Abstract
Many agricultural products are susceptible to contamination by aflatoxin-producing species from Aspergillus section Flavi. The objectives of this study were to determine the occurrence of Aspergillus section Flavi in four agricultural products, such as pistachio, walnut, hazelnut, and dried fruits, collected from market and retail shops in various areas of Kerman County and obtain information on the relationships between isolation source and ability to produce sclerotia and potential for aflatoxin production. Aspergillus species were identified based on morphological characteristics as well as subsequent sequencing of the parts of the β-tubulin and calmodulin genes. From 207 isolated strains, the following species were identified: A. flavus, A. tamarii A. nomius, A. parasiticus, A. arachidicola, A. caelatus, A. pseudotamarii, and A. leporis. To the best of our knowledge, this is the first report of A. pseudotamarii and A. arachidicola with the potential to produce aflatoxins from dried apricots and hazelnuts, respectively. Sclerotial type was significantly different between isolates from different isolation sources. From 192 tested isolates, 38% were aflatoxin producer from which 5% were scored as strong aflatoxin producers and 33% as average aflatoxin producers. A significant difference in the population of aflatoxin-producing strains across the isolation sources was observed which may reflect host adaptation and thereby different vulnerabilities to aflatoxin-producing species among the examined products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aspergillus section Flavi contains more than 34 species many of which produce Aflatoxins. Aflatoxin is important for its liver toxicity and carcinogenicity. Aflatoxins cause chronic and serious health hazards and are genotoxic, carcinogenic, and teratogenic to both humans and animals. Agricultural crops are often contaminated by aflatoxins resulting in substantial economic loss worldwide [1, 2].
Species in Aspergillus section Flavi especially A. flavus show variations in their ability to produce aflatoxins [3] which is important because the atoxigenic strains are being successfully used as biological control agents to reduce the risk of occurrence of aflatoxigenic strains [4,5,6]. Relationships between factors affecting aflatoxin production including phenotype of sclerotial production [7], geographic origin [8], vegetative compatibility [9], and genetic diversity [10] have been studied. The relationships between sources of isolation of Aspergillus section Flavi to the distribution of L and S strains and also to the occurrence of aflatoxin/non-aflatoxin-producing species have been investigated on cotton [11], corn, peanut, rice, and soil [7]. The relationships between sources of isolation and strain distribution in terms of sclerotial type and aflatoxin-producing ability on dried fruits and nuts are less studied.
Most available research in Iran has focused on aflatoxigenic mycoflora of pistachios because of the economic importance of this horticultural product. There is little information available on Aspergillus section Flavi in other dry agricultural goods marketed in Iran. Pistachio, walnut, hazelnut, and dried fruits (mostly consumed as mixtures of apricot, peach and plum) are very popular in Iran and consumed throughout the country by many people. These agricultural products can be colonized and contaminated by several fungi among which Aspergillus section Flavi are the most important as they are able to produce aflatoxins. Dried fruits and nuts sold commercially in market and retail shops are reported to be contaminated with aflatoxins at different levels [12,13,14]. The objectives of this research were, thus, to study the occurrence of Aspergillus section Flavi in pistachio, walnut, hazelnut, and dried fruits (mostly sold as mixtures of dried apricot, peach and plum) collected from market and retail shops in Kerman county (Iran) and access the ability of key species to produce aflatoxin and sclerotium. The relationships of isolation source and their ability to produce sclerotia and potential for aflatoxin production were evaluated.
Materials and Methods
Fungal Isolation
A total of 200 samples of pistachio, walnut, hazelnut, and dried fruits (mixture of apricot, peach and plum) (50 of each crop) were collected from market and retail shops in various areas in Kerman County during 2018 and 2019. None of the pistachio, walnut, and hazelnut samples were roasted or salted. Direct plating technique [15] was used to assess the colonization of samples by Aspergillus species. Samples were surface disinfected with 70% ethanol for 10 s, rinsed in sterile distilled water (SDW) for 10 s, then submerged for 1 min in 1% NaOCl, rinsed in SDW and placed on sterile filter paper to dry. Pieces of pistachio, walnut, hazelnut, and dried fruits were plated on dichloran Rose Bengal chloramphenicol (DRBC) agar [16]. Plates were incubated for 5–10 days at 25 °C. All isolates with the appearance of belonging to Aspergillus section Flavi i.e., yellow green colony color were transferred on AFPA (A. flavus and parasiticus Agar; [17]) to see colony characteristics and the possible production of an orange pigment visible on the underside of colonies [17]. The Putative isolates of Aspergillus section Flavi were sub-cultured onto CYA (Czapek yeast extract agar; [18]) for subsequent identification of species. Isolates were deposited in the KGUT Fungal Culture Collection at Kerman Graduate University of Advanced Technology, Kerman, Iran, stored in 10% glycerol at – 20 °C for short-term and at – 80 °C for long-term preservation.
