel virus, SARS-CoV-2 (Severe acute respiratory symptom coronavirus 2), which has been identified as a sister/ closely related to the earlier reported human SARS-CoV virus [2]. This infection dominated the health sector globally by the end of 2019 and the entire of 2020, with many associated comorbidities such as strokes, coronary and systemic vasculitis, severe pneumonia, kidney failure, and cardiomyopathy [3]. As of March 23rd, 2023 about 761,071,826 cases were positive with 44,696,984 cases from India [4] Of these, 6,879,677 people have succumbed to this deadly disease worldwide, with 530, 808 mortalities reported from India [4].
As SARS-CoV-2 was a novel virus, therefore data regarding collective signs and symptoms, and the associated risk factors which could enhance the probability of co-infections were not readily available and is still insufficient. Lack of immediate effective vaccine and antiviral therapy has led to various treatment options to combat COVID-19 infection. For instance, the use of immunosuppressive drugs and systemic glucocorticoids to treat critically ill patients have showed improvement in the survival rate of COVID-19 patients [5]. However, unfortunately, their excessive use has eventually led to the uncontrolled hyperglycemic condition as well as secondary infections caused either by bacteria (Mycoplasma pneumoniae, Pseudomonas aeruginosa, and Haemophilus influenzae) or fungi (aspergillosis and candidiasis) [6, 7]. One such fungal infection, mucormycosis, has emerged at an unprecedented rate in various states of India, raising alarm bells throughout the world and thereby posing a serious threat to human health.
Mucormycosis is an opportunistic fungal angioinvasive (marked by or causing infiltration of blood vessels) disorder caused by filamentous fungi such as Mucor, Rhizopus, Absidia, Rhizomucor, and Cunninghamella belonging to the Order Mucorales and Phylum Mucoromycota. These species are ubiquitous, widely distributed in the environment, and are frequently found in soil, dead and decaying organic substrates, such as rotten fruit, vegetables, animal excreta, and compost. It is the third most frequent invasive fungal infection after candidiasis and aspergillosis associated with high morbidity and mortality [8]. The members of Order Mucorales have a unique characteristic of angio-invasion, also known as vascular invasion, which results in vasculitis and thrombosis of blood vessels that eventually lead to necrosis and infarction [9]. Based on the site of infection, mucormycosis can be categorized as rhino-cerebral, pulmonary, cutaneous, central nervous system, renal and others.
The entry of these molds into the human body is common either through the skin or respiratory tract, less commonly through gastrointestinal route, which ultimately results in a severe inflammatory response [10]. Environmental habitat, both outdoor and indoor, and various contaminated food items possibly have contributed to the proliferation of Mucorales in a diverse range of environmental conditions [11]. Studies have also reported that Mucorales-contaminated foodstuff could be a possible source of acquiring mucormycosis, especially in immunocompromised patients [12]. Since they require high moisture content for their growth and development, conditions like humidity and high temperature in tropical and sub-tropical countries provide a congenial environment for their prevalence.
Various risk factors are associated with the acquisition of mucormycosis, with diabetic ketoacidosis and the use of steroids being the most common. Others include acidic medium either due to metabolic acidosis or diabetic ketoacidosis, increased ferritin, and prolonged hospitalization (with or without mechanical ventilators). Moreover, decreased phagocytic activity of WBC (due to immune suppression caused by SARS-CoV-2) also aided in the acquisition of fungal infections such as mucormycosis in patients suffering or recovering from COVID-19 infection [13]. Despite immediate medical and surgical interventions, the mortality rates due to CAM (Covid-19-associated mucormycosis) remained high almost exceeding 50% [14].
In recent years, mucormycosis has been commonly considered a community-acquired disease and has been recognized as a nosocomial disease, which could be attributed to the release of a large number of fungal spores in healthcare-associated devices and contaminated air filters [15, 16]. For instance, in one such study from India, the origin of mucormycosis in 9% of all the cases studied was found to be nosocomial [17]. Similarly, another study revealed 15.4% of cases of acquired nosocomial infections [18].
Although mucormycosis has been mostly found to be associated with individuals with immunocompromised status, there are reports of such infections in immunocompetent patients, the incidence of the latter is however rare [19,20,21]. In addition, outbreaks of mucormycosis have often been linked to natural disasters like tsunami, tornado, or a volcanic eruption [22,23,24]. Variation was seen in the average time gap between COVID-19 diagnosis and emergence of mucormycosis. In one case, the average time gap between the diagnosis of COVID-19 and first-emergence of mucormycosis was 15 days [25, 26]. However, in other cases, a time gap of 42 days or 90 days has been observed [25, 26]. The mortality rate associated with mucormycosis is around 20–50% in cases of localized infection (mainly because of delay in diagnosis, seeking medical facilities, and therapy) while in cases of disseminated infection the mortality rate varies from 70 to 80% [17, 19, 27, 28].
Even prior to the emergence of COVID-19, an alarming rise in the number of cases of mucormycosis in developing countries, including India was reported [17, 29, 30]. A survey of published literature revealed a significant variation between developed and developing countries with respect to etiological agents, risk factors, clinical presentations, and prevalence of mucormycosis [31, 32]. The latter has also been shown to vary with respect to seasonal variation in terms of rainfall, temperature, and humidity [33].
In recent times, an upsurge in the number of cases of mucormycosis all over the world, especially in the patients of COVID-19 and those with underlying diabetes is a matter of concern and research. COVID-19 pandemic has led to a substantial loss of health and economy worldwide. In fact, increased frequency of incidences of diseases associated with COVID-19 such as CAM, further complicated the management of COVID-19 disease. This review article encompasses an updated summary of the available information on COVID-19-associated mucormycosis from their historical aspects, mode of acquisition of fungal spores, epidemiology, etiological agents, various risk factors associated, pathogenesis, types of mucormycosis and their clinical presentation, seasonal variation, diagnosis, treatment, and future prospects in the management of CAM (nanotechnology, MALDI–TOF–MS, T2 Magnetic Resonance, biosensors, probiotics, the possible role of zinc and statin in CAM therapy). We have focused on the incidence of mucormycosis at the global level as well as in India along with the post-COVID-19 scenario.
Historical Aspects
The disease mucormycosis was first reported by Friedrich Kuchenmeister in 1855 [34]. Furbinger (1876) first described the disease involving the lungs (pulmonary mucormycosis) in a patient who died due to cancer, with his right lung showing fungal hyphae and sporangia of Mucorales [35]. Later, Lichtheism (1884) established the occurrence of disease in rabbits caused by two fungal species, Lichtheimia and Rhizopus [14]. Similarly, Arnold Paltauf (1885) coined the term “Mycosis mucorina” for disseminated mucormycosis and published the first case of human disease by Mucorales [36]. In 1943, three cases were reported affecting the eyes, sinus, and brain showing the association of the disease with diabetes (poorly controlled) [37]. In 1957, an American pathologist, Baker coined the term “mucormycosis” for the infections caused by the genus Rhizopus [38]. In India, Balasubrahmanyan and Chaudhuri (1963) reported the first case of mucormycosis (pulmonary mucormycosis) [39]. Later, Grover et al. (1966) made the first antemortem clinical diagnosis of the disease followed by the identification of fungal agent during postmortem [40]. Similarly, Hazarika et al. (1984) came up with the description of a case of rhino-cerebral mucormycosis [41]. Since then, several mucormycosis cases have been reported to be increasing worldwide. Recently, this disease has become a health emergency due to its emergence and prevalence in COVID-19 patients, especially in India. Due to the upsurge in the number of cases of mucormycosis, many Indian state governments have acknowledged it as an epidemic and its association with immunocompromised patients affected by COVID-19 [42].
Mode of Acquisition of Fungal Spores
Inhalation of the fungal spores which are produced abundantly is the primary cause of exposure and acquiring mycormycosis [43]. Various other modes include acquisition through ingestion of spores or via injured skin by trauma, direct injection, catheters or burns [44]. Ingestion of bread products, fermented milk, and other substrates contaminated with fungal species are likely to have a role in causing gastrointestinal mucormycosis [45]. In developing countries, like India water used in oxygen humidifiers can also be one of the potential sources of dissemination of fungal spores (including Mucorales) [16].
Epidemiology
As far as the epidemiology of mucormycosis in India is concerned, variations have been observed with respect to other countries of the world, including the United States and European countries [17, 19, 29, 46]. For example, in a population-based study (1992–1993) in San Francisco (California), the annual incidence was reported to be 1.7 cases/million persons i.e., 500 cases/year [47]. On the other hand, a study from Spain revealed a lower incidence of mucormycosis with 0.43 cases per million [8]. From France, during the analysis of hospital records, an incidence of 0.7 cases per million was reported in the year 1996 rising to 2.6 per million in 2006 [48]. Later, Roden et al. (2005) made the first extensive review in which 929 published cases (1940–2003) were analyzed, where in addition to mucormycosis, cases of entomophthoramycosis were also included [27]. Similarly, Diwakar et al. (2007) reviewed 461 cases of mucormycosis in India, where 70% of the cases were reported from a single medical center [49].
In a similar study from Belgium, an upsurge in the number of cases of mucormycosis from 0.019 per 10,000 patients during 2000 to 0.148 per 10,000 patients during 2009 was observed [50]. Studies and surveys have reported an increasing number of cases of mucormycosis from 1969 to 1989 (0.01–0.16%) and 2008–2014 (9.7–23.7%) from Japan and Iran, respectively [51, 52].
However, in India, population-based studies are lacking, thus making it difficult to calculate the exact number of cases. Based on the availability of data from certain groups of patients, a greater incidence of mucormycosis in India has been reflected. As per a study, 35 cases out of 22,316 screened diabetic patients were reported to be positive for mucormycosis [53]. A prevalence rate of 1.2% mucormycosis was observed in patients who had undergone renal transplantion [54] and 20% in patients with enterocolitis [55]. In addition, three consecutive studies on mucomycosis during 1990–1999, 2000–2004, and 2006–2007 involving 12.9 cases/year, 35.6 cases/year, and 50 cases/year, respectively, clearly showed the rise in the number of cases of mucormycosis [17, 31, 32]. However, recently in a multicenter study during 2013–2015, 89 cases of mucormycosis per year were reported [20].
Etiological Agents
The Order Mucorales include 55 genera and 261 species, of which 38 genera are of medical interest and are involved in causing human infections(mucormycosis) [56, 57]. The spectrum of fungal species responsible for causing mucormycosis is broad. These include Mucor irregularis [58], Rhizopus arrhizus [59], Apophysomyces variabilis [60], R. microsporus [61], R. homothallica [62], and Thamnostylum lucknowense [63]. Of these, R. arrhizus is the most dominant etiological agent worldwide [59, 64]. The second most frequent etiological agent is Lichtheimia corymbifera followed by Mucor racemosus [17] Though Apophysomyces elegans is a less commonly isolated species worldwide, in India, it is the second most commonly isolated causal agent and is responsible for many cutaneous and subcutaneous infections in humans [17, 32] Moreover, this species has also been reported to cause renal, pulmonary, and disseminated or systemic mucormycosis [64]. Recently, Singh et al. (2021) have highlighted the significance of the genus Syncephalastrum as the causal agent of mucormycosis [13].
In a global review, it was reported that Mucor, Lichtheimia, and Rhizopus contributed to 75% of all the cases studied [24]. There are also reports that Rhizopus microsporus, Cunninghamella bertholletiae, and Rhizomucor pusillus, though rare, can also initiate mucormycosis in healthy or immunocompetent individuals [65,66,67]. In India, there are reports of increasing cases of mucormycosis due to Rhizopus homothallica and Rhizopus microsporus [20, 21, 68]. The prevalence rate of mucormycotic agents has been found to vary with respect to geographical location. For example, in a study from Europe, Rhizopus and Lichtheimia were isolated in 34% and 19% of cases, respectively [19], whereas, in another similar study from France, the prevalence rate of Rhizopus and Lichtheimia was 52% and 29%, respectively [69]. The various etiological agents isolated from different geographical locations are depicted in Fig. 1.
Risk Factors
The various risk factors associated with this infection show variation with respect to different countries. For example, in India, the disease is prevalent in patients with uncontrolled diabetes with or without ketoacidosis (ketosis = accumulation of ketone bodies in blood; acidosis = increased acidity in blood). However, in a study (2006), type I diabetes showing a prevalence rate of 10–15% has also been observed [32]. In developed countries, like USA and Europe its prevalence is common in patients with hematological malignancies, or those undergoing chemotherapy and transplant recipient [48, 60, 71]. After China, India has the second largest population of 77 million diabetic patients, which could be one of the reasons for diabetes being the most prevalent risk factor for mucormycosis [72]. Also, the most susceptible patients for mucormycosis include those with uncontrolled hyperglycemia along with ketoacidosis [73] but those with metabolically controlled diabetes rarely suffer from mucormycosis [53].
