Abstract
The gut microbiota has long been of research interests due to its nutritional importance for many insects. It has been demonstrated that diversity of gut microbiota in insects can be modulated by many factors, including habitats, feeding preference, etc. Besides, the community structure of gut microbiota could also be altered during the different life stages of host insects. With development of conventional culture-dependent technologies and advanced culture-independent technologies, comprehensive and deep understanding of the functions of gut microbiota and their relationship with host insects were achieved, especially for the nutrient metabolic process mediated by them. In this review, we summarized the gut microbiota composition, major methods for gut microbiota characterization, and vital nutrient metabolic process mediated by gut microbiota in different insects. The increasing knowledge on the modulation of gut microbiota will help us for the comprehension of the contribution of gut microbiota to the nutritional metabolism of insects, prompting their growth and health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The research of gut microbiota is important for in-depth understanding of relationships between the gut microbiota and its host. Most studies on gut microbiota were performed in mammals, especially in human. However, gut microbiota also exists in insects, functioning on conveying beneficial nutrition and protection to their hosts. In fact, considering the potential disease control, pollination of many food crops, and mediators of biogeochemical cycling (such as nitrogen cycle) for insects in medicine, agriculture and ecology, the intricate relationship of gut microbiota with insect host, such as termites (Isoptera), cockroaches (blatta), bees (Anthophila), black solider fly (Hermetia illucens) etc., has been catching great interests in recent years [1, 2]. From an ecological perspective, gut microbiota play vital roles in the co-evolution of insect symbiotic interactions mediated by secondary metabolites [3, 4]. In agreement, gut microbiota also play crucial roles in the life cycles of insect, including insect growth, development, reproduction, and inevitable damage in the process of metabolism [5,6,7,8,9,10]. The increasing knowledge on the composition and functions of gut microbiota in insects and the link between a balanced gut microbiota and insects could prompt the use of gut microorganisms for insect growth, thus benefiting the environment and further applications on human health.
Considering the unique roles of gut microbiota in insects, this review will mainly encompass features of gut microbiota from insects (composition, diversity and integrity), methods to study gut microbiota, and potential applications of insect gut microbiome. We believe this will prompt the further comprehension of nutrition-transformation relationship of endosymbiont gut microbiota community with their hosts and shed light of a vision of how this knowledge may lead to novel nutrient control strategies.
Features of Gut Microbiota from Insect
Composition of Gut Microbiota in Insects
The insect gut is separated into three major parts, including anterior midgut, posterior midgut, and hindgut. The composition of gut microbiota may be interfered by several factors, including insect development, biochemical conditions in different intestinal regions [11, 12], and the insect ability of acquiring available resources [13]. Among these parts, hindgut of insects, as extension part of the body cavity, contains food waste. As a consequence, it provides gut microbiota with abundant nutrient environment, thus prompting their growth and diversity [14, 15].
Gut microbiota in insect include protists, fungi, archaea and bacteria. In the lower termites, protists occupy more than 90% portion of the hindgut. For example, bacteria and archaea are found in the guts of both lower and higher termites [16]. Researchers reported that the guts of honey bee (Apis mellifera) adult workers are dominated by a distinctive set of nine bacterial species (Five of these, Snodgrassella alvi, Gilliamella apicola, two species of Lactobacillus, and a Bifidobacterium species) [17]. Research also showed that gut microbiota was rarely in direct contact with intestinal epithelial cells due to their special localization. Normally, bacteria residing within the gut appear to be localized to the lumen within the endoperitrophic space, which is delimited by the peritrophic matrix, a chitinous barrier that lines the midgut [18]. It was reported that the insect gut microbiota were dominated by Proteobacteria (62.1% of the total reads, including 14.1% Wolbachia sequences) and Firmicutes (20.7%) [19].