Morphological Characterization
Morphological characterization was performed according to Klich [19]. Spore suspensions (in 0.2% agar) were inoculated at three points on 9 cm plates of MEA (Malt Extract Agar), CYA, CY20S (Czapek Agar with 20% sucrose), and CZ (Czapek's agar). Plates were incubated upside down for 7 d in the dark at 25°C. Additional plate of CYA was incubated in 37°C and 42°C. All media were prepared according to Samson et al. [20]. Colony diameter, color, pigmentations, and exudates were observed after 7 days. Microscopic examinations were made in cotton blue and 50% lactic acid from colonies grown on MEA medium and a drop of alcohol was added to help spreading conidia and removing air bubbles. Digital images of these structures were made using a Dino-eye microscope camera USB lens (The Microscope Store, LLC, USA). All isolates were cultured on AFPA for 72 h in the dark to identify Aspergillus flavus, Aspergillus parasiticus, and their related species colonies based on the orange color in the reverse of the colony. All isolates were preliminarily identified based on their morphological features. The identifications were further confirmed by molecular analysis.
DNA Extraction, PCR, and Sequencing
The isolates used for the molecular studies were inoculated into bottles containing 50 ml PDB and incubated in a shaker (180 rpm) at room temperature for 48 h. Mycelia mats were collected by vacuum filtration and washed in sterile water. Fungal mats were homogenized using liquid nitrogen. The cells were lysed using CTAB solution and the DNA extraction was performed using DNGTM-Plus solution (Sinaclon, Iran) following the manufacturer’s instructions. The DNA concentrations were quantified using a NanoDrop spectrophotometer (NanoDrop Technologies, USA).
A 580 bp portion of calmodulin gene was amplified from Aspergillus isolates using the primers cmd5 (5ˊ CCG AGT ACA AGG AGG CCT TC 3ˊ) and cmd6 (5ˊ CCG ATA GAG GTC ATA ACG TGG 3ˊ) [21]. Amplification of part of (550bp) the ß-tubulin gene was performed using Bt2a (5ˊ GGT AAC CAA ATC GGT GCT TTC 3ˊ) and Bt2b (5ˊACC CTC AGT GTA GTG ACC CTT GGC 3ˊ) [22]. Twenty-five μL PCR reactions contained 1X reaction buffer, 0.4 mM of each primer (Metabion, Germany), 200 mM dNTPs, 2.5 mM MgCl2, 20 ng of DNA, and 1 unit of Taq polymerase. PCR amplifications were performed in a Biometra TAdvanced Thermal Cycler (Biometra, Göttingen, Germany) with the cycling conditions consisting of 95 °C for 3 min, followed by 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min, and then 5 min at 72°C. The sequencing was carried out by Macrogen (Macrogen Inc, South Korea). The unique β-tubulin and calmodulin sequences were deposited at the GenBank nucleotide sequence database under accession numbers MN986404-MN986425 for calmodulin and MT009226-MT009233 and MN993901- N993914 for β-tubulin (Table 1).
Molecular Identification and Phylogenetic Analysis
The results of sequencing were used to perform homology searches made in NCBI/Genbank database using Basic Local Alignment Search Tool (BLAST). The obtained DNA sequences were edited and aligned with Geneious version 7 (Biomatters, USA). Furthermore, a number of DNA sequences was retrieved from GenBank according to the reliable published papers and included in the phylogenetic analyses (Table 1). Bayesian phylogenetic analyses were performed with MrBayes v3.2.2 [23]. Aspergillus montevidensis CBS 119376 was used as outgroup. JModeltest v.2.1.4 [24] and the Bayesian information criterion (BIC) ratio test were applied to select the best fit model. Four Markov chain Monte Carlo (MCMC) analyses were run for 10,000,000 generations with a burn-in fraction of 25%. The resulted trees were viewed in FigTree v1.4.0. Maximum parsimony phylogenies were applied using heuristic searches in PAUP v. 4.0a133 [25].
Sclerotial production
For sclerotia production, conidial suspensions of isolates were inoculated onto 9 cm Petri dishes (three replicate per isolate) containing Czapek agar (CZ) supplemented with 3% NaNO3 and incubated for 2 weeks at 30 °C in darkness [11]. The conidia were washed with 95% ethyl alcohol followed by rinsing with running tap water. The mean diameter of sclerotia was measured using a 10X Zeiss Stereo Microscope connected to a Dino-eye USB lens. Ten sclerotia of each replicate were measured. The isolates were classified as S strain if small (diameter <400 μm) conidia were observed and L strain if large (diameter >400 μm) conidia were observed.
Assessment of Aflatoxin Production with Ammonium Vapor Assay
For Ammonium vapor test, isolates of A. flavus, A. parasiticus, and A. nomius were cultured on coconut agar (COA) medium and incubated at 30 °C for 3 days in the dark according to Saito and Machida [26]. COA was prepared as follows: 100 g of shredded coconut was homogenized for 3 min with 300 ml of hot distilled water and filtered through four layers of cheese cloth, agar was added (1.5%), and autoclaved. To test the color change in the colony reverse side, a drop of 25 % ammonia solution (Merck, Germany) was added to the lid of the dishes while they were upside down. The isolates that their colony reverse turned to pink immediately after exposure to ammonium vapor were recorded as aflatoxin producing, while the isolates with no color change were recorded as non-aflatoxin producing [26, 27]. All isolates were tested in triplicates to confirm results. Based on visual intensity of the pink color, the isolates were grouped in three categories (strong aflatoxin-producing ability, average aflatoxin-producing ability and non-aflatoxin producing).