Similarly, other countries like Iran and Mexico have also shown diabetes to be a predominant risk factor for mucormycosis [74, 75]. In addition, studies from trans-European countries, such as France, Belgium, and Italy have also revealed diabetes as a risk factor with prevalence rates of 17%, 16%, 6%, and 18%, respectively [19, 48, 50, 76]. Other predisposing factors that contribute to mucormycosis involve immunocompromised status, stem cell transplants, neutropenia, illicit intravenous drug use, use of corticosteroids, malnourishment, and iron overload [16, 77, 78]. On the other hand, there are also few reports on HIV as one of the predisposing factors for mucormycosis [77]. In recent years, chronic kidney disorder has emerged as a new risk factor for mucormycosis [20, 32]. The prevalence of this risk factor in patients varied from 9 to 32% [17, 20, 21]. In Turkey, a similar study revealed that 18% of mucormycotic patients had underlying chronic renal insufficiency [79]. A study conducted in India (2013–2015) revealed that in addition to uncontrolled diabetes (56.8%), trauma (10.2%) was among the most common risk factors [20, 77]. Similarly, French investigators have also reported trauma as an emerging risk factor for mucormycosis [80]. Also, some studies have claimed that prolonged stays in hospitals with or without mechanical ventilators may also make patients susceptible to mucormycosis [13].
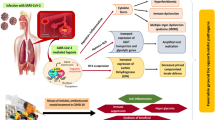
As discussed earlier, one of the probable contributing factors to COVID-19-associated mucormycosis can be inappropriate and overuse of antibiotics especially during the first phase of COVID-19 pandemic. India is the top consumer of antibiotics worldwide [81]. As per a study in India, approximately 216 million doses of antibiotics were used to treat COVID-19 patients, of which azithromycin usage alone was 6.2 million [82]. Various mechanisms by which different risk factors can enhance the susceptibility of patients to mucormycosis are shown in Fig. 2.
Pathogenesis
Phagocytes (Greek phagein = to eat, cyte = cell) play a crucial role in innate immunity by eliminating pathogenic microbes such as fungi, bacteria, and dead and dying cells by the process of phagocytosis. Experimental and clinical data support the idea that individuals lacking phagocytes (immune cells) or having impaired function pose a high risk of mucormycosis. Both mononuclear and polymorphonuclear phagocytes of the host are involved in killing pathogens belonging to the group Mucorales by producing oxidative metabolites and defensins (cationic peptides) [83]. Underlying conditions, such as hyperglycemia and low/acidic pH of the serum (blood plasma without clotting factors) in patients with diabetic ketoacidosis lead to dysfunctional phagocytes. This is accompanied by hindered chemotaxis which results in the defective killing of pathogen (intracellular) via both non-oxidative and oxidative mechanisms [84]. The exact mechanisms responsible for impaired or dysfunctional phagocytes in patients with diabetic mellitus and ketoacidosis are not known and still need elaborative explorations. However, it has been observed that in a group of hematological patients suffering from mucormycosis, Mucorales specific T cells (CD4 + and CD8 +) present in the host produces cytokines such as IL-4, IL-10, IL-17, and IFN-γ which are specifically involved in the damage of hyphae of the fungal agent [85].
It has recently been observed that an increased level of available serum iron is associated with enhanced susceptibility to mucormycosis. Studies have demonstrated that the amount of available unbound iron in serum plays a critical role in making the patient (with diabetic ketoacidosis) prone [86]. Usually in the host, iron bind to the carrier proteins viz., ferritin, transferrin, and lactoferrin which protects against the toxic effects of free iron [87]. Opportunistic fungi, such as members of Mucorales can access iron from the mammalian host either by employing high-affinity iron permeases or by secreting low-molecular weight iron chelators or iron carriers known as siderophores [87]. This has been exemplified by experiments performed on animal models that have revealed that extraordinary iron is essential for the pathogenicity of Rhizopus sp. and also that the enhanced iron uptake by the pathogenic fungi is directly proportional to its growth and proliferation in the host [86].
As the growth of R. oryzae has been observed to be poor in normal serum and gets accelerated with the addition of exogenous iron, the major universal host defense mechanism against pathogenic microbes, particularly in Mucorales is therefore, by limiting the iron availability to them [86]. Many similar studies have confirmed that patients with systemic acidosis mainly diabetic ketoacidosis are at higher risk. This has been implicated in the higher levels of available serum iron which may probably be due to the acidosis that causes the release of iron from the carrier proteins in the host [88]. As per one of the studies, neutrophils when get exposed to the hyphae of R. oryzae marks the upregulation in the expression of Toll-like receptor 2 and proinflammatory gene expression along with rapid activation of genes related to NF-kB pathway [89].
As discussed earlier, an important event in mucormycosis is the extensive angioinvasion which is characterized by the formation of blood clot within a vessel (vessel thrombosis) and further death of the body tissues (tissue necrosis). This event of angioinvasion is associated with the potential of members of Mucorales to spread or disseminate hematogenously from their original infection site to other organs in their host. Therefore, the most crucial step in the microbe’s pathogenetic strategy is to damage the blood vessel followed by penetration through the endothelial cells lining them. For instance, the ability of R. oryzae spores to adhere to the sub-endothelial matrix proteins, such as type IV collagen and laminin have been observed [90]. However, this study was conducted in-vitro and pregerminated spores (germlings) didn’t show the same results. In a similar study, spores and hyphae of R. oryzae showed variation with respect to adherence to the subendothelial matrix protein [91]. For example, spores of this fungus showed significantly better adherence as compared to the hyphae but there was no such variation while adhering to the human umbilical endothelial vein. This indicates that the adhesins proteins of R. oryzae binding to endothelial cells and those binding to subendothelial matrix protein were dissimilar [91]. Pathogenesis of the Order Mucorales in immunocompromised and immunocompetent host is shown in Fig. 3.
Pathogenesis of Mucorales a) in an immunocompetent host active defense mechanism inhibit the fungi from proliferation, causing infection and its spread to other parts of the body. Neutrophils play a crucial role in the process. Under normal pH, no free iron is available due to their sequestration by the IBP (Iron binding protein). Endothelial cells remain intact, functional and regulate permeability and the vascular tone b) in an immunocompromised host (due to diabetes, corticosteroid therapy or other conditions) host defense mechanism becomes defective. Neutropenia and functional defects in neutrophils result in uncontrolled fungal proliferation. Under acidic/high pH condition (due to diabetes ketoacidosis), free iron is available due to the release of iron from IBP which further supports the multiplication of the fungus. Function of endothelial cell also get disturbed and the fungus eventually penetrate the endothelial cells through angioinvasion, thrombosis, ischemia, necrosis and eventually disseminate to various body parts.
Deep insights into the molecular mechanisms of mucormycosis have been revealed by many researchers. For example, it has been observed that the unique interaction between GPR78, glucose protein receptor 78 which is present on the surface of the host endothelial cell and Cot H (present on the surface of spore/germling of Mucorales) lead to host cell damage and further dissemination of the fungi hematogenously [92]. Moreover, high levels of iron, glucose, or BHB (ketone bodies) support fungal growth and increase the expression of Cot H and GRP78 which eventually enhances the capability of Mucorales to occupy their host tissues. However, the interaction between GPR78 and Cot H is not the only mechanism responsible for the invasive infection by Mucorales. Certain secondary metabolites (excreted directly by Mucorales) help the pathogen to facilitate its interaction with the host [93]. The ability of Mucorales with pathways for the synthesis of secondary metabolites, such as L-tryptophan dimethylallyl transferases, polyketide synthases and non-ribosomal peptide synthetases have been reported [94]. The interaction of Mucorales with the endothelial cells of the host which eventually leads to the spread of fungal infection and the effect of some host factors on these interactions and the immune response is shown in Fig. 4.
The interaction of Mucorales with the endothelial cells leading to the spread of fungal infection and the effect of some host factors on these interactions and immune response. A) High glucose content (hyperglycemia) and acidifying factors such as ketoacidosis leads to the release of iron bound to serum sequestering proteins via the process of glycosylation and protonation, respectively. B) Free iron and ketone bodies (such as BHB (hydroxy butyrate) support the fungal proliferation by negatively affecting the immune response of the host but NaHCO3 reverse the negative affect by neutralizing acidity and preventing the release of iron from SSP. C) Stress elicited due to free iron, BHB, and high glucose enhances the expression of glucose-regulator protein (GRP78). D) Moreover, high glucose, BHB, and free iron also enhances the expression of Cot H, located on the surface of fungal cell. E) Interaction between these two receptors eventually result in invasion of the endothelium and proliferation of fungi.
Types of Mucormycosis and Their Clinical Presentation
The clinical presentation of mucormycosis varies with respect to the location of the disease and it can manifest different sites of human body. As per a survey in Spain, the most frequently affected site was observed to be the sinuses with a prevalence rate of 39% [8]. It is followed by the lungs (24%), skin or cutaneous (19%), brain (9%), gastrointestinal tract (7%), disseminated form (6%), and other sites (6%) [8]. On the contrary, in India the most common clinical type was found to be rhino-orbito- cerebral with a prevalence rate of 48–55% followed by cases of cutaneous (13–15%), pulmonary (7–17%), disseminated (5–12%), gastrointestinal (5–13%), and renal mucormycosis (5–14%) [17, 31,32,33]. In Europe, a study involving 230 cases of zygomycosis found pulmonary mucormycosis to be the most common clinical presentation [19].
The different clinical types of mucormycosis and the associated clinical symptoms of mucormycosis are briefly discussed as follows:
Rhino-Cerebral Mucormycosis
Most of the published studies have reported rhino-cerebral mucormycosis to be one of the most common clinical types especially in diabetic patients [18, 31, 32, 48]. Clinical manifestation initiates with necrosis of the sinuses or palate that later may advance toward the orbit before reaching the intracranial structures. Frequent symptoms include fever, proptosis, epistaxis, amaurosis, obnubilation, invasion of the trigeminal nerve, and facial paralysis [95]. In severe or unresolved cases, thrombosis of the cavernous sinuses and cranial invasion may occur. Rhino-cerebral mucormycosis exhibit a mortality rate ranging from 30 to 69% [33, 96, 97].
Pulmonary or Respiratory Mucormycosis
Infection of the lungs, due to inhalation of fungal spores is another common type of mucormycosis. Clinical symptoms of this type of pulmonary mucormycosis include fever, pleural pain, and hemoptysis [98]. The radiological results show similarity with that of invasive aspergillosis. In both cases, vascular necrosis and thrombosis eventually lead to the necrosis of the tissues. Other clinical presentations include pulmonary nodules, cuneiform pulmonary infiltrates, and cavitated lesions [99]. The mortality rate associated with this type of mucormycosis is around 60% [99].
Central Nervous System
This type of mucormycosis can be a part of the advancement of the disease through the rhino-orbital route or may be the clinical manifestation restricted to the central nervous system [100]. More the delay in diagnosing the disease, the greater the chances of mortality.
Cutaneous Mucormycosis
Over the last few years, there has been an upsurge in the cases of cutaneous and soft tissue mucormycosis. Clinical manifestation may occur either on intact skin or broken skin due to rupture of the protective epidermal barriers through burns, trauma, and surgery [99]. Other possible routes that have been implicated include insect bites, or catheter insertion sites, or intramuscular injections [101]. In this type, lesions are visible on the affected areas, hence they can be diagnosed early and thereby lowering the rate of mortality in patients with cutaneous mucormycosis.
Renal Mucormycosis
Though rare, some of the studies in the literature have reported cases of renal mucormycosis (where kidneys are affected). For instance, in a study, around 22% cases of disseminated mucormycosis were observed where kidneys were involved [102]. Clinical symptoms include hematuria or anuria, fever, and flank pain [17]. On the other hand, in India patients suffering from renal mucormycosis have been reported to be healthy and without any underlying disease [17, 31, 32] while case reports in China showed some risk factors or immunocompromised status of the individuals (except pediatric population) [103]. Moreover, cases of isolated renal mucormycosis have been reported in developing countries like Egypt, Singapore, India, Kuwait, and Saudi Arabia having warm climates and in patients using intravenous drugs [104, 105].
Miscellaneous/Other Types
In addition to the types described above, mucormycosis can also affect the digestive route/tract. As per a study involving 25 published case reports, the mortality rate in patients with other types of mucormycosis was 98% [99]. Some studies have reported that endocarditis in natural or prosthetic valves may eventually cause mucormycosis resulting in invasion and blockage of the vessels [106]. Clinical symptoms in this type of mucormycosis are rare and extremely high rate of mortality has been observed [107]. Another rare form is osteomyelitis mucormycosis affecting the joints of the patient [108]. A survey of the literature showed other uncommon types which include peritoneal mucormycosis [109] hepatic mucormycosis [110], and gastrointestinal mucormycosis [111]. Different types of mucormycosis (based on the site of infection) in various studies in India and abroad are shown in Fig. 5.