Diversity of Insect Gut Symbionts
The highest ratios of total gut microbial biomass to host mass are found in some litter and wood-feeders, including termites, crickets, and cockroaches. However, insects such as Drosophila, mosquitoes and aphids, contain relatively small communities in relation to host body mass [1, 20]. This may be resulted from highly compartmentalized guts in litter and wood-feeders. The most commonly studied gut bacterial communities in insects are those groups feeding on wood, decaying matter, or detritus, such as termites, cockroaches, crickets, and some beetles [21]. Bacteria and archaea are found in the guts of both lower and higher termites. The most frequently found archaea are methanogens, which are tolerant of hypoxia environment as well as some gut protists such as Dinenympha parva and spirotrichonympha leidyi [22]. The dominant gut microbiota in different insect hosts that feed on different foods, including lower termites, higher termites, cockroaches, crickets, beetles, black soldier fly, drosophila, house fly, bees, butterfly and wasps, were summarized (Table 1). As well known, abundant lignocellulose exits in wood. Although lower and higher termites both feed on wood, the degradative process differed from higher to lower termites. And quite possibly, lignocellulose digestion is conducted by different microbiota in lower and higher termites. In lower termites, this process is mostly accomplished by bacteria, while mostly by protists in higher termites. In fact, research indicated that endoglucanases and β-glucosidases secreted by protists in higher termites might be responsible for the lignocellulose digestion [16]. Different from termites, beetles mostly feed on grass, thus leading to the occupation of Hypocreales, Streptomyces, Firmicutes and some proteobacteria in the gut of beetles [23, 24]. Also, cockroaches possess diverse gut microorganisms because they can feed on feces, utilizing complicated components in the feces.
Bacteria diversity was significantly higher in the guts of insects characterized as omnivores than in those of carnivores or herbivores [19]. Hence, feeding preference of host is a vital factor which impacts the diversity of gut microbiota in the insects. Except some obvious factors such as feeding preference of host, diversity of gut microbiota is also associated with the environmental habitat of the host. The environmental habitat could be divided into sky, ground, underground, and aquatic. Due to the difference of oxygen levels in different habitats, aerobes were more abundant in the gut of the terrestrial insects (sky, ground and underground) than in those of the aquatic insects. Also, the abundance of anaerobes was significantly higher in the guts of aquatic insects than those of terrestrial insects [19]. The abundance variation of anaerobes was most likely related to the levels of oxygen in the different environmental habitats, thus impacting the community diversity of the insect gut microbiota.
16S rRNA sequence is still the widely used standard for classifying gut microbiota. However, although massive amounts of 16S rRNA databases, such as Genomes OnLine Database (GOLD), SILVA, GreenGenes (GG), and the Ribosomal Database Project (RDP), have been developed for taxonomy predictions and annotations [25, 26], classification of the taxonomic in the gut microbiota are limited by the taxonomic depth and the reference databases. More and more researchers refer the SILVA as the best suited database since it contains the largest number of sequences and it is regularly updated. With these powerful datasets, it’s convenient for exploring the gut community structure of insects.
Other than these, beta diversity that incorporates additional information to variation in different species or samples, is now being used by many researchers to describe the relative abundances of species [27]. For instance, age-related changes in the composition of Drosophila gut microbiota were studies, revealing an increase in the relative abundance of Proteobacteria and a decrease in the proportion of Firmicutes [28, 29]. Another example, 16S rRNA sequencing results of Hermetia illucens showed strong difference between the soybean (SD) and insect diet (ID) groups in both type and relative abundance (beta diversity) of microbial species [30]. In particular, Bacteroides plebeius, Elusimicrobium minutum, Alkaliphilus transvaalensis, Christensenella minuta, Vallitalea guaymasensis and Flavonifractor plautii represented the principal contributors of changes in gut microbiota composition of ID group [30]. These application instances demonstrated that beta diversity is a significant criterion revealing the relative abundances of species.
Change of Gut Microbiota During the Different Life Stages of Hosts
Another interesting finding is that the community structure of gut microbiota in hosts could be changed along with the growth of host. For example, Lactobacillus and Bifidobacterium populations decreased significantly along with the aging of short-lived (worker) honey bees, while other bacteria, such as Proteobacteria, Bartonella apis and Acetobacteraceae increased, and consistent with a suite of host senescence markers. In contrast, long-lived (queen) honey bees maintained youthful cellular function with accumulated Lactobacillus firm5, Lactobacillus kunkeei and Bifidobacterium spp., and decreased Proteobacteria, Parasaccharibacterapium and Acetobacteraceae 2.1 [31, 32]. Along with the increased oxidative damage, gram-positive bacteria decrease in workers but increase in queen [31, 33].
In addition, high-throughput sequencing of the gut microbiota also indicated the similar results. Composition of gut bacterial community with variation in V4 region of 16S rRNA gene of Chrysomya megacephala under different ages (eggs, 1-day-old larvae, 5-day-old larvae, pupae, adult females and males) were compared using Illumina MiSeq technology [31, 34]. Their results suggested that gut bacteria in C. megacephala varied across life stages, in which Alphaproteobacteria, Bacilli, Bacteroidia, Betaproteobacteria, Flavobacteriia and Gammaproteobacteria represented the majority in C. megacephala. Eight species were identified to have significantly different abundance between 1-day larvae and 5-day larvae and took 28.95% of shared species between these two groups. Seven of the identified species decreased from 1-day-Larvae to 5-day-Larvae groups, only one species Pseudoclavibacter bifida increased [34]. Gut microbiota community in these honey bees or flies both showed some difference at detailed taxonomic levels across the life stages. Whether larva insect or adult insect, they might share some of core harbored gut bacteria results from overlap of food range.