HPLC Analysis
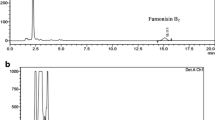
In order to confirm the results of ammonium vapor assay, high-performance liquid chromatography (HPLC) was applied to 20 randomly selected strains. Strains were grown on 9 cm Petri dishes containing Yeast Extract Sucrose (YES) medium [28] and incubated at 28–30 °C for 10 days in the dark. Three plugs of 6 mm diameter were cut from colonies using a cork drill. The plugs were transferred to a 4 ml vial and were extracted with a solution of methanol: water (80:20 v/v) on a mechanical shaker for 1 h. Then, 500 µl of the methanolic extract was diluted with an equal amount of sterile distilled water, filtered through a 0.45 µm cellulose filters, and analyzed by HPLC. Aflatoxin production was determined using Thermo Scientific™ UltiMate™ 3000 HPLC system, the analytical column was C18 (Thermo Scientific) equipped with a Dionex Fluorescence Detector RF-2000. The column was thermostated in 36 °C. The mobile phase was water/methanol/ acetonitrile (65:15:20) with a flow rate of 0.8 ml/min. The excitation and emission wavelengths for detection were 360 nm and 450 nm, respectively. One hundred μl of the preparation was injected into the HPLC apparatus. Aflatoxins were measured by comparing the peak areas with a calibration curves obtained by aflatoxin pure standard solutions (Sigma-Aldrich, Italy).
Data Analysis
All the experiments were done in triplicates. The statistical analyses were performed by SPSS version 16 program for Windows (SPSS Inc. Chicago). Chi-square Test of Independence was used to evaluate the distribution of sclerotial types and aflatoxin production among sources and the correlation of aflatoxin and sclerotia production. P<0.05 was considered statistically significant.
Results
Fungal Species Identification
In total 207 Aspergillus section Flavi strains were isolated over a 2-year study (2018-2019) from 200 samples of four different agricultural products including pistachio, walnut, hazelnut, and dried fruits (mixture of apricot, peach and plum) from local markets in Kerman County, Iran. The species were identified using morphology combined with DNA sequencing data as A. flavus, A. parasiticus, A. tamari, A. pseudotamarii, A. nomius, A. arachidicola, A. caelatus, and A. leporis. The frequently isolated species was A. flavus (75%) followed by A. tamarii (9%), A. nomius (7%), and A. parasiticus (4%) (Fig. 1). In addition, A. pseudotamarii (three isolates) and A. leporis (two isolates) were isolated from dried fruits and A. arachidicola (two isolates), A. caelatus (one isolate) was isolated from hazelnut, and A. transmontanensis (one isolate) was isolated from walnut. In this survey, a number of other Aspergillus species were isolated such as species belonging to sections Nigri, Aspergillus, and Nidulantes which were excluded from the study.
Phylogenetic Analysis
The species identification and genetic relatedness of section Flavi isolates was confirmed through PCR amplification and sequencing of parts of the β-tubulin and calmodulin genes. Obtained sequences were submitted to GenBank under the accession numbers in Table 1. The final concatenated aligned dataset had a total of 1136 characters (558 characters for calmodulin and 578 for β-tubulin) of which 454 were phylogenetically informative.
The General time reversible inverse gamma rates (GTR+G+I) model was determined to be the best for both loci in Bayesian analyses. The topology of the trees generated from Bayesian and maximum parsimony analyses were congruent. The Bayesian tree is presented (Fig. 2). The tree is rooted to A. montevidensis (CBS 119376).
Maximum parsimony phylogeny inferred from a concatenated nucleotide data set (partial β-tubulin and calmodulin) using heuristic searches in PAUP v. 4.0a133. Bootstrap values are presented at the nodes above the branches. The bar indicates the number of substitutions per site. The phylogram is rooted to A. montevidensis (CBS 119376)
Based on our phylogenetic analysis, strains of section Flavi isolated from pistachio, walnut, hazelnut, and dried fruits in Kerman County were identified as nine different species which are resided in four of eight series of Aspergillus section Flavi including Flavi, Kitamyces, Nomiarum and Leporum series. Most isolates belonged to Flavi serie (Fig. 2).
Sclerotia Formation
Sclerotia production was determined in isolates of A. flavus. Isolates were categorized as S strain (average diameter <400 μm), L strain (average diameter >400 μm), and non-sclerotial. In total, 61% of the tested isolates did not produce sclerotia in culture. Of all sclerotial isolates, 23% were S and 77% were L strains (Fig. 3). Sclerotial type was significantly different between isolates from different sources (P < 0.05). None of the tested walnut isolates (24 isolates) produced sclerotia in culture. Twenty-four percent of the dried fruits isolates produced sclerotia all of which were L strains and no S strains were observed. The highest percent of sclerotial isolates (62%) was observed in hazelnut isolates followed by pistachio isolates (48%). However, pistachio isolates had more S strains (18%) compared to hazelnut isolates (9%). The majority of S strains were observed in isolates from pistachio (Fig. 3).
Production of Aflatoxins
The frequently isolated species of Aspergillus section Flavi from pistachio, walnut, hazelnut, and dried fruits including A. flavus, A. nomius, and A. parasiticus were screened for their ability to produce aflatoxins in culture by ammonium vapor method. From 172 tested isolates, the colony reverse side of 71 isolates (41% of all tested isolates) turned pink after exposure to 25% ammonium vapor and were scored as toxigenic. Based on visual intensity of the pink color, 5% (9 isolates) were scored as strong aflatoxin producers and 36% (62 isolates) as average aflatoxin producers, while 59% (101 isolates) had no color change upon exposure to ammonium vapor which were designated as non-aflatoxin producers (Figs. 4 and 5).