Gender
Although fungal infections including mucormycosis can occur in both genders, their prevalence has been observed to be more in males compared to females. A global review by Roden et al. (2005) highlighted the predominance of mucormycosis in males (65%) [27]. Similarly, another study revealed 72% of the cases of mucormycosis studied were of males and 28% of females [18]. In India, studies by Sachdeva (2013) and Patel et al. (2020) also supported the male preponderance in cases of mucormycosis [21, 112]. In a study over a period of 5 years (2010–2014) by Chander et al. (2018) similar trend was observed with 55 cases of males and 27 of females [113]. A study from Mexico reported cases of mucormycosis were greater in males [88]. In some of the studies from Iran, male dominance was observed [114] but other studies from Iran have shown females to be more commonly affected than males [73, 114].
Environmental Factors
In tropical and sub-tropical countries, variations in humidity and temperature in different seasons directly affect the growth and proliferation of Mucorales and eventually leading to the fluctuations in the number of mucormycosis cases. For example, in India post-rainy season and autumn months have been reported to be more favorable for the incidence of mucormycosis [33]. This could be ascribed to a greater load of air borne spores of fungi in the autumn season compared to the summer season. In countries like Japan, Iran, and Israel, it has been reported to be more frequent in autumn season [115, 116]. Similarly, in Middle East countries autumn was found to be the most favorable season while in Europe the incidence of mucormycosis reached its peak in autumn and winter season. In Khuzestan, Iran the incidence was also observed to be maximum in the winter season (40%) and least in the spring season (15%) [114]. Another study found a negative correlation with temperature while a positive one with humidity [117].
Although clinically relevant Mucorales species occupy diverse ecological niches, data on the distribution of their spores in patients’ residential areas and the contribution of environmental contamination in causing the disease and eventually its outbreak are not well documented. However, in one of the studies, the distribution of mucoralean spores in the residential environment of a patient was evaluated. It was found that in the majority (68%) of the cases Mucorales species isolated from the clinical samples and that of patient’s environment were the same [118]. Similarly, a multicenter study was conducted in a hospital to assess the role of the hospital environment in the CAM outbreak [119]. It revealed that Mucorales species were isolated from outdoor air samples, indoor air samples, and air conditioning vents with a prevalence rate of 53.8%, 21.7%, and 11.1%, respectively [119].
Recently, a review highlighted the impact of improper waste disposal during COVID-19 and various safe, secure, and innovative disposal [120]. Contaminated PPE (personnel protective equipments) such as gloves, facemasks, when not disposed off appropriately ended up as hazardous biological waste posing danger to the well-being of individuals and adversely affect the environment [120]. In addition, viral-loaded waste, when not dumped properly may reach the open fields, where the ragpickers may get exposed, get infected, and even further spread the virus [121].
Diagnosis
Early diagnosis can help in improving the outcome of mucormycosis. It has been established that early diagnosis either help in enhancing the survival of the patient [109] or may reduce the requirement for disfigurement and surgical resection, thereby further lessening the sufferings and complications [122]. However, the diagnosis at an early stage remains baffling due to the absence of appropriate diagnostic tools and biomarkers and also that sample collection from deep tissues is not easy [123]. Although recently PCR showed good results in the evaluation and diagnosis of mucormycosis at an early stage but a standard commercial kit for routine use is still lacking [124].
The pathological hallmark of mucormycosis include cell invasion, thrombosis and necrosis of the tissues underlying them. Therefore, correct mycological identification with characters specific to Mucorales will help in distinguishing them from other fungal infections. Smith and Krichner’s (1950) proposed a criteria for the diagnosis of mucormycosis that is still considered standard which include clinical signs like (a) facial pain and blood tinged nasal discharge, both on same side of the face (b) black necrotic nasal turbinate (easily mistaken for dried blood) (c) soft periorbital or perinasal swelling with discoloration and induration (d) lethargy and loss of corneal reflex, mostly observed late in the process of invasion by a fungal pathogen (e) ptosis of the eyelid, proptosis of the eyeball and complete ophthalmoplegia (d) cranial nerve palsies not related to documented lesions [125].
Most of the species of Mucorales are fast growing and are capable of growing at 37 °C temperature within 24–48 h. For their identification purpose, growth, temperature, cultural, and microscopic characteristics are taken into consideration [122]. In addition, DNA sequencing (ITS) helps in the identification of unidentified fungal isolates and confirming the identity of already identified fungal species [126]. The trio of histopathology, direct microscopy and cultural studies can be helpful in routine laboratory diagnosis. Histopathology proves beneficial especially in the case of pulmonary mucormycosis as it demarcates the presence of fungus as a pathogen from a contaminant in culture and is vital in delining if there is blood vessel invasion [127].
Direct microscopy with KOH using fluorescent dyes such as, calcofluor or blankophor white is an inexpensive method for rapid diagnosis of fungal infection and clearly defining surgical margins for fungal infection (invasive) during operations [122]. Though it is not possible to identify the fungus upto species level using direct microscopy but characteristic fungal hyphae with enhanced visualization (due to fluorescent dyes) help in confirming the presence of fungi and hence the fungal infection. Moreover, immunohistochemistry (using monoclonal antibodies) against R. arrhizus can also help in distinguishing mucormycosis from aspergillosis and has proved beneficial in diagnosis even when the culture is negative [128]. In molecular methods, ITS sequencing is a reliable method and is recommended for the identification of Mucorales upto species level [126]. In addition, PCR-based techniques have also been developed for the detection of Mucorales in the affected tissues [129].
As the clinical signs and symptoms of mucormycosis are not specific, therefore as per some of the studies autopsy is considered the gold standard method for diagnosis. Based on the autopsy, in Japan, 4% of the total cases of invasive fungal infection [52] while in USA 7% of cases were found to be of mucormycosis [89]. Computed tomography (chest and sinus) and MRI imaging have also been proven to be beneficial in diagnosing the disease especially when multiple infarcts are visible [18].
For the management of mucormycosis, guidelines at the national and global level stressing the optimum use of steroids and controlling diabetes followed by surgical intervention (guided by radiology) were provided [130]. Globally, the most commonly followed guidelines on mucormycosis in the year 2019 were of the European Confederation of Medical Mycology (ECMM) and Mycoses Study Group Education and Research Consortium which helped in spreading awareness among people regarding the screening, diagnosis, and management of mucormycosis [131].
Treatment
As we are well versed with the phrase “prevention is better than cure.” Likewise, proper precautions and symptomatic management would lessen the probability of acquiring acute mucormycosis. For the management of such disease in the best possible way, surgical debridement along with medical treatment (antifungal treatment) and controlling the predisposing factors can prove beneficial in saving the lives of the patients [31, 75]. In one of the studies in India involving 53 patients, 16 patients survived following surgery and conventional amphotericin B therapy, 5 survived due to surgery only, 56% survived by surgery + liposomal amphotericin B therapy, 3 survived by amphotericin B treatment + surgery + controlling underlying disease and 1 survived by amphotericin B treatment + controlling underlying diseases [17]. In most of the cases, amphotericin B was the only antifungal to be licensed and verified to be the drug of choice. However, lipid formulations of amphotericin B are known to cause less kidney dysfunction as compared to conventional amphotericin B [132]. As per one of the studies in India, 50% of the patients suffering from mucormycosis survived when treated with the antifungal drug amphotericin B (conventional or liposomal formulations) [113].
Other antifungal drugs, such as posaconazole have also gained popularity in the recent times and can be used in place of amphotericin B [133]. Similarly, isavuconazole can be used as primary and in salvage therapy as it shows the same results as shown by amphotericin B [134]. In second-line treatment combination of lipid amphotericin B with caspofungin or posaconazole, is also recommended [133]. Although drugs, amphotericin B and posaconazole were found to be the most effective against fungal infection especially during COVID times, an in-vitro study reported species-specific variations [135]. Therefore, correct identification of fungal species is important for accurate diagnosis of the disease and also for developing species-specific antifungal preparations.
Hyperbaric oxygen, especially in diabetic patients has been recommended as a promising treatment for mucormycosis [136]. Oxygen in high concentration possess antifungal property and can hinder the growth of fungal species causing this disease in in-vitro studies (Mucorales). In addition, it can enhance the activity of neutrophil cells and wound healing process by supplying an improved flow of oxygen to ischemic tissues [136, 137]. In a study, the survival rate of patients using standard therapy of mucormycosis increased from 22 to 83% when hyperbaric oxygen was used along with standard therapy [136]. However, due to the limited clinical and experimental data the use of hyperbaric oxygen in treating mucormycosis is limited.
As discussed above, the most likely phenomenon helping the fungus to survive therapy is ischemic necrosis due to angioinvasion by the respective mucormycetes. Hence, surgery is considered essential to reduce a load of fungus in the damaged tissues. It also enhances the entry of antifungal drugs in sufficient concentrations by removing the necrotic tissue in massive amounts. Moreover, awareness of the general public about mucormycosis and related complications can lessen the possibility of fungal infection, particularly by the members of Mucorales. As discussed, uncontrolled diabetes and hyperglycemic conditions are mainly responsible for mucormycosis, thus controlled glycemic level and other predisposing factors can help in managing mucormycosis [138].
Future Prospects for Management of Mucormycosis
Despite the use of nanotechnology in medicine since 1980’s, its adequate usage in fungal diagnostic purpose has not been made. Recently, studies have reported the use of gold nanoparticles in detecting A. niger causing cutaneous fungal infections in humans in a short time [139]. Similarly, for the effective treatment of human fungi infections, a novel nanoemulsion formulation was utilized which also minimized the risk of drug resistance development [140]. Therefore, Myconanotechnology is an emerging field to tackle fungal infections by making use of nanotechnology and have scope in treating mucormycosis as well [141]. For the rapid identification of species-specific fungal peptides, MALDI–TOF–MS (Matrix-assisted laser desorption ionization-time of flight mass spectrometry) is a commercially available technique which requires 30 min to identify the fungal isolate [142]. Another commercially available technique is T2MR (T2 Magnetic Resonance) for the detection of pathogenic Candida species within 5 h [143]. Therefore, the above-mentioned techniques can also prove useful tools for rapid detection of mucormycosis so that treatment can be initiated at an early stage. Similarly, biosensors are self-contained integrated analytical devices that detects and measures chemical and biological reactions by generating electrochemical or optical signals proportional to the concentration of a bio-analyte. Some of the studies have used biosensor technologies for the direct detection of fungal pathogenic species such as A. fumigatus and C. albicans [144, 145]. As biosensors have not been extensively explored for detecting fungal isolates, therefore making use of biosensors for fungal diagnostic purpose could be an active area for research especially with respect to rapid detection of mucormycosis. Moreover, it would also prove helpful in following the treatment response based on specific marker analytes.
Probiotics are living entities that, when administered orally in an appropriate quantity, provide a health benefit to the host [146]. The emerging studies suggest their potential for regulating the host’s immune response. Besides showing anti-inflammatory activity, they also possess antimycotoxigenic and antifungal activities [147, 148]. In both animal experiments and clinical trials, the effectiveness of probiotic bacteria in the treatment of oral candidiasis has been shown [149]. The probable mechanism could be the competition between the probiotic species and the human pathogen for food and binding receptors, which eventually upsets the growth of Candida species [149]. There are also reports on experimental studies stressing on the significance of probiotics in treating COVID-19 infections [146]. However, despite their well-known role as antifungal agents, no experimental evidence or reports assessing their role in treating CAM are available in the literature to date. Therefore, future research focusing on the use of probiotics either singly or as an adjunct to the existing management strategies could prove beneficial in the effective treatment of CAM.
Whether Zinc Has an Association with CAM?
There is no clear explanation for the rapid increase in mucormycosis associated with COVID-19 pandemic. One of the speculations in this regard include zinc supplement used for the management of COVID-19 that might have initiated the growth and proliferation of Mucorales, eventually resulting in CAM [150]. There are reports in literature revealing the role of zinc in the virulence of harmful fungi, such as Aspergillus, Candida. Zinc, a micronutrient known to regulate the fungal proteins, is involved in the infection of mammalian hosts and thereby influences various mechanisms responsible for fungal pathogenesis [151].
Both in-vitro and in-vivo studies have revealed the ability of zinc chelators in restricting the growth of different fungi, including members of Mucorales [152]. In addition, deprivation of zinc by the mammalian host showed antifungal activity [153]. Using large doses of zinc for a long term is found to be associated with adverse effects, which include neurological deficit and copper deficiency [154].