Gut microbiota in hosts likely originate from insect-vectored plant and animal pathogens or from direct insect pathogens. During the different life stages of insect hosts, these pathogens often also require entry to host cell to complete life cycles (Origin, birth, middle age, old age, death and rebirth), resulting in interplay between hosts, pathogens and host-beneficial gut microbiota [35].
Methods for Exploring Gut Microbiota
Depending on the source of the microbial samples, approaches to determine microorganism taxonomy of insect gut microbiota can be classified into culture-dependent or culture-independent assays (Table 2). Culture-dependent assays focused on enriching previously determined component organisms, which may be used for further characterization. In comparison, culture-independent assays are strongly recommended for the initial taxonomic profiling of microbial communities as many species may not be easily cultured [36].
Culture-Dependent Assays
Studying the composition of gut microbiota helps understand their functions and relationship with the hosts. Normally, microbial characterization is the first step for systemic studies of the gut microbiota [37]. Conventional methods were normally performed by experimental culturing followed up by phenotype characterization using morphological and biochemical characteristics. Notably, the culture-dependent assay encompasses many advantages, such as the availability of pure cultures of bacteria, the ability to study living bacteria for specific functions, and the detection of specific intestinal pathogens [38].
Culture-Independent Assays
The major defects of culture-dependent assay are that many bacteria could not be cultured under laboratory conditions, and the symbiotic relationship among different bacteria is difficult to be studied. Along with the fast development of molecular biotechnologies, culture-independent assays for analyzing the gut microbiota composition have been largely developed, mainly including PCR-based methods, fluorescent in situ hybridization (FISH), flow cytometry, DNA sequencing, etc. Among these PCR-based methods, the quantitative PCR (Q-PCR) is a major representative approach. For example, using PCR primers targeting 16S rRNA genes of the gut microbiota could amplify the 16S rRNA sequences from all gut bacteria. Combining with the subsequent quantification steps using fluorophores or DNA binding dyes, the composition of the gut microbiota could be deciphered. Using this technology, the gut microbiota of honeybee were analyzed and assessed changes in the abundance of major bacterial groups of the honeybee core microbiota [39]. Since Q-PCR assays do not require time-consuming post-PCR procedures, such as gel electrophoresis and staining, effective results could be obtained with reduced contaminations in contrast to traditional PCR analysis [37].
Moreover, non-PCR-based methods, such as FISH and flow cytometry, were also used to aid the exploration of microbial diversity in insects. Fluorescein-labeled oligonucleotide probes targeting 16S rRNA genes are frequently used for FISH analysis. Through hybridization, bacterial cells associated with gut tissues or feces are labeled with a fluorescent probe and can be analyzed by flow cytometry [40]. Recently, due to the fast development of next generation sequencing (NGS) technology in biological research, it became a very powerful method to characterize the diversity of gut microbiota in insects based on its high efficiency and accuracy. The NGS technologies have been applied largely in metatranscriptomics and metagenomics. In recent research, metagenomic and metatranscriptomics technologies were applied for illuminating the diversity of lignocellulolytic enzymes within the termite gut [41]. Metagenomic studies make it possible to explore the potential functions of the specific microorganisms within the gut communities [42]. Besides, it’s also of great significance for investigating key metabolites associated with host-gut microbiota interaction. Moreover, Mass Spectrometry-based metabolomics approaches further facilitated the exploration of the metabolic networks between the hosts and gut microbiota.
Culture-dependent and culture-independent methods have different strength (Table 2). Therefore, new methods that integrated both strategies were also developed to evaluate the effect of gene, protein and metabolite on the composition and function of gut microbiota. For instance, Ogué-Bon, etc., reported that stirred, pH-controlled anaerobic batch cultures in combination with FISH assay could be used to investigate the in vitro effects of galacto-oligosaccharide alone or combined with the probiotic Bifidobacterium bifidum 02 450B on fecal microbiota [43]. Additionally, Lin et al. reported that metabolic capacities of the gut microbiota in a fungus-cultivating Termite through in vitro assays of colonic fermentation with in vitro assays of DGGE and metagenomics [44].