Color change in colony reverse side of Aspergillus flavus isolates on coconut agar medium upon exposure to 25% ammonium vapor. Left to right: dark pink (strong aflatoxin-producing strain), pale pink (average aflatoxin-producing strain), and no color change (non-aflatoxin-producing strain) (Color figure online)
Production of Aflatoxins by A. flavus, A. nomius, and A. parasiticus isolates obtained from four different sources determined by ammonium vapor assay separated by source. Isolates evaluated included pistachio (67), walnut (24), hazelnut (40), and dried fruits (41). A significant difference in the population of aflatoxin-producing strains across the isolation sources is observed
In total, the incidence of non-aflatoxin-producing strains was higher (59%) than that of aflatoxin-producing strains. A significant difference in the population of aflatoxin-producing strains across the isolation sources was observed (Fig. 5). Dried fruits had the highest (46%) aflatoxin-producing strains, followed by pistachio (43%), hazelnut (35%), and walnut (8%). Hazelnuts had the highest percentage (8%) of strong aflatoxin-producing isolates, while the most frequent average aflatoxin-producing isolates were dried fruits isolates (38%). The distribution of aflatoxin-producing species was different among the four isolation sources. The population of aflatoxigenic A. nomius was predominant on dried fruits (one strong and 5 average aflatoxin-producing strains out of 8 isolates). HPLC assays were applied to 20 A. flavus strains and showed that the toxigenic status of them was correctly determined by Ammonium vapor assay on COA medium.
Aflatoxin-producing ability of the obtained strains of A. pseudotamarii and A. arachidicola was determined using Ammonium vapor assay. One strain of A. pseudotamarii and two strains of A. arachidicola had color change (pale pink) in ammonium vapor test indicating average aflatoxin-producing ability.
Discussion
The results of this study have provided comprehensive information on identifying structures of Aspergillus section Flavi in four agricultural products, pistachio, walnut, hazelnut, and dried fruits, from market and retail shops in Kerman County, Iran. The occurrence of Aspergillus section Flavi as well as the information on the relationships between isolation source and ability to produce sclerotia and potential for aflatoxin production has been discussed. A. flavus was the most common species found. Seventy-one percent of the isolates belonged to A. flavus followed by A. tamarii (9%) A. nomius (7%), and A. parasiticus (4%). Two aflatoxin-producing species, A. pseudotamarii and A. arachidicola, were isolated in this study from dried apricots and hazelnuts, respectively, and are reported in this study for the first time from these substrates. The occurrence of A. flavus in nuts in our study was comparable to studies of Fani et al. [29] and Houshyarfard et al. [30] in Iran. Although research has been done on determination of aflatoxins in walnut, hazelnut, and dried fruits [12, 13, 31], no specific study has attempted to determine occurrence of Aspergillus section Flavi on these products.
We used concatenated sequences of calmodulin and β-tubulin to confirm morphological identifications and determine the genetic relatedness of section Flavi isolates obtained in this study. The resulting tree topology was in agreement with the latest phylogenies of Aspergillus section Flavi [2]. The obtained isolates were resided in four of eight Series (clades) of Aspergillus section Flavi including Flavi, Kitamyces, Nomiarum, and Leporum series.
Approximately, 39% of A. flavus isolates were able to produce sclerotia in culture. The percent of Sclerotial isolates was lower than those reported on many other crops. Wicklow et al. [32] observed that 98% of the A. flavus from a cornfield in Illinois were sclerotial. Giorni et al. [33] found that sixty-two percent of A. flavus strains isolated from northern Italy were able to produce sclerotia. In addition, Mamo et al. [34] reported that 85% of isolates obtained from different regions of China produced sclerotia.
Regarding sclerotial size, 9% were S strain and 30% were L strains. Our results showing predominance of L strains are in agreement to several similar surveys in the world [11, 35, 36]. Sclerotial type was significantly different between isolates from different sources. All of the isolates obtained from dried fruits were L strains. Pistachio isolates were greater in S strain incidence than hazelnut isolates, while none of the tested dried fruits isolates were of S strain type. Pistachio may have some characteristics that give competitive advantage to S strains which favors their isolation. There are researchers that have related the distribution of L and S strains to the source of isolation. Horn and Dorner [11] reported that cotton growing regions had the highest occurrence of S strains. Abbas et al. [7] have examined the distribution of L and S strains in corn, peanut, rice, and soil and found that isolates from rice had a higher percentage of S strains than other sources. They suggested that A. flavus population is not randomly distributed, instead particular niches differ in strain composition. However, the exact selective forces favoring one type of strain over the other are not understood yet. Identification of the putative factors and mechanisms may be crucial in developing strategies to forecast the incidence of S strains and take action when needed.