Recently, in a preliminary in-vitro study, the contribution of zinc in the multiplication of R. arrhizus (isolated from CAM patients) has been evaluated [155]. However, large- scale in-vivo studies are required to ascertain the above hypothesis.
Introducing Statin into CAM Therapy
The therapeutic benefit of involving statin into CAM therapy came into light in the recent times [156]. Several in-vitro studies have shown the positive response of statin against various pathogenic fungi. For example, rosuvastatin and fluvastatin were effective against Rhizopus and Rhizomucor, while lovastatin resulted in apoptosis of Mucor racemosus [130]. Combination therapy involving amphotericin B and lovastatin/atorvastatin had better efficacy against R. arrhizus as compared to amphotericin B alone [157] The probable role of statin include the induction of cytoprotective GRP 78 expression, lowering the risk of infection and synergizing or coordinating the level of anti-CAM drugs in plasma [130]. However, more extensive in-vivo studies and clinical trials are required to prove the worthiness of statin against CAM.
Global Scenario
Globally, the incidence of mucormycosis ranges from 0.005 to 1.7 per million population while the estimated prevalence of mucormycosis (0.02 to 9.5 per 100,000 persons) in India is 80 times more as compared to the global data [57, 113]. The prevalence of 14 cases per 100,000 people was estimated based on a computational model in India [12]. From India, a rising trend of mucormycosis was observed during the periods of 1990–1999, 2000–2004, and 2006–2007 with an annual incidence of 12.9 cases, 35.6 cases, and 50 cases per year, respectively [17, 31, 32]. In a study from Tamil Nadu over a period of 10 years (2005–2015), an annual incidence of 18.4 cases per year was reported [158] while the same state during 2015–2019 showed an annual incidence of 9.5 cases per year [159].
There has been an upsurge in the incidence of mucormycosis worldwide with higher number of cases in India, and China especially in patients with uncontrolled diabetes [57]. As per a systematic review and meta-analysis of 851 cases (2000–2007), Europe has a higher burden (34%) of mucormycosis compared to Asia (31%) followed by North or South America (28%), Africa (3%), New Zealand and Australia (3%) [24]. In another study, the incidence of 16 cases per 1000 patients having diabetes (35 cases suffering from mucormycosis with diabetes out of total 22,316 cases) was reported [53]. Recently, in a study by Prakash et al. (2019), a high prevalence of diabetic ketoacidosis (90%) was reported in North India compared to South India, where the prevalence was only 10%. In a review on mucormycosis, 101 cases of CAM patients were considered [20]. It included cases from India (82), USA (9), Iran (3), and one case each from UK, Brazil, Italy, France, Turkey, Austria, and Mexico [13]. Likewise, 15 more cases were reported from Iran (April to September, 2020). In 2021, from India, 2826 cases were reported from 98 centers (January 1 to May 26, 2021) reflecting the upsurge in the number of cases and thereby increasing the burden of disease [26, 160].
Diabetes has also been reported as one of the most important risk factors worldwide with the prevalence rate of around 70% in different countries, such as Mexico with 72% [75, 88] and Iran with 74% [69]. However, in India, three major case series reported diabetes as risk factor in 50% of patients with mucormycosis [17, 31, 32]. In European countries, like Italy (18%), France (23%), and Greece (29%) a lower prevalence rate of diabetes as the risk factor varying from 17 to 29% has been observed [15, 76, 161]. As per WHO, the incidence is higher in patients with pre-existing diabetes or those using systemic corticosteroids and in patients with COVID-19 or those who got recovered from the infection.
Scenario After COVID-19
COVID-19 is a fatal viral disease associated with a wide spectrum of opportunistic bacterial and fungal infections [162]. Cases of co-infection due to Candida and Aspergillus have been reported post-COVID-19 pandemic [128]. This has been observed with an upsurge in the number of cases of mucormycosis in COVID-19 patients from different countries and more from India. This can be due to a number of factors altogether forming a congenial and ideal environment, such as hypoxia (low oxygen level), hyperglycemia (high glucose either due to diabetes, or new-onset hyperglycemia or steroid-induced hyperglycemia), increased ferritins (leading to high iron levels), acidosis (metabolic or diabetic leading to acidic medium) and immunosuppression conditions for the growth of Mucorales spores. These conditions may lead to reduced activity of WBCs and hence their immunity (either due to SARS-COV-2 medication or steroid mediated or background comorbidities) [13].
So far, India has the highest burden of mucormycosis with nearly 140 cases per million population in comparison to other countries [20]. A survey of published literature from December 2019 to April 2021, showed that India has contributed around 71% of global cases of mucormycosis in COVID-19 patients [163]. This could be possible as India has the second largest population with diabetes mellitus after China, which remain the leading risk factor in India associated with the fungal infection including mucormycosis [164]. Another reason could be the prolonged use of corticosteroids which is often linked to various opportunistic fungal infections such as aspergillosis and mucormycosis. Although rare, a few cases of mucormycosis are also reported to be due to short-term steroid therapy (5–14 days) [165]. Globally, the mortality rate of 46% has been found in COVID-19 patients with diabetes mellitus as the risk factor [24]. As of July 15, 2021, from India 45,432, cases of mucormycosis have been reported of which 84.4% cases had a history of COVID-19. Of the total affected people in India, 21,085 individuals are under treatment and 4,252 have died due to mucormycosis associated with COVID-19. In the reported cases, rhinocerebral has been the most common presentation (77.6%) followed by cutaneous (4.3%) and pulmonary (3%) infections [166]. In one of the studies from India six cases of mucormycosis (rhino-orbital cerebral mucormycosis) following COVID-19 infections were reported recently [26]. In the year 2022 Mumbai recorded the first case of mucormycosis in a 70 years old male amidst the third wave of corona virus [167]. Recently, a systemic review on mucormycosis in mainland China has been presented by Wie et al. (2022) [168]. In India, a clinico-mycological study over a period of 10 months reported 42.66% cases of mucormycosis in post-COVID-19 patients, where the most common etiological agent was R arrhizus and rhino-orbital-cerebral as predominant clinical form [169]. Likewise, a rare case of post-COVID-19 mucormycosis of kidney was reported in a 34 years old male from Delhi [170]. Indian and Global scenarios of mucormycosis in COVID-19 patients is represented in Tables 1 and 2, respectively.
Conclusion
As per the data available so far, it seems that the trio conditions of diabetes, excessive use of corticosteroids, and COVID-19 provided a congenial environment for the emergence of mucormycosis in recent times. Despite the increase in awareness among physicians regarding mucormycosis, associated morbidity and mortality are still increasing probably due to patients’ delay in seeking medical facilities, as a result of which diagnosis could not be established on time. Judicious use of steroids is the need of the hour to avoid the rise in fungal infections and also to check the increase in blood glucose. A coordinated effort i.e., proper awareness and precautions on the part of individuals, and expertise and competence in mycological diagnosis on the part of clinicians can help in curbing the higher incidence of such infections. Early recognition, diagnosis, and subsequent appropriate antifungal treatment or surgical debridement or both are essential for improving the outcome of individuals suffering from mucormycosis.
For the development of effective treatment strategies, novel antifungal drug discovery with potential to cure mucormycosis, multicenter studies and registries are of supreme importance. Moreover, in order to better understand the pathogenesis of COVID-19- associated mucormycosis, detailed and elaborative studies for evaluating the possible link between COVID-19 and mucormycosis need to be established. Various other possible factors (environmental or genetic) contributing to the outbreak of mucormycosis during COVID period and difference in pathogenicity and virulence of fungal strains causing mucormycosis isolated during COVID-19 period and those isolated before the pandemic need to be evaluated. Moreover, large-scale studies on the association of zinc with mucormycosis or the use of statin to cure mucormycosis and to ascertain the role of mechanical ventilation in enhancing the role of mucormycosis need to be conducted. Various techniques such as, nanotechnology, biosensors, and probiotics can prove beneficial in tackling mucormycosis. Also, the discovery of new and novel diagnostic biomarkers for early diagnosis of the disease is required urgently so that treatment can be initiated accordingly, improving the patients chances of survival.
References
Farnoosh G, Alishiri G, Hosseini Zijoud SR, Dorostkar R, Jalali FA (2020) Understanding the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease (COVID-19) based on available evidence—a narrative review. J Mil Med 22:1–11
Wang X, Ding H, Chen Z et al (2020) CARD 9 deficiency in a Chinese man with cutaneous mucormycosis, recurrent deep dermatophytosis and a review of the literature. Mycopathologia 185:1041–1050. https://doi.org/10.1007/s11046-020-00487-0
Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, Satlin MJ, Campion TR et al (2020) Clinical characteristics of COVID-19 in New York City. N Engl J Med 382:2372–2374. https://doi.org/10.1056/nejmc2010419
World Health Organisation (WHO) (2023) Coronavirus (COVID-19) Dashboard. https://covid19.who.int/ Accessed 23 Mar 2023
Goursaud S, Descamps R, Daubin C, du Cheyron D, Valette X (2020) Corticosteroid use in selected patients with severe acute respiratory distress syndrome related to COVID-19. J Infect 81:e89–e90. https://doi.org/10.1016/j.jinf.2020.05.023
Peman J, Ruiz-Gaitan A, Garcia-Vidal C, Salavert M, Ramirez P, Puchades F et al (2020) Fungal co-infection in COVID-19 patients: should we be concerned? Rev Iberoam Micol 37:41–46. https://doi.org/10.1016/j.riam.2020.07.001
Moorthy A, Gaikwad R, Krishna S, Hegde R, Tripathi KK, Kale PG et al (2021) SARS-CoV-2, uncontrolled diabetes and corticosteroids-an unholy trinity in invasive fungal infections of the maxillofacial region? a retrospective, multi-centric analysis. J Maxillofac Oral Surg 20:418–425. https://doi.org/10.1007/s12663-021-01532-1
Torres-Narbona M, Guinea J, Martinez-Alarcon J et al (2007) Impact of mucormycosis on microbiology overload: a survey study in Spain. J Clin Microbiol 45:2051–2053. https://doi.org/10.1128/JCM.02473-06