Nutrient Metabolic Process Mediated by Insect Gut Microbiota
Protein Hydrolysis, Sugar Fermentation and Amino Acid Metabolism
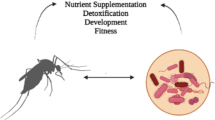
Uptake systems and catabolism of proteins/amino acids in gut microbiota bacteria are vital metabolic process, which benefit both gut microbiota and host insects (Fig. 1). Many microbial species such as Clostridium spp., Bacteroides spp., Lactobacillus spp. etc., in gut microbiota contain varieties of proteases involved in catabolism of proteins. In the well characterized proteolytic system of Lactic acid bacteria (LAB), proteinase, peptide transporters and peptidases function cooperatively. In general, protein hydrolysis by LAB is initiated by a cell-envelope proteinase (CEP) that degrades the protein into oligopeptides, which are subsequently taken up by the cells via specific peptide transporters, and further degraded by various intracellular peptidases into shorter peptides and amino acids [45, 46].
As one major source of energy, sugar fermentation is another important metabolic process catalyzed by gut microbiota. It has been reported that LAB acquired the ability to recognize sugars, including xylose, cellobiose, glucose, and fructose [46, 47], thus playing essential roles in Lactic fermentation [45]. Through protein degradation and sugar fermentation, symbiotic gut microbes could provide essential amino acids (EAAs) to their host insects. Birkle et al. reported that the symbiotic bacteria Buchnera could provide tryptophan and other essential amino acids to their aphid hosts due to shortage of EAAs in the food ingredients of aphid [48]. Additionally, gut transcriptomic studies of A. glabripennis revealed the presence of complete bacterial and fungal biosynthetic pathways for several EAAs [49]. The potential mechanism of EAAs synthesis is that nitrogen is recycled and fixed as the form of NH4+, which is then routed into end-products, such as EAAs and non-EAAs [50]. Since wood is a poor resource of nitrogen, this is very important to wood-feeding insects because they are unable to synthesize EAAs de novo.
Lipid Metabolism
Lipid metabolites produced in gut microbes is a source of carbon and energy storage for host insects. Extensive microbial fermentation of polysaccharides exists in the hindgut of insects, in which large amounts of short-chain fatty acids (SCFAs) are produced. Zheng et al. found that microbial metabolism markedly reduces the pH and redox potential in gut through the production of SCFAs. In the hindgut of honey bee, SCFAs including acetate, propionate, and butyrate are main metabolites produced by the resident gut bacteria, which affects its weight [51]. Other than SCFAs, polyhydroxyalkanoates (PHAs) are also a class of important lipid metabolites produced by gut microbes in insects. For instance, PHAs can be transformed into carbon and energy storage by gut microbes in yellow mealworm [52]. Moreover, some new aminoglycolipids were identified from a Deinococcus sp. strain isolated from the gut of queen carpenter ants (Camponotus japonicus), among which four new aminoglycolipids, annotated as deinococcucins A-D, showed functional ability of inducing the quinone reductase production in host cells [53].
Cellulose Digestion and Nitrogen Fixation
Insects which feed on woods usually possess gut bacteria with cellulose digestion ability. For example, Bombyx mori that feeds on mulberry leaves mainly depends on the digestive enzymes produced by its gut bacteria to degrade carbohydrates, such as pectin, xylan, cellulose and starch [54]. Under normal condition, these gut bacteria provide digestive enzymes in a synergic manner and contribute to the larval growth. Similar cases exist in phytophagous and xylophagous insects, which are the most efficient ecosystems to degrade lignocellulose. Studies of gut microbiota of Ergates faber (beetle), Potosia cuprea (chafer), Gromphadorrhina portentosa (cockroach), Locusta migratoria (locust), and Gryllus bimaculatus (cricket) in anaerobic batch reactors indicated that many gut microbiota possess the degradation ability of lignocellulose [55]. Another example is termites, which was recently aimed for converting wood into biofuels due to their strong cellulose degradation ability by their gut microbiota. Degradations of cellulose into hexose and pentose oligomers, and a series of biofuel derivatives are catalyzed by a number of macromolecular complexes, which result from synergism of a number of microbial communities in gut [56].
Other than cellulose digestion, studies to date reported that symbiotic nitrogen fixation was also vital metabolic process for insect nutrition and development. It was identified that the nitrogen nutrition of termites was mainly obtained from their symbiotic gut microbes instead of food [57,58,59]. Interestingly, the nitrogen fixation ability of the gut microbes is determined by termite feeding. Termites that feed on soil generally do not have strong nitrogen fixation ability, while termites that mainly feed on wood have more intestinal bacteria (Citrobacter freundii and Enterobacter