Several techniques including high-performance liquid chromatography (HPLC), enzyme-linked immunosorbent assay (ELISA), thin layer chromatography (TLC), and PCR-based methods have been used for detection of aflatoxins production; however, these methods are expensive, time consuming, and labor intensive [37]. We used a cultural method to screen Aspergillus section Flavi strains for their ability to produce aflatoxins. Ammonium vapor assay is a useful method for screening Aflatoxin-producing strains of Aspergillus section Flavi. This method is based on the color change of anthraquinones associated with the aflatoxin biosynthetic pathway which act as pH indicator dyes [38]. The frequency of false negatives and false positives have been reported to be low [26, 29] and it is a suitable technique when large number of strains are tested and the resources are limited [27]. We used COA medium which have higher color change intensity than PDA and YES media [26, 29]. Fani et al. [29] showed Ammonium vapor assay using COA medium was in 100% agreement with TLC in terms of reliability and precision. They reported that the intensity of the color response in ammonium vapor method correlated with the amount of aflatoxin produced by the isolates.
In the present study, 41% of the total tested strains were able to produce aflatoxin in COA medium from which 5% were strong aflatoxin producers and 36% were average aflatoxin producers. The percentages of aflatoxin-producing isolates among Aspergillus section Flavi isolates vary greatly in different surveys. In our results percentages of aflatoxin-producing isolates were lower than those obtained by Jamali et al., Amani et al. and Fani et al. [29, 39, 40] in Iran, all of which had reported more than 60% isolates were aflatoxigenic. Our finding was significant because atoxigenic strains are able to competitively displace naturally occurring aflatoxin-producing strains. Importantly, recent biocontrol technologies are focused on using mixtures of diverse genotypes of atoxigenic strains instead of a single atoxigenic strain [41]. Therefore, the high incidence of naturally occurring atoxigenic strains in the studied products which are adapted to the host is an effective long- term preventive tool established naturally against toxigenic strains especially in walnuts and hazelnuts. All of the obtained atoxigenic isolates which produced L-type sclerotia were selected to be examined as potential biocontrol agents against aflatoxin contamination in future studies.
Few studies have reported aflatoxin production by A. tamari. Goto et al. [42] reported the production of aflatoxins B1 and B2 by A. tamarii isolated from soil collected from a tea field in Japan. Klich et al. [43] also reported aflatoxin production in A. tamarii and reported strong similarities in the aflatoxin biosynthetic pathway genes of A. tamarii and A. flavus. However, these reports are due to misidentification of A. pseudotamarii strains which are known to be aflatoxin producers to A. tamarii strains which are not aflatoxin producers.
In this study, we have obtained A. pseudotamarii and A. arachidicola from dried apricots and hazelnuts respectively. Using ammonium vapor assay, one strain of A. pseudotamarii and two strains of A. arachidicola had color change (pale pink) in ammonium vapor test indicating average aflatoxin-producing ability which is reported in this study for the first time.
The distribution of aflatoxin-producing species was different among the four isolation sources. The highest relative incidence of aflatoxin-producing isolates were detected in dried fruits isolates followed by pistachios isolates which might be explained by the generic nutrient constitution and processing techniques of these substrates that have favored aflatoxigenic species occurrence [44]. Apricot, peach and plum are harvested with high water content and slowly air-dried that may allow fungal growth during the processing stage. Fungal growth and mycotoxin production have been proved to be affected by substrate composition, temperature and pH [45]. Liu et al. [46] found that in addition to lipids as one of most important factors supporting aflatoxigenic isolates, sucrose, glucose, maltose, arginine, glutamic acid, aspartic acid, and zinc significantly support aflatoxin production and fungal growth of A. flavus. Our results are consistent with Abbas et al. [7] who showed that A. flavus populations isolated from corn, rice, peanut and soil in Mississippi Delta were not randomly distributed, but preferentially isolated from particular sources. The high occurrence of toxigenic strains on dried fruits highlight the need to take measurements to monitor post-harvest procedures such as drying techniques and storage conditions of this agricultural product. It would be interesting for future studies to assess the impact of processing practices and storage conditions on fungal community of nuts and dried fruits which are sold in market and retail shops.
We did not observe any relationship between the sclerotial type and aflatoxigenicity. Several studies have tried to relate sclerotia formation to aflatoxigenicity, but their reported results are inconsistent. Various authors have reported positive correlation between aflatoxigenecity and small sclerotia formation [47, 48] while others have found large type sclerotia producers to produce higher amounts of aflatoxins [7, 9, 49]. In the present study, the aflatoxin-producing strains were randomly distributed among S strains, L strains and non-sclerotial strains. Our findings are in agreement with Rodrigues et al. [50] who found that there was no correlation between sclerotia production and aflatoxigenicity in Aspergillus section Flavi originating from Portuguese almonds. In accordance with our results, Okoth et al. [51] could not relate sclerotia formation to the amount of aflatoxins produced both in vivo and in vitro.
The fungal contamination of nuts and dried fruits can occur both during the pre- and post-processing phases. In this study, we documented the occurrence and toxigenicity of A. flavus, A. parasiticus and A. nomius from pistachio samples collected from orchards and market of Kerman which is the major pistachio producing region of Iran. The incidence of the three species in the orchard samples was higher than market samples. This can be explained by the large differences in fruit physiology between orchards and market phases which would provide different host environments for fungal colonization. Temperature, relative humidity and moisture content affect the ability of Aspergillus section Flavi to grow on a substrate [52].