Ben-Ami R, Luna M, Lewis RE, Walsh TJ, Kontoyiannis DP (2009) A clinicopathological study of pulmonary mucormycosis in cancer patients: extensive angioinvasion but limited inflammatory response. J Infect 59:134–138. https://doi.org/10.1016/j.jinf.2009.06.002
Mantadakis E, Samonis G (2009) Clinical presentation of zygomycosis. Clin Microbiol Infect 15:15–20. https://doi.org/10.1111/j.1469-0691.2009.02974.x
Richardson MD, Rautemaa-Richardson R (2020) Biotic environments supporting the persistence of clinically relevant mucormycetes. J Fungi 6:4. https://doi.org/10.3390/jof6010004
Mousavi B, Botterel F, Costa JM, Arne P, Guillot J, Dannaoui E (2019) Occurrence and species diversity of human-pathogenic Mucorales in commercial food-stuffs purchased in Paris area. Med Mycol 57(6):739–744. https://doi.org/10.1093/mmy/myy121
Singh AK, Singh R, Joshi SR, Misra A (2021) Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Meta Syndr 15:102146. https://doi.org/10.1016/j.dsx.2021.05.019
Sharma K, Mishra S, Gautam A, Malviya R (2021) Mucormycosis—a fungal infection in patient recovered from COVID-19. Lette Appl Nanosci 11:3802–3810. https://doi.org/10.33263/LIANBS113.38023810
Petrikkos G, Skiada A, Sambatakou TA, Vaiopoulos G, Giannopoulou M, Katsilambros N (2003) Mucormycosis: Ten-year experience at a tertiary-care center in Greece. Eur J Clin Microbiol Infect Dis 22:753–756. https://doi.org/10.1007/s10096-003-1035-y
Rammaert B, Lanternier F, Zahar JR et al (2012) Healthcare-associated mucormycosis. Clin Infect Dis 54:S44–S54. https://doi.org/10.1093/cid/cir867
Chakrabarti A, Chatterjee S, Das A et al (2009) Invasive zygomycosis in India: experience in a tertiary care hospital. Postgrad Med J 85:573–581. https://doi.org/10.1136/pgmj.2008.076463
Bala K, Chander J, Handa U, Puni RS, Attri AK (2015) A prospective study of mucormycosis in North India: experience from a tertiary care hospital. Med Mycol 53:248–257. https://doi.org/10.1093/mmy/myu086
Skiada A, Pagano L, Groll A, Zimmerli S, Dupont B, Lagrou K, Lass-Florl C, Bouza E, Klimko N, Gaustad P et al (2011) Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European confederation of medical mycology (ECMM) working Group on zygomycosis between 2005 and 2007. Clin Microbiol Infect 17:1859–1867. https://doi.org/10.1111/j.1469-0691.2010.03456.x
Prakash H, Ghosh AK, Rudramurthy SM, Singh XI, Savio J, Pamidimukkala U, Jillwin J, Varma S, Das A et al (2019) A prospective multicenter study on mucormycosis in India: Epidemiology, diagnosis, and treatment. Med Mycol 57:395–402. https://doi.org/10.1093/mmy/myy060
Patel A, Kaur H, Xess I, Michael JS, Savio J, Rudramurthy S, Singh R, Shastri P, Umabala P, Sardana R et al (2020) A multi-center observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin Microbiol Infect 26:944.e9-944.e15. https://doi.org/10.1016/j.cmi.2019.11.021
Fanfair RN, Benedict K, Bos J, Bennett SD, Lo YC, Adebanjo T, Etienne K, Deak E, Derado G, Shieh WJ, Drew C, Zaki S, Sugerman D, Gade L, Thompson EH, Sutton DA, Engelthaler DM, Schupp JM, Brandt ME, Harris JR, Lockhart SR, Turabelidze G, Park BJ et al (2012) Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med 367:2214–2225. https://doi.org/10.1056/NEJMoa1204781
Benedict K, Park BJ (2014) Invasive fungal infections after natural disasters. Emerg Infect Dis 20:349. https://doi.org/10.3201/eid2003.131230
Jeong W, Keighley C, Wolfe R et al (2019) The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect 25:26–34. https://doi.org/10.1016/j.cmi.2018.07.011
Khatri A, Chang KM, Berlinrut I, Wallach F (2021) Mucormycosis after coronavirus disease 2019 infection in a heart transplant recipient—case report and review of literature. J Med Mycol 31:101125. https://doi.org/10.1016/j.mycmed.2021.101125
Sen M, Honavar SG, Bansal R et al (2021) Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India-collaborative OPAI-IJO study on mucormycosis in COVID-19 (COSMIC), report 1. Indian J Ophthalmol 69:1670–1692. https://doi.org/10.4103/ijo.IJO_1565_21
Roden MM, Zaoutis TE, Buchanan WL et al (2005) Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 41:634–653. https://doi.org/10.1086/432579
Zilberberg MD, Shorr AF, Huang H, Chaudhari P, Paly VF, Menzin J (2014) Hospital days, hospitalization costs, and inpatient mortality among patients with mucormycosis: a retrospective analysis of US hospital discharge data. BMC Infect Dis 14:310. https://doi.org/10.1186/1471-2334-14-310
Meis JF, Chakrabarti A (2009) Changing epidemiology of an emerging infection: zygomycosis. Clin Microbiol Infect 15:10–14. https://doi.org/10.1111/j.1469-0691.2009.02973.x
Queiroz-Telles F, Nucci M, Colombo AL, Tobon A, Restrepo A (2011) Mycoses of implantation in Latin America: an overview of epidemiology, clinical manifestations, diagnosis and treatment. Med Mycol 49:225–236. https://doi.org/10.3109/13693786.2010.539631
Chakrabarti A, Das A, Sharma A et al (2001) Ten years’ experience in zygomycosis at a tertiary care centre in India. J Infect 42:261–266. https://doi.org/10.1053/jinf.2001.0831
Chakrabarti A, Das J, Mandal J et al (2006) The rising trend of invasive zygomycosis in patients with uncontrolled diabetes mellitus. Med Mycol 44:335–342. https://doi.org/10.1080/13693780500464930
Nithyanandam S, Jacob MS, Battu RR et al (2003) Rhino-orbito-cerebral mucormycosis. A retrospective analysis of clinical features and treatment outcomes. Indian J Ophthalmol 51:231–236
Chander J (2018) Mucormycosis Textbook of Medical Mycology, 4th edn. Jaypee Brothers Medical Publishers Ltd, New Delhi, pp 534–596
Furbinger P (1876) Observations on lungenmycose beinmeschen. Arch Pathol Anat Physiol Klin Med 66:330–365
Paltauf A (1885) Mycosis mucorina, Archiv. Fur. Pathologische. Anatomie. Und Physiologie Und Fur Klinische Medicin 102:543–564
Gregory JE, Golden A, Haymaker W (1943) Mucormycosis of the central nervous system: a report of three cases. Bull Johns Hopkins Hosp 73:405–419
Baker RD (1957) Mucormycosis—a new disease? J Am Med Assoc 163:805–808
Balasubrahmanyan M, Chaudhuri S (1963) A case of pulmonary mucormycosis. Indian J Pathol Bacteriol 6:60–62
Grover S, Naidu A, Jonarkar RV (1966) Rhinocerebral phycomycosis (a case report). Indian J Pathol Bacteriol 9:264
Hazarika P, Ravikumar V, Nayak RG, Rao PS, Shivananda PG (1984) Rhinocerebral mycosis. Ear Nose Throat J 63:464–468
Singh CM, Naik BN, Pandey S, Singh PK, Rajath Rao UR, Kokkayil P, Bhavana K, Singh PK (2022) Investigation of an acute surge of COVID-19 associated mucormycosis (CAM) cases reported to a tertiary health care institution in Bihar. India J Family Med Prim Care 11(6):2802–2810. https://doi.org/10.4103/jfmpc.jfmpc_1909_21
Ribes JA, Vanover-Sams CL, Baker DJ (2000) Zygomycetes in human disease. Clin Microbiol Rev 13:236–301. https://doi.org/10.1128/CMR.13.2.236
Skiada A, Petrikkos G (2009) Cutaneous mucormycosis. Clin Microbiol Infect 15:41–45. https://doi.org/10.1111/j.1469-0691.2009.02979.x
Hutter RVP (1959) Phycomycetous infection (mucormycosis) in cancer patients: a complication of therapy. Cancer 12:330–350
Kontoyiannis DP, Yang H, Song J et al (2016) Prevalence, clinical and economic burden of mucormycosis-related hospitalizations in the United States: a retrospective study. BMC Infect Dis 16:730. https://doi.org/10.1186/s12879-016-2023-z
Rees JR, Pinner RW, Hajjeh RA, Brandt ME, Reingold AL (1998) The epidemiological features of invasive mycotic infections in the San Francisco Bay area, 1992–1993: results of population-based laboratory active surveillance. Clin Infect Dis 27:1138–1147
Bitar D, Van Cauteren D, Lanternier F et al (2009) Increasing incidence of zygomycosis (mucormycosis), France, 1997–2006. Emerg Infect Dis 15:1395–1401. https://doi.org/10.3201/eid1509.090334
Diwakar A, Dewan RK, Chowdhary A, Randhawa HS, Khanna G, Gaur SN (2007) Zygomycosis—a case report and overview of the disease in India. Mycoses 50:247–254. https://doi.org/10.1007/s12262-012-0429-4
Saegeman V, Maertens J, Meersseman W et al (2010) Increasing incidence of mucormycosis in University Hospital, Belgium. Emerg Infect Dis 16:1456–1458. https://doi.org/10.3201/eid1609.100276
Yamazaki T, Kume H, Murase S et al (1999) Epidemiology of visceral mycoses: analysis of data in annual of the pathological autopsy cases in Japan. J Clin Microbiol 37:1732–1738. https://doi.org/10.1128/jcm.37.6.1732-1738.1999
Dolatabadi S, Ahmadi B, Rezaei-Matehkolaei A, Zarrinfar H, Skiada A, Mirhendi H, Niknejad NF, Nazeri M, Rafiei A et al (2018) Mucormycosis in Iran: A six-year retrospective experience. J Mycol Med 28:269–273. https://doi.org/10.1016/j.mycmed.2018.02.014
Bhansali A, Bhadada S, Sharma A, Suresh V, Gupta A, Singh P, Chakarbarti RA, Dash J (2004) Presentation and outcome of rhino-orbital-cerebral mucormycosis in patients with diabetes. Postgrad Med J 80:670–674. https://doi.org/10.1136/pgmj.2003.016030
Godara SM, Kute VB, Goplani KR et al (2011) Mucormycosis in renal transplant recipients: predictors and outcome. Saudi J Kidney Dis Transpl 22:751–756
Patra S, Vij M, Chirla DK et al (2012) Unsuspected invasive neonatalgastrointestinal mucormycosis: a clinicopathological study of six cases from a tertiary care hospital. J Indian Assoc Pediatr Surg 17:153–156. https://doi.org/10.4103/0971-9261.102329
Walther G, Wagner L, Kurzai O (2019) Updates on the taxonomy of mucorales with an emphasis on clinically important taxa. J Fungi 5:106. https://doi.org/10.3390/jof5040106
Sharma B, Nonzom S (2021) Superficial mycoses, a matter of concern: global and Indian scenario-an updated analysis. Mycoses 64:890–908. https://doi.org/10.1111/myc.13264
Hemashettar BM, Patil RN, O’Donnell K, Chaturvedi V, Ren P, Padhye AA (2011) Chronic rhinofacial mucormycosis caused by Mucor irregularis (Rhizomucor variabilis) in India. J Clin Microbiol 49:2372–2375. https://doi.org/10.1128/JCM.02326-10
Prakash H, Chakrabarti A (2019) Global epidemiology of mucormycosis. J Fungi 5:26
Kokkayil P, Pandey M, Agarwal R, Kale P, Singh G, Xess I (2017) Rhizopus homothallicus causing invasive infections: series of three cases from a single center in North India. Mycopathologia 182:921–926. https://doi.org/10.1007/s11046-017-0153-5
Tsyrkunou AV, Ellison RT, Akalin A, Wiederhold N, Sutton DA, Lindner J et al (2014) Multifocal Rhizopus microsporus lung infection following brush clearing. Med Mycol Case Rep 6:14–17. https://doi.org/10.1016/j.mmcr.2014.08.001
Chakrabarti A, Singh R (2011) The emerging epidemiology of mould infections in developing countries. Curr Opin Infect Dis 24:521–526. https://doi.org/10.1097/QCO.0b013e32834ab21e
Xess I, Mohapatra S, Shivaprakash MR, Chakrabarti A, Benny GL, O’Donnell K et al (2012) Evidence implicating Thamnostylum lucknowense as an etiological agent of rhino-orbital mucormycosis. J Clin Microbiol 50:1491–1494. https://doi.org/10.1128/JCM.06611-11
Chakrabarti A, Marak RS, Shivaprakash MR, Gupta S, Garg R, Sakhuja V et al (2010) Cavitary pulmonary zygomycosis caused by Rhizopus homothallicus. J Clin Microbiol 48:1965–1969. https://doi.org/10.1128/JCM.01272-09