Conclusion
This study provides the first comprehensive investigation of the Aspergillus section Flavi occurrence in four agricultural products, such as pistachio, walnut, hazelnut, and dried fruits (apricot, peach and plum), from market and retail shops in Kerman County, Iran while accessing relationships of isolation source to the ability of key species to produce aflatoxin and sclerotium. Here, we report high contamination of dried fruits with toxigenic strains of Aspergillus section Flavi in market and retail shops in Kerman. Two aflatoxin-producing species, A. pseudotamarii and A. arachidicola, are reported for the first time from dried apricots and Hazelnuts, respectively. These data highlight the need to take proper management practices during pre- and post-harvest in agricultural products to reduce the risk of infestation with toxigenic molds. The potential of atoxigenic L-type isolates obtained in this study as a management tool for biocontrol of aflatoxin contamination will be examined in future work.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Perrone G, Susca A, Cozzi G, Ehrlich K, Varga J, Frisvad JC, Meijer M, Noonim P, Mahakarnchanakul W, Samson RA (2007) Biodiversity of Aspergillus species in some important agricultural products. Stud Mycol 59:53–66. https://doi.org/10.3114/sim.2007.59.07
Houbraken J, Kocsubé S, Visagie C, Yilmaz N, Wang X-C, Meijer M, Kraak B, Hubka V, Bensch K, Samson RF, JC. (2020) Classification of Aspergillus, Penicillium, Talaromyces and related genera (Eurotiales): an overview of families, genera, subgenera, sections, series and species. Stud Mycol 95:5–169. https://doi.org/10.1016/j.simyco.2020.05.002
Okoth S, De Boevre M, Vidal A, Diana Di Mavungu JL, Schoot S, Kyallo M, Njuguna J, Harvey J, De Saeger S (2018) Genetic and toxigenic variability within Aspergillus flavus population isolated from maize in two diverse environments in Kenya. Front Microbiol 9:57. https://doi.org/10.3389/fmicb.2018.00057
Agbetiameh D, Ortega-Beltran A, Awuah RT, Atehnkeng J, Elzein A, Cotty PJ, Bandyopadhyay R (2020) Field efficacy of two atoxigenic biocontrol products for mitigation of aflatoxin contamination in maize and groundnut in Ghana. Biol Control 150:104351. https://doi.org/10.1016/j.biocontrol.2020.104351
Aikore MOS, Ortega-Beltran A, Eruvbetine D, Atehnkeng J, Falade TDO, Cotty PJ, Bandyopadhyay R (2019) Performance of broilers fed with maize colonized by either toxigenic or atoxigenic strains of Aspergillus flavus with and without an aflatoxin-sequestering agent. Toxins 11(10):565. https://doi.org/10.3390/toxins11100565
Bandyopadhyay R, Atehnkeng J, Ortega-Beltran A, Akande A, Falade TDO, Cotty PJ (2019) “Ground-Truthing” efficacy of biological control for aflatoxin mitigation in farmers’ fields in Nigeria: from field trials to commercial usage, a 10-year study. Front Microbiol 10:2528. https://doi.org/10.3389/fmicb.2019.02528
Abbas HK, Weaver MA, Zablotowicz RM, Horn BW, Shier WT (2005) Relationships between aflatoxin production and sclerotia formation among isolates of Aspergillus section Flavi from the Mississippi Delta. Eur J Plant Pathol 112:283–287. https://doi.org/10.1007/s10658-004-4888-8
Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Allameh A, Kazeroon-Shiri A, Ranjbar-Bahadori S, Mirzahoseini H, Rezaee MB (2006) A survey on distribution of Aspergillus section Flavi in corn field soils in Iran: population patterns based on aflatoxins, cyclopiazonic acid and sclerotia production. Mycopathologia 161(3):183–192. https://doi.org/10.1007/s11046-005-0242-8
Novas MV, Cabral D (2002) Association of mycotoxin and sclerotia production with compatibility groups in Aspergillus flavus from peanut in Argentina. Plant dis 86(3):215–219. https://doi.org/10.1094/PDIS.2002.86.3.215
Acur A, Arias RS, Odongo S, Tuhaise S, Ssekandi J, Muhanguzi D, Kiggundu A (2020) Genetic diversity of aflatoxin-producing Aspergillus flavus isolated from groundnuts in selected agro-ecological zones of Uganda. BMC Microbiol 20:252. https://doi.org/10.1186/s12866-020-01924-2
Horn BW, Dorner JW (1998) Soil populations of Aspergillus species from section Flavi along a transect through the peanut-growing regionsof the United States. Mycologia 90:767–776. https://doi.org/10.1080/00275514.1998.12026969
Amiri MJ, Karami M, Sadeghi E (2013) Determination of AFB1 in peanut, almond, walnut, and hazelnut in kermanshah markets, Iran. Int J Agri Crop Sci 6(17):1199–1202
Heshmati A, Zohrevand T, Khaneghah AM, Nejad ASM, Sant’Ana AS, (2017) Co-occurrence of aflatoxins and ochratoxin A in dried fruits in Iran: Dietary exposure risk assessment. Food Chem Toxicol 106:202–208. https://doi.org/10.1016/j.fct.2017.05.046
Ait Mimoune N, Arroyo-Manzanares N, Gámiz-Gracia L, García-Campaña AM, Bouti K, Sabaou N, Riba A (2018) Aspergillus section Flavi and aflatoxins in dried figs and nuts in Algeria. Food Addit Contam Part B Surveill 11:119–125. https://doi.org/10.1080/19393210.2018.1438524
Pitt JI, Hocking AD (2009) Fungi and food spoilage, 3rd edn. Springer, New York
King AD, Hocking AD, Pitt JI (1979) Dichloran-rose bengal medium for enumeration and isolation of molds from foods. Appl Environ Microbiol 37(5):959–964. https://doi.org/10.1128/aem.37.5.959-964.1979
Pitt JI, Hocking AD, Glenn DR (1983) An improved medium for the detection of Aspergillus flavus and Aspergillus parasiticus. J Appl Bacteriol 54:109–114. https://doi.org/10.1111/j.1365-2672.1983.tb01307.x
Pitt JI (1979) The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces. Academic Press Inc, London
Klich MA (2002) Identification of common Aspergillus species. Centraalbureau voor Schimmelcultures, Utrecht
Samson RA, Visagie CM, Houbraken J, Hong SB, Hubka V, Klaassen CHW, Perrone G, Seifert KA, Susca A, Tanney JB, Varga J, Szigeti KS, G, Yaguch T, Frisvad JC, (2014) Phylogeny, identification and nomenclature of the genus Aspergillus. Stud Mycol 78:141–173. https://doi.org/10.1016/j.simyco.2014.07.004
Hong SB, Cho HS, Shin HD, Frisvad JC, Samson RA (2006) Novel Neosartorya species isolated from soil in Korea. Int J Syst Evol 56:477–486. https://doi.org/10.1099/ijs.0.63980-0
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61:1323–1330. https://doi.org/10.1128/AEM.61.4.1323-1330.1995
Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. https://doi.org/10.1093/sysbio/sys029
Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25:1253–1256. https://doi.org/10.1093/molbev/msn083
Swofford DL (2002) PAUP*: phylogenetic analysis using parsimony (*and other methods). Sinauer Associates, Sunderland
Saito M, Machida S (1999) A rapid identification method for aflatoxin-producing strains of Aspergillus flavus and A. parasiticus by ammonia vapor. Mycoscience 40:205–208. https://doi.org/10.1007/BF02464300
Abbas HK, Zablotowicz RM, Weaver MA, Horn BW, Xie W, Shier WT (2004) Comparison of cultural and analytical methods for determination of aflatoxin production by Mississippi Delta Aspergillus isolates. Can J Microbiol 50:193–199. https://doi.org/10.1139/w04-006
Davis ND, Diener UL, Eldridge DW (1966) Production of Aflatoxins B1 and G1 by Aspergillus flavus in a semisynthetic medium. Appl Environ Microbiol 14:378–380. https://doi.org/10.1128/AEM.14.3.378-380.1966
Fani SR, Moradi M, Probst C, Zamanizadeh HR, Mirabolfathy M, Haidukowski M, Logrieco AF (2014) A critical evaluation of cultural methods for the identification of atoxigenic Aspergillus flavus isolates for aflatoxin mitigation in pistachio orchards of Iran. Eur J Plant Pathol 140:631–642. https://doi.org/10.1007/s10658-014-0499-1
Houshyarfard M, Rouhani H, Falahati-Rastegar M, Malekzadeh-Shafaroudi S, Mehdikhani-Moghaddam E, Probst C (2014) Characterization of Aspergillus section Flavi from pistachio soils in Iran. J Plant Prot Res. https://doi.org/10.2478/jppr-2014-0053
Ostadrahimi A, Ashrafnejad F, Kazemi A, Sargheini N, Mahdavi R, Farshchian M et al (2014) Aflatoxin in raw and salt-roasted nuts (pistachios, peanuts and walnuts) sold in markets of Tabriz. Iran Jundishapur J Microbiol 7(1):8674. https://doi.org/10.5812/jjm.8674
Wicklow DT, Mcalpin CE, Platis CE (1998) Characterization of the Aspergillus flavus population within an Illinois maize field. Mycol Res 102:263–268. https://doi.org/10.1017/S0953756297004851
Giorni P, Magan N, Pietri A, Bertuzzi T, Battilani P (2007) Studies on Aspergillus section Flavi isolated from maize in northern Italy. Int J food Microbiol 113:330–338. https://doi.org/10.1016/j.ijfoodmicro.2006.09.007
Mamo FT, Shang B, Selvaraj JN, Wang Y, Liu Y (2018) Isolation and characterization of Aspergillus flavus strains in China. J Microbiol 56(2):119–127. https://doi.org/10.1007/s12275-018-7144-1
Perrone G, Haidukowski M, Stea G, Epifani F, Bandyopadhyay R, Leslie JF, Logrieco A (2014) Population structure and Aflatoxin production by Aspergillus Sect. Flavi from maize. Food Microbiol 41:52–59. https://doi.org/10.1016/j.fm.2013.12.005
Okun DO, Khamis FM, Muluvi GM, Ngeranwa JJ, Ombura FO, Yongo MO, Kenya EU (2015) Distribution of indigenous strains of atoxigenic and toxigenic Aspergillus flavus and Aspergillus parasiticus in maize and peanuts agro-ecological zones of Kenya. Agric Food Secur 4(1):14. https://doi.org/10.1186/s40066-015-0033-5
Matabaro E, Ishimwe N, Uwimbabazi E, Lee BH (2017) Current immunoassay methods for the rapid detection of aflatoxin in milk and dairy products. Compr Rev Food Sci Food Saf 16(5):808–820. https://doi.org/10.1111/1541-4337.12287