Kimura M, Udagawa S, Makimura K, Satoh K, Toyazaki N, Ito H (2009) Isolation and identification of Rhizomucor pusillus from pleural zygomycosis in an immunocompetent patient. Med Mycol 47:869–873. https://doi.org/10.3109/13693780903059485
Gomes MZR, Lewis RE, Kontoyiannis DP (2011) Mucormycosis caused by unusual mucormycetes, non-Rhizopus, Mucor and Lichtheimia species. Clin Microbiol Rev 24:411–445. https://doi.org/10.1128/CMR.00056-10
Verma R, Nair V, Vasudevan B, Vijendran P, Behera V, Neema S (2014) Rare case of primary cutaneous mucormycosis of the hand caused by Rhizopus microsporus in an immunocompetent patient. Int J Dermatol 53:66–69. https://doi.org/10.1097/QCO.0b013e32834ab21e
Pandey M, Singh AR, Dabas Y, Jyotsna VP, Kumar R et al (2018) Emerging Rhizopus microsporus infections in India. J Clin Microbio 56:1–5. https://doi.org/10.1128/JCM.00433-18
Lanternier F, Dannaoui E, Morizot G, Elie C, Garcia-Hermoso D, Huerre M, Bitar D, Dromer F et al (2012) A global analysis of mucormycosis in France: the retro zygo study (2005–2007). Clin Infect Dis 54:S35–S43. https://doi.org/10.1093/cid/cir880
Kennedy KJ, Daveson K, Slavin MA, van Hal SJ, Sorrell TC, Lee A, Marriott DJ, Chapman B, Halliday CL, Australia and New Zealand Mycoses Interest Group of the Australasian Society for Infectious Diseases et al (2016) Mucormycosis in Australia: contemporary epidemiology and outcomes. Clin Microbiol Infect 22:775–781. https://doi.org/10.1016/j.cmi.2016.01.005
Stelzmueller I, Lass-Floerl C, Geltner C et al (2008) Zygomycosis and other rare filamentous fungal infections in solid organ transplant recipients. Transpl Int 21:534–546
International Diabetes Federation (IDF) Diabetes Atlas. https:// diabetesatlas.org/en/resources/2019s 10 May 2021
Greenberg RN, Scott LJ, Vaughn HH, Ribe JA (2004) Zygomycosis (mucormycosis): emerging clinical importance and new treatments. Curr Opin Infect Dis 17:517–525. https://doi.org/10.1097/00001432-200412000-00003
Vaezi A, Moazeni M, Rahimi MT et al (2016) Mucormycosis in Iran: a systematic review. Mycoses 59:402–415. https://doi.org/10.1111/myc.12474
Corzo-Leon DE, Chora-Hernandez LD, Rodriguez-Zulueta AP, Walsh TJ (2018) Diabetes mellitus as the major risk factor for mucormycosis in Mexico: epidemiology, diagnosis, and outcomes of reported cases. Med Mycol 56:29–43. https://doi.org/10.1093/mmy/myx017
Pagano L, Valentini CG, Posteraro B, Girmenia C, Ossi C, Pan A, Candoni A, Nosari A, Riva M, Cattaneo C et al (2009) Zygomycosis in Italy: A survey of FIMUA-ECMM (Federazione Italiana di Micopatologia Umana ed Animale and European Confederation of Medical Mycology). J Chemother 21:322–329. https://doi.org/10.1179/joc.2009.21.3.322
Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP (2012) Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis 54:S23–S34. https://doi.org/10.1093/cid/cir866
Serris A, Danion F, Lanternier F (2019) Disease entities in mucormycosis. J Fungi 5:23. https://doi.org/10.3390/jof5010023
Kursun E, Turunc T, Demiroglu Y, Alıskan HE, Arslan AH (2015) Evaluation of 28 cases of mucormycosis. Mycoses 58:82–87. https://doi.org/10.1111/myc.12278
Lelievre L, Garcia-Hermoso D, Abdoul H et al (2014) Posttraumatic mucormycosis. Medicine (Baltimore) 93:373–382. https://doi.org/10.1097/MD.0000000000000221
Sulis G, Daniels B, Kwan A, Gandra S, Daftary A, Das J et al (2020) Antibiotic overuse in the primary health care setting: a secondary data analysis of standardised patient studies from India China and Kenya. BMJ Glob Health 5(9):e003393. https://doi.org/10.1136/bmjgh-2020-003393
Sulis G, Batomen B, Kotwani A, Pai M, Gandra S (2021) Sales of antibiotics and hydroxychloroquine in India during the COVID-19 epidemic: an interrupted time series analysis. PLoS Med 18:e1003682. https://doi.org/10.1371/journal.pmed.1003682
Waldorf AR (1989) Pulmonary defense mechanisms against opportunistic fungal pathogens. Immunol Ser 47:243–271
Chinn RY, Diamond RD (1982) Generation of chemotactic factors by Rhizopus oryzae in the presence and absence of serum: relationship to hyphal damage mediated by human neutrophils and effects of hyperglycemia and ketoacidosis. Infect Immun 38:1123–1129. https://doi.org/10.1128/iai.38.3.1123-1129.1982
Potenza L, Vallerini D, Barozzi P, Riva G, Forghieri F, Zanetti E, Quadrelli C, Candoni A, Maertens J, Rossi G et al (2011) Mucorales-specific T cells emerge in the course of invasive mucormycosis and may be used as a surrogate diagnostic marker in high-risk patients. Blood 118:5416–5419. https://doi.org/10.1371/journal.pone.0149108
Boelaert JR, de Locht M, Van Cutsem J et al (1993) Mucormycosis during deferoxamine therapy is a siderophore-mediated infection: in-vitro and in-vivo animal studies. J Clin Invest 91:1979–1986. https://doi.org/10.1172/JCI116419
Howard DH (1999) Acquisition, transport, and storage of iron by pathogenic fungi. Clin Microbiol Rev 12:394–404. https://doi.org/10.1128/CMR.12.3.394
Reid DW, Lam QT, Schneider H, Walters EH (2004) Airway iron and iron-regulatory cytokines in cystic fibrosis. Eur Respir J 24:286–291
Chamilos G, Lewis R, Lamaris G, Walsh TJ, Kontoyiannis DP (2008) Zygomycetes hyphae trigger an early, robust proinflammatory response in human polymorphonuclear neutrophils through toll-like receptor 2 induction but display relative resistance to oxidative damage. Antimicrob Agents Chemother 52:722–724. https://doi.org/10.1128/AAC.01136-07
Bouchara P, Oumeziane NA, Lissitzky JC, Larcher G, Tronchin G, Chabasse D (1996) Attachment of spores of the human pathogenic fungus Rhizopus oryzae to extracellular smatrixcomponents. Eur J Cell Biol 70:76–83
Ibrahim AS, Spellberg B, Avanessian V, Fu Y, Edwards JE (2005) Rhizopus oryzae adheres to, is phagocytosed by, and damages endothelial cells in-vitro. Infect Immun 73(2):778–783. https://doi.org/10.1128/IAI.73.2.778-783.2005
Gebremariam T, Liu M, Luo G, Bruno V, Phan QT, Waring AJ et al (2014) CotH3 mediates fungal invasion of host cells during mucormycosis. J Clin Invest 124:237–250. https://doi.org/10.1172/JCI71349
Lee SC, Billmyre RB, Li A, Carson S, Sykes SM, Huh EY et al (2014) Analysis of a food-borne fungal pathogen outbreak: virulence and genome of a Mucor circinelloides isolate from yogurt. mBio 5(4):e01390-14. https://doi.org/10.1128/mBio.01390-14
Voigt K, Wolf T, Ochsenreiter K, Nagy G, Kaeger K, Shelest E et al (2016) Genetic and metabolic aspects of primary and secondary metabolism of the zygomycetes, In: D. Hoffmeister (Eds.), The Mycota Vol III: Biochemistry and Molecular Biology, 3rd Edition III ed: Springer Verlag, Berlin, pp. 361–385
Effat KG, Karam M, El-Kabani A (2005) Pott’s puffy tumour caused by mucormycosis. J Laryngol Otol 119:643–645. https://doi.org/10.1258/0022215054516304
Gleissner A, Schilling A, Anagnostopolous I, Siehl I, Thiel E (2004) Improved outcome of zygomycosis in patients with hematological diseases? Leuk Lymphoma 45:1351–1360. https://doi.org/10.1080/10428190310001653691
Prabhu RM, Patel R (2004) Mucormycosis and entomophthoramycosis: a review of the clinical manifestations, diagnosis and treatment. Clin Microbiol Infect 10:31–47. https://doi.org/10.1111/j.1470-9465.2004.00843.x
Dobrilovic N, Wait MA (2005) Pulmonary mucormycosis. Ann Thorac Surg 79:354. https://doi.org/10.1016/S0003-4975(03)01287-6
Jaju MR, Sagar RV, Srivastav SK, Singh R, Kumar KV, Faisal M (2020) Different types of mucormycosis: case series. Int J Res Med Sci 8:2284–2296. https://doi.org/10.18203/2320-6012.ijrms20202281
Dubey A, Patwardhan RV, Sampth S, Santosh V, Kolluri S, Nanda A (2005) Intracranial fungal granuloma: analysis of 40 patients and review of the literature. Surg Neurol 63:254–260. https://doi.org/10.1016/j.surneu.2004.04.020
Paparello SF, Robert L, Dougald CMG, Nadine B, Douglas LM (1992) Hospital-acquired wound mucormycosis. Clin Infect Dis 14:350–352. https://doi.org/10.1093/clinids/14.1.350
Ingram CW, Sennesh J, Cooper JN, Perfect JR (1989) Disseminated zygomycosis: report of four cases and review. Rev Infect Dis 11:741–754. https://doi.org/10.1093/clinids/11.5.741
Yu J, Li RY (2006) Primary renal zygomycosis due to Rhizopus oryzae. Med Mycol 44:461466. https://doi.org/10.1080/13693780500338951
Weng DE, Wilson WH, Little R, Walsh TJ (1998) Successful medical management of isolated renal mucormycosis: case report and review. Clin Infect Dis 26:601–605. https://doi.org/10.1086/514562
Chkhotua A, Yussim A, Tovar A, Weinberger M, Sobolev V, Bar-Nathan N, Shaharabani E, Shapira Z, Mor E (2001) Mucormycosis of the renal allograft: case report and review of the literature. Transpl Int 14:438–441. https://doi.org/10.1007/s001470100010
Chen L, Xiao Y, Wang X (2005) Successful treatment of mucormycosis in the pulmonary artery after cardiac surgery. J Card Surg 20:186–188. https://doi.org/10.1111/j.0886-0440.2005.200382q.x
Zhang R, Zhang JW, Szerlip HM (2002) Endocarditis and hemorrhagic stroke caused by Cunninghamella bertholletiae infection after kidney transplantation. Am J Kidney Dis 40:842–846. https://doi.org/10.1053/ajkd.2002.35698
Wanishsawad C, Kimbrough RC, Chinratanalab S, Nugent K (1996) Mucormycotic osteolytic rib lesion presenting as subacute pleural effusion. Clin Infect Dis 22:715–716. https://doi.org/10.1093/clinids/22.4.715
Serna JH, Wanger A, Dosekun AK (2003) Successful treatment of mucormycosis peritonitis with liposomal amphotericin B in a patient on long-term peritoneal dialysis. Am J Kidney Dis 42:14–17. https://doi.org/10.1016/s0272-6386(03)00797-2
Oliver MR, Van Voorhis WC, Boeckh M et al (1996) Hepatic mucormycosis in a bone marrow transplant recipient who ingested naturopathic medicine. Clin Infect Dis 22:521–524. https://doi.org/10.1093/clinids/22.3.521
Garg PK, Gupta N, Gautam V, Hadke NS (2008) Gastric mucormycosis: unusual cause of gastric perforation in an immunocompetent patient. South Med J 101:449–450. https://doi.org/10.1097/smj.0b013e318167bb31
Sachdeva K (2013) Rhino-oculo-cerebral mucormycosis with multiple cranial nerve palsy in diabetic patient: review of six cases. Indian J Otolaryngol Head Neck Surg 65:375–379. https://doi.org/10.1007/s12070-013-0659-1
Chander J, Kaur M, Singla N, Punia RPS, Singhal SK, Attri AK et al (2018) Mucormycosis: battle with the deadly enemy over a five-year period in India. J Fungi 4:46. https://doi.org/10.3390/jof4020046
Sarvestani AS, Pishdad G, Bolandparvaz S (2013) Predisposing factors for mucormycosis in patients with diabetes mellitus; An experience of 21 years in southern Iran. Bull Emerg Trauma 1:164–170
Funada H, Matsuda T (1996) Pulmonary mucormycosis in a hematology ward. Intern Med 35:540–544. https://doi.org/10.2169/internalmedicine.35.540
Shpitzer T, Keller N, Wolf M et al (2005) Seasonal variations in rhinocerebral Mucor infection. Ann Otol Rhinol Laryngol 114:6958. https://doi.org/10.1177/000348940511400907
El-Herte RI, Baban TA, Kanj SS (2012) Mucormycosis: a review on environmental fungal spores and seasonal variation of human disease. Adv Infect Dis 2:76–81. https://doi.org/10.4236/aid.2012.23012
Ghosh AK, Singh R, Reddy S, Singh S, Rudramurthy SM, Kaur H, Choudhary H, Chakrabarti A (2022) Evaluation of environmental Mucorales contamination in and around the residence of COVID-19-associated mucormycosis patients. Front Cell Infect Microbiol 12:953750. https://doi.org/10.3389/fcimb.2022.953750
Biswal M, Gupta P, Kanaujia R, Kaur K, Kaur H, Aruna V et al (2022) Evaluation of hospital environment for presence of mucorales during COVID-19 associated mucormycosis outbreak in India—a multi-centre study. J Hosp Infect 122:173–179. https://doi.org/10.1016/J.JHIN.2022.01.016
Iyer M, Tiwari S, Renu K, Pasha MY, Pandit S, Singh B, Raj N, Krothapalli S, Kwak HJ, Balasubramanian V, Jang SB, Kumar GD, Uttpal A, Narayanasamy A, Kinoshita M, Subramaniam MD, Nachimuthu SK, Roy A, Valsala Gopalakrishnan A, Ramakrishnan P, Cho SG, Vellingiri B (2021) Environmental survival of SARS-CoV-2—a solid waste perspective. Environ Res 197:111015. https://doi.org/10.1016/j.envres.2021.111015
Sarkodie SA, Owusu PA (2020) Impact of COVID-19 pandemic on waste management. Environ Dev Sustain 23(5):7951–7960. https://doi.org/10.1007/s10668-020-00956-y
Walsh TJ, Gamaletsou MN, McGinnis MR, Hayden RT, Kontoyiannis DP (2012) Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis). Clin Infect Dis 54:S55–S60. https://doi.org/10.1093/cid/cir868
Kontoyiannis DP, Lewi RE, Lortholary O et al (2012) Future directions in mucormycosis research. Clin Infect Dis 54:79–85. https://doi.org/10.1093/cid/cir886
Millon L, Herbrecht R, Grenouillet F, Morio F, AlanioLetscher-Bru A, Cassaing Sophie et al (2016) Early diagnosis and monitoring of mucormycosis by detection of circulating DNA in serum: retrospective analysis of 44 cases collected through the French Surveillance Network of Invasive Fungal Infections (RESSIF). Clin Microbiol Infect 22:810.e1-810.e8. https://doi.org/10.1016/j.cmi.2015.12.006
Smith HW, Kirchner JA (1950) Cerebral mucor-mycosis: a report of 3 cases. Arch Otolaryng (Chicago) 68:715–726. https://doi.org/10.1001/archotol.1958.00730020739010
Prakash H, Ghosh AK, Rudramurthy S, Paul RA, Gupta S, Negi V, Chakrabarti A (2016) The environmental source of emerging Apophysomyces variabilis infection in India. Med Mycol 54:567–575. https://doi.org/10.1093/mmy/myw014
Wang L, Wang C, Shen Y et al (2015) Phaeohyphomycosis caused by Exophiala sinifera: an increasing disease in young females in mainland China? two case reports and review of five cases reported from mainland China. Mycoses 5:193–196. https://doi.org/10.1111/myc.12295
Song G, Liang G, Liu W (2020) Fungal Co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia 185:599–606. https://doi.org/10.1007/s11046-020-00462-9
Zaman K, Rudramurthy SM, Das A, Panda N, Honnavar NP, Kaur H, Chakrabarti A (2017) Molecular diagnosis of rhino-orbito-cerebral mucormycosis from fresh tissue samples. J Med Microbiol 66:1124–1129. https://doi.org/10.1099/jmm.0.000560
Aranjani JM, Manuel A, Abdul Razack HI, Mathew ST (2021) COVID-19–associated mucormycosis: evidence-based critical review of an emerging infection burden during the pandemic’s second wave in India. PLoS Negl Trop Dis 15:e0009921. https://doi.org/10.1371/journal.pntd.0009921
Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SC, Dannaoui E, Hochhegger B, Hoenigl M, Jensen HE, Lagrou K, Lewis RE, Mellinghoff SC (2019) Global guideline for the diagnosis and management of mucormycosis: an initiative of the European confederation of medical mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis 19:e405–e421. https://doi.org/10.1016/S1473-3099(19)30312-3
Shoham S, Magill SS, Merz WG, Gonzalez C, Seibel N, Buchanan WL, Knudsen TA, Sarkisova TA, Walsh TJ (2010) Primary treatment of zygomycosis with liposomal amphotericin B: analysis of 28 cases. Med Mycol 48:511–517. https://doi.org/10.3109/13693780903311944
Skiada A, Lanternier F, Groll AH, Pagano L, Zimmerli S, Herbrecht R, Lortholary O, Petrikkos GL (2013) Diagnosis and treatment of mucormycosis in patients with haematological malignancies: guidelines from the 3rd European Conference on Infections in Leukemia (ECIL3). Haematologica 98:492–504. https://doi.org/10.3324/haematol.2012.065110
Ellsworth M, Ostrosky-Zeichner L (2020) Isavuconazole: Mechanism of action, clinical efficacy and resistance. J Fungi 6:324. https://doi.org/10.3390/jof6040324
Macedo A, Leonardelli F, Dudiuk C, Vitale RG, Del Valle E, Giusiano G, Gamarra S, Garcia-Effron G (2019) In vitro and in vivo evaluation of voriconazole-containing antifungal combinations against Mucorales using a Galleria mellonella model of mucormycosis. J Fungi 5:5. https://doi.org/10.3390/jof5010005
John B, Chamilos G, Kontoyiannis D (2005) Hyperbaric oxygen as an adjunctive treatment for zygomycosis. Clin Microbiol Infect 11:515–517. https://doi.org/10.1111/j.1469-0691.2005.01170.x
Dokmetas HS, Canbay YS et al (2002) Diabetic ketoacidosis and rhino-orbital mucormycosis. Diabetes Res Clin Prac 57:139–142. https://doi.org/10.1016/s0168-8227(02)00021-9
Cornely OA, Arikan-Akdagli S, Dannaoui E, Groll AH, Lagrou K, Chakrabarti A, Lanternier F, Pagano L, Skiada A, Akova M, Arendrup MC, Boekhout T, Chowdhary A, Cuenca-Estrella M, Freiberger T, Guinea J, Guarro J, de Hoog S, Hope W et al (2014) ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis. Clin Microbiol Infect 3:5–26. https://doi.org/10.1111/1469-0691.12371
Sojinrin T, Conde J, Liu K, Curtin J, Byrne HJ, Cui D et al (2017) Plasmonic gold nanoparticles for detection of fungi and human cutaneous fungal infections. Anal Bioanal Chem 409:4647–4658. https://doi.org/10.1007/s00216-017-0414-7
Garcia A, Fan YY, Vellanki S, Huh EY, Vanegas D, Wang SH et al (2019) Nanoemulsion as an effective treatment against human-pathogenic fungi. mSphere 4(6):e00729-19. https://doi.org/10.1128/mSphere.00729-19
Rai M, Yadav A, Bridge P, Gade A (2009) Myconanotechnology: A new and emerging science. In: Rai M, Bridge PD (eds) Appl Mycol. CABI, Wallingford, pp 258–267
Lau AF, Walchak RC, Miller HB et al (2019) Multicenter study demonstrates standardization requirements for mold identification by MALDI-TOF MS. Front Microbiol 10:2098. https://doi.org/10.3389/fmicb.2019.02098
Zacharioudakis IM, Zervou FN, Mylonakis E (2018) T2 magnetic resonance assay: overview of available data and clinical implications. J Fungi 4:45. https://doi.org/10.3390/jof4020045
Bhatnagar I, Mahato K, Ealla KKR et al (2018) Chitosan stabilized gold nanoparticle mediated self-assembled gliP nanobiosensor for diagnosis of invasive Aspergillosis. Int J Biol Macromol 110:449–456
Asghar W, Sher M, Khan NS et al (2019) Microfluidic chip for detection of fungal infections. ACS Omega 4:7474–7481. https://doi.org/10.1021/acsomega.9b00499
Gohil K, Samson R, Dastager S, Dharne M (2021) Probiotics in the prophylaxis of COVID-19: something is better than nothing. 3 Biotech 11(1):1. https://doi.org/10.1007/s13205-020-02554-1
Arasu MV, Al-Dhabi NA (2017) In-vitro antifungal, probiotic, and antioxidant functional properties of a novel Lactobacillus paraplantarum isolated from fermented dates in Saudi Arabia. J Sci Food Agric 97:5287–5295. https://doi.org/10.1002/jsfa.8413
Azizkhani M, Saris PEJ, Baniasadi M (2021) An in-vitro assessment of antifungal and antibacterial activity of cow, camel, ewe, and goat milk kefir and probiotic yogurt. J Food Meas Charact 15:406–415. https://doi.org/10.1007/s11694-020-00645-4
Hu L, Zhou M, Young A et al (2019) In-vivo effectiveness and safety of probiotics on prophylaxis and treatment of oral candidiasis: a systematic review and meta-analysis. BMC Oral Health 19:140. https://doi.org/10.1186/s12903-019-0841-2
Thomas S, Patel D, Bittel B et al (2021) Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Netw Open 4(2):e210369. https://doi.org/10.1001/jamanetworkopen.2021.0369
Staats CC, Kmetzsch L, Schrank A, Vainstein MH (2013) Fungal zinc metabolism and its connections to virulence. Front Cell Infect Microbiol 3:65. https://doi.org/10.3389/fcimb.2013.00065
Leonardelli F, Macedo D, Dudiuk C, Theill L, Cabeza MS, Gamarra S et al (2019) In-vitro activity of combinations of zinc chelators with amphotericin B and posaconazole against six Mucorales species. Antimicrob Agents Chemother 63(5):e00266. https://doi.org/10.1128/AAC.00266-19
Lulloff SJ, Hahn BL, Sohnle PG (2004) Fungal susceptibility to zinc deprivation. J Lab Clin Med 144:208–214. https://doi.org/10.1016/j.lab.2004.07.007
Nath S, Baidya DK (2021) Mucormycosis in COVID-19: is zinc a silent killer in India? Indian. J Crit Care Med 25:1079–1080. https://doi.org/10.5005/jp-journals-10071-23938
Muthu V, Kumar M, Paul RA et al (2021) Is there an association between zinc and COVID-19–associated mucormycosis? results of an experimental and clinical study. Mycoses 64:1291–1297. https://doi.org/10.1111/myc.13365
Vuorio A, Kovanen PT (2021) Statins as adjuvant therapy for COVID-19 to calm the stormy immunothrombosis and beyond. Front Pharmacol 11:579548. https://doi.org/10.3389/fphar.2020.579548
Chatterjee S, Vardhan B, Singh DK, Maitra A, Ojha UK (2021) Should statins be considered for the management of mucormycosis in COVID-19? Diabetes Metab Syndr 15(4):102162. https://doi.org/10.1016/j.dsx.2021.05.035
Manesh A, Rupali P, Sullivan MO, Mohanraj P, Rupa V, George B, Michael JS (2019) Mucormycosis—a clinicoepidemiological review of cases over 10 years. Mycoses 62:391–398. https://doi.org/10.1111/myc.12897
Priya P, Ganesan V, Rajendran T, Geni VG (2020) Mucormycosis in a tertiary care center in South India: a 4-Year experience. Indian J Crit Care Med 24:168–171. https://doi.org/10.5005/jp-journals-10071-23387
Pakdel A, Ahmadikia K, Salehi M et al (2021) Mucormycosis in patients with COVID-19: a cross-sectional descriptive multicentre study from Iran. Mycoses 64:1238–1252. https://doi.org/10.1111/myc.13334
Lortholary O, Petrikkos G, Akova M, Arendrup MC, Arikan-Akdagli S, Bassetti M, Bille J, Calandra T, Castagnola E, Cornely OA et al (2012) ESCMID guideline for the diagnosis and management of Candida diseases 2012: patients with HIV infection or AIDS. Clin Microbiol Infect 18(7):68–77. https://doi.org/10.1111/1469-0691.12042
Kubin CJ, McConville TH, Dietz D et al (2021) Characterization of bacterial and fungal infections in hospitalized patients with COVID-19 and factors associated with healthcare-associated infections. Open Forum Infect Dis 8:ofab201. https://doi.org/10.1093/ofid/ofab201
John TM, Jacob CN, Kontoyiannis DP (2021) When uncontrolled diabetes mellitus and severe COVID-19 converge: the perfect storm for mucormycosis. Journal of Fungi 7(4):298. https://doi.org/10.3390/jof7040298
Sharma B, Nonzom S (2021) Talaromyces stipitatus, a novel agent causing superficial mycosis in a diabetic patient from North India. Microbes Infect 24(2):104887. https://doi.org/10.1016/j.micinf.2021.104887
Hoang K, Abdo T, Reinersman JM, Lu R, Higuita NIA (2020) A case of invasive pulmonary mucormycosis resulting from short courses of corticosteroids in a well-controlled diabetic patient. Med Mycol Case Rep 29:22–24. https://doi.org/10.1016/j.mmcr.2020.05.008
The Hindustan Times, 2021. India reported over 45,000 black fungus cases so far, says Mandaviya in RS. https://www.hindustantimes.com/india-news/india-reported-over-45-000-black-fungus-cases-so-far-says-mandaviya-in-rs-101626781531292.html.