Shier WT, Lao Y, Steele TW, Abbas HK (2005) Yellow pigments used in rapid identification of aflatoxin-producing Aspergillus strain are anthraquinones associated with the aflatoxin biosynthetic pathway. Bioorg Chem 33:426–438. https://doi.org/10.1016/j.bioorg.2005.09.002
Jamali M, Ebrahimi MA, Karimipour M, Shams-Ghahfarokhi M, Dinparast-Djadid N, Kalantari S, Pilehvar-Soltanahmadi Y, Amani A, Razzaghi-Abyaneh M (2012) An insight into the distribution, genetic diversity, and mycotoxin production of Aspergillus section Flavi in soils of pistachio orchards. Folia Microbiol 57(1):27–36. https://doi.org/10.1007/s12223-011-0090-5
Amani S, Shams-Ghahfarokhi M, Banasaz M, Razzaghi-Abyaneh M (2012) Mycotoxin-producing ability and chemotype diversity of Aspergillus section Flavi from soils of peanut-growing regions in Iran. Indian J Microbiol 52:551–556. https://doi.org/10.1007/s12088-012-0275-x
Ortega-Beltran A, Moral J, Picot A, Puckett RD, Cotty PJ, Michailides TJ (2019) Atoxigenic Aspergillus flavus isolates endemic to almond, fig, and pistachio orchards in California with potential to reduce aflatoxin contamination in these crops. Plant Dis 103(5):905–912. https://doi.org/10.1094/PDIS-08-18-1333-RE
Goto T, Wicklow DT, Ito Y (1996) Aflatoxin and cyclopiazonic acid production by a sclerotium-producing Aspergillus tamarii strain. Appl Environ Microbiol 62(11):4036–4038
Klich M, Mullaney E, Daly C, Cary J (2000) Molecular and physiological aspects of aflatoxin and sterigmatocystin biosynthesis by Aspergillus tamarii and A. ochraceoroseus. Appl Microbiol Biotechnol 53(5):605–609. https://doi.org/10.1007/s002530051664
Kheiralla ZH, Hassanin NI, Amra H (1992) Effect of incubation time, temperature and substrate on growth and aflatoxin production. Int Biodeterior Biodegrad 30(1):17–27. https://doi.org/10.1016/0964-8305(92)90021-F
Magan N, Aldred D (2007) Why do fungi produce mycotoxins? In: Dijksterhuis J, RA, Samson, (eds) Food mycology: a multifaceted approach to fungi and food. CRC Press, Boca Raton, FL, pp 121–133
Liu J, Sun L, Zhang N, Zhang J, Guo J, Li C, Rajput SA, Qi D (2016) Effects of nutrients in substrates of different grains on aflatoxin B1 production by Aspergillus flavus. Biomed Res Int. https://doi.org/10.1155/2016/7232858
Chang PK, Bennett JW, Cotty PJ (2001) Association of aflatoxin biosynthesis and sclerotial development in Aspergillus parasiticus. Mycopathologia 153:41–48. https://doi.org/10.1023/a:1015211915310
Hua SST, McAlpin CE, Chang PK, Sarreal SBL (2012) Characterization of aflatoxigenic and non-aflatoxigenic Aspergillus flavus isolates from pistachio. Mycotoxin Res 28(1):67–75. https://doi.org/10.1007/s12550-011-0117-4
Astoreca A, Dalcero A, Pinto VF, Vaamonde G (2011) A survey on distribution and toxigenicity of Aspergillus section Flavi in poultry feeds. Int J food Microbiol 146(1):38–43. https://doi.org/10.1016/j.ijfoodmicro.2011.01.034
Rodrigues P, Venâncio A, Kozakiewicz Z, Lima N (2009) A polyphasic approach to the identification of aflatoxigenic and non-aflatoxigenic strains of Aspergillus section Flavi isolated from Portuguese almonds. Int J Food Microbiol 129(2):187–193. https://doi.org/10.1016/j.ijfoodmicro.2008.11.023
Okoth S, Nyongesa B, Joutsjoki V, Korhonen H, Ayugi V (2016) Sclerotia formation and toxin production in large sclerotial Aspergillus flavus isolates from Kenya. Adv Microbiol 06(01):47–56. https://doi.org/10.4236/aim.2016.61005
Norlia M, Jinap S, Nor-Khaizura M, Radu S, Samsudin NIP, Farah FA (2019) Aspergillus section Flavi and aflatoxins: occurrence, detection, and identification in raw peanuts and peanut-based products along the supply chain. Front Microbiol 10:2602. https://doi.org/10.3389/fmicb.2019.02602
Funding
This work was supported by the Iran National Science Foundation (INSF) under grant number 96000908 and the Institute of Science and High Technology and Environmental Sciences, Graduate University of Advanced Technology, Kerman, Iran, under Grant No. 97/532.
Author information
Authors and Affiliations
Contributions
The study concept and design were performed by AH and DA; The experiments, data analysis, and the manuscript drafting were performed by AH; the funds were collected by AH; all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest regarding the publication of this paper.
Consent to Participate
The authors have agreed to be listed and approved the submitted version of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Habibi, A., Afzali, D. Aspergillus Section Flavi from Four Agricultural Products and Association of Mycotoxin and Sclerotia Production with Isolation Source. Curr Microbiol 78, 3674–3685 (2021). https://doi.org/10.1007/s00284-021-02620-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-021-02620-8