COVID: Mumbai records first mucormycosis cases in 2022 amid omicron surge. https://www.republicworld.com/india-news/city-news/mumbai-records-first mucormycosis-case-as-india-continues-to-grapple-with-covid-articleshow.html
Wei LW, Zhu PQ, Chen XQ et al (2022) Mucormycosis in mainland China: a systematic review of case reports. Mycopathologia 187:1–14. https://doi.org/10.1007/s11046-021-00607-4
Tak K, Rastogi V, Verma P, Gehlot V, Gupta M, Rawat D, Prajapati S, Parihar G (2022) A clinico-mycological study of suspected mucormycosis in post-COVID patients attending a tertiary care hospital. Med Mycol 60:myac072P299. https://doi.org/10.1093/mmy/myac072.P299
Tyagi A, Gupta M, Mittal A, Sengupta A (2022) A rare case of post-COVID mucormycosis of the kidney. Curr Med Res Pract 12:35–37. https://doi.org/10.4103/cmrp.cmrp_85_21
Meshram HS, Kute VB, Chauhan S, Desai S (2021) Mucormycosis in post-COVID-19 renal transplant patients: a lethal complication in follow-up. Transpl Infect Dis 23(4):e13663. https://doi.org/10.1111/tid.13663
Rao VU, Arakeri G, Madikeri G, Shah A, Oeppen RS, Brennan PA (2021) COVID-19 associated mucormycosis (CAM) in India: a formidable challenge. Br J Oral Maxillofac Surg 59:1095–1098. https://doi.org/10.1016/j.bjoms.2021.06.013
Mishra N, Mutya VSS, Thomas A, Rai G, Reddy B, Mohanan AA, Ray S, Thiruvengadem AV, Siddini V, Hegde R (2021) A case series of invasive mucormycosis in patients with COVID-19 infection. Int J Otorhinolaryngol Head Neck Surg 7:867–870. https://doi.org/10.18203/issn.2454-5929.ijohns20211583
Satish D, Joy D, Ross A, Balasubramanya, (2021) Mucormycosis coinfection associated with global COVID-19: a case series from India. Int. J. Otorhinolaryngol Head Neck Surg 7:815–820. https://doi.org/10.18203/issn.2454-5929.ijohns20211574
Garg D, Muthu V, Sehgal IS, Ramachandran R, Kaur H, Bhalla A, Puri GD, Chakrabarti A, Agarwal R (2021) Coronavirus disease (Covid-19) associated mucormycosis (CAM): case report and systematic review of literature. Mycopathologia 186:289–298. https://doi.org/10.1007/s11046-021-00528-2
Krishna V, Morjaria J, Jalandari R, Omar F, Kaul S (2021) Autoptic identification of disseminated mucormycosis in a young male presenting with cerebrovascular event, multi-organ dysfunction and COVID-19 infection. IDCases 25:e01172. https://doi.org/10.1016/j.idcr.2021.e01172
Saldanha M, Reddy R, Vincent MJ (2021) Paranasal mucormycosis in COVID-19 patient. Indian J Otolaryngol Head Neck Surg. https://doi.org/10.1007/s12070-021-02574-0
Revannavar SM, Supriya PS, Samaga L, Vineeth V (2021) COVID-19 triggering mucormycosis in a susceptible patient: a new phenomenon in the developing world? BMJ Case Rep 14(4):e241663. https://doi.org/10.1136/bcr-2021-241663
Maini A, Tomar G, Khanna D, Kini Y, Mehta H, Bhagyasree V (2021) Sino-orbital mucormycosis in a COVID-19 patient: a case report. Int J Surg Case Rep 82:105957. https://doi.org/10.1016/j.ijscr.2021.105957
Mehta S, Pandey A (2020) Rhino-orbital mucormycosis associated with COVID-19. Cureus 12(9):e10726. https://doi.org/10.7759/cureus.10726
Sharma S, Grover M, Bhargava S, Samdani S, Kataria T (2021) Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol 135(5):442–447. https://doi.org/10.1017/S0022215121000992
Sarkar S, Gokhale T, Choudhury SS, Deb AK (2021) COVID-19 and orbital mucormycosis. Indian J Ophthalmol 69:1002. https://doi.org/10.4103/ijo.IJO_3763_20
Nehara HR, Puri I, Singhal V, Sunil IH, Bishnoi BR, Sirohi P (2021) Rhinocerebral mucormycosis in COVID-19 patient with diabetes a deadly trio: Case series from the north-western part of India. Indian J Med Microbiol 39:380–383. https://doi.org/10.1016/j.ijmmb.2021.05.009
Kandasamy S, Muthuraju S, Vasugi A, Chandrasekar M, Murugan R, Inbasekaran P, Prabhu R (2022) Clinicopathological study of mucormycosis in COVID-19 patients: experience from a tertiary care center in South India. Cureus 14(3):e23016. https://doi.org/10.7759/cureus.23016
Chakravarty J, Gupta MK, Tilak R, Kumar R, Maurya RP, Kumar N, Aggarwal SK, Siva S, Sharma NK, Dhiman NK, Chaubey M, Singh V, Verma A, Banerjee T, Agrawal NK, Prasad RS (2022) COVID-19-associated Mucormycosis: a clinico-epidemiological study. J Diabetes Complications 36(9):108284. https://doi.org/10.1016/j.jdiacomp.2022.108284
Viswamithra P, Muralikrishna V, Sultana SS, Sindusha K, Rani BS (2023) Rhino-orbito-cerebral mucormycosis in a pregnant woman: a case report. Indian J Ophthalmol Case Rep 3:167–169. https://doi.org/10.4103/ijo.IJO_1734_22
Zurl C, Hoenigl M, Schulz E, Hatzl S, Gorkiewicz G, Eller RP, Prattes J (2021) Autopsy proven pulmonary mucormycosis due to Rhizopus microsporus in a critically ill COVID-19 patient with underlying hematological malignancy. Journal of Fungi 7(2):88. https://doi.org/10.3390/jof7020088
Monte Junior ESD, Santos M, Ribeiro IB, Luz GO, Baba GO, Hirsch BS et al (2020) Rare and fatal gastrointestinal mucormycosis (Zygomycosis) in a COVID-19 patient: a case report. Clin Endosc 53:746–749. https://doi.org/10.5946/ce.2020.180
Bonates P, Joao GAP, Cruz KS, Ferreira MS, Baia-da-Silva DC, Farias MEL et al (2021) Fatal rhino-orbito-cerebral mucormycosis infection associated with diabetic ketoacidosis post-COVID-19. Rev Bras Med Trop 54:1–6. https://doi.org/10.1590/0037-8682-0358-2021
Ashour MM, Abdelaziz TT, Ashour DM, Askour A, Saleh MI, Mahmoud MS (2021) Imaging spectrum of acute invasive fungal rhino-orbital-cerebral sinusitis in COVID-19 patients: a case series and a review of literature. J Neuroradiol 48:319–324. https://doi.org/10.1016/j.neurad.2021.05.007
El-Kholy NA, El-Fatta AMA, Khafagy YW (2021) Invasive fungal sinusitis in post COVID-19 patients: a new clinical entity. Laryngoscop 131:2652–2658. https://doi.org/10.1002/lary.29632
Riad A, Shabaan AA, Issa J, Ibrahim S, Amer H, Mansy Y, Kassem I, Kassem AB, Howaldt HP, Klugar M, Attia S (2021) COVID-19-Associated mucormycosis (CAM): case-series and global analysis of mortality risk factors. J Fungi (Basel, Switzerland) 7(10):837. https://doi.org/10.3390/jof7100837
Youssif SF, Abdelrady MM, Thabet AA, Abdelhamed MA, Gad MOA, Abu-Elfatth AM, Saied GM, Goda I, Algammal AM, Batiha GE, Abd El-Rady NM, Hetta HF, Kasem SM (2022) COVID-19 associated mucormycosis in Assiut University Hospitals: a multidisciplinary dilemma. Sci Rep 12(1):10494. https://doi.org/10.1038/s41598-022-13443-3
Bellanger P, Navellou JC, Lepiller Q, Brion A, Brunel AS, Millon L, Berceanu A (2021) Mixed mold infection with Aspergillus fumigatus and Rhizopus microsporus in a severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) patient. Infect Dis Now 51:633–635. https://doi.org/10.1016/j.idnow.2021.01.010
Danion F, Letscher-Bru V, Guitard J, Sitbon K, Delliere S, Angoulvant A, Desoubeaux G, Botterel F, Bellanger AP et al (2022) COVID-Mucor study group, coronavirus disease 2019-associated mucormycosis in France: a rare but deadly complication. Open Forum Infect Dis 9:566. https://doi.org/10.1093/ofid/ofab566
Evert K, Dienemann T, Brochhausen C et al (2021) Autopsy findings after long-term treatment of COVID-19 patients with microbiological correlation. Virchows Arch 479:97–108. https://doi.org/10.1007/s00428-020-03014-0
Karimi-Galougahi M, Arastou S, Haseli S (2021) Fulminant mucormycosis complicating coronavirus disease 2019 (COVID-19). Int Forum Allergy Rhinol 11:1029–1030. https://doi.org/10.1002/alr.22785
Veisi A, Bagheri A, Eshaghi M, Rikhtehgar MH, Rezaei Kanavi M, Farjad R (2022) Rhino-orbital mucormycosis during steroid therapy in COVID-19 patients: a case report. Eur J Ophthalmol 32(4):NP11–NP16. https://doi.org/10.1177/11206721211009450
Mohammadi F, Badri M, Safari S, Hemmat N (2021) A case report of rhino-facial mucormycosis in a non-diabetic patient with COVID-19: a systematic review of literature and current update. BMC Infect Dis 2:1–7. https://doi.org/10.1186/s12879-021-06625-3
Balushi AA, Ajmi AA, Sinani QA, Menon V, Berieki ZA, Shezawi AA, Azri SA, Rashdi AA, Jardani AA, Baluki TA, Ghaithi SA, Reesi AA, Al-Zaabi AT, Al Balushi MA, Maqbali TA (2022) COVID-19-Associated Mucormycosis: an opportunistic fungal infection. a case series and review. Int J Infect Dis 121:203–210. https://doi.org/10.1016/j.ijid.2022.05.005
Pasero D, Sanna S, Liperi C, Piredda D, Branca GP, Casadio L, Simeo R, Buselli A, Rizzo D, Bussu F, Rubino SA (2021) A challenging complication following SARS-CoV-2 infection: a case of pulmonary mucormycosis. Infection 49:1055–1060. https://doi.org/10.1007/s15010-020-01561-x
Waizel-Haiat S, Guerrero-Paz JA, Sanchez-Hurtado L, Calleja-Alarcon S, Romero-Gutierrez L (2021) A case of fatal rhino-orbital mucormycosis associated with new onset diabetic ketoacidosis and COVID-19. Cureus 13(2):e13163. https://doi.org/10.7759/cureus.13163
Buil Jochem B, van Zanten Arthur R H, Bentvelsen Robbert G, Rijpstra Tom A, Goorhuis Bram, van der Voort Sanne, Wammes Linda J, Janson Jeroen A, Melchers Max, Heusinkveld Moniek, Melchers Willem JG, Kuijper Ed J, Verweij Paul E (2021) Case series of four secondary mucormycosis infections in COVID-19 patients the Netherlands December 2020 to May 2021. Euro Surveill. 26(23): 2100510. https://doi.org/10.2807/1560-7917.ES.2021.26.23.2100510
Sargin F, Akbulut M, Karaduman S, Sungurtekin H (2021) Severe rhinocerebralmucormycosis case developed after COVID 19. J Bacteriol Parasitol 12:386
Hanley B, Naresh KN, Roufosse C (2020) Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe 1:e245–e253. https://doi.org/10.1016/S2666-5247(20)30115-4
Werthman-Ehrenreich A (2021) Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg Med 42:264.e5-264.e8. https://doi.org/10.1016/j.ajem.2020.09.032
Placik DA, Taylor WL, Wnuk NM (2020) Bronchopleural fistula development in the setting of novel therapies for acute respiratory distress syndrome in SARS-CoV-2 pneumonia. Radiol Case Rep 15:2378–2381. https://doi.org/10.1016/j.radcr.2020.09.026
Alekseyev K, Didenko L, Chaudhr B (2021) Rhinocerebral mucormycosis and COVID-19 pneumonia. J Med Cases 12:85–89. https://doi.org/10.14740/jmc3637
Kanwar A, Jordan A, Olewiler S, Wehberg K, Cortes M, Jackson BR (2021) A fatal case of Rhizopus azygosporus pneumonia following COVID-19. J Fungi 7:174. https://doi.org/10.3390/jof7030174
Johnson AK, Ghazarian Z, Cendrowski KD, Persichino JG (2021) Pulmonary aspergillosis and mucormycosis in a patient with COVID-19. Med Mycol Case Rep 32:64–67. https://doi.org/10.1016/j.mmcr.2021.03.006
Dallalzadeh LO, Ozzello DJ, Liu CY, Kikkawa DO, Korn BS (2021) Secondary infection with rhino-orbital cerebral mucormycosis associated with COVID-19. Orbit. https://doi.org/10.1080/01676830.2021.1903044
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or nonprofit sectors.
Author information
Authors and Affiliations
Contributions
Both the authors have contributed to the manuscript. BS: Conceptualization, Resources and Writing-original draft. SN: Supervision, Review and Editing.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, B., Nonzom, S. Mucormycosis and Its Upsurge During COVID-19 Epidemic: An Updated Review. Curr Microbiol 80, 322 (2023). https://doi.org/10.1007/s00284-023-03430-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03430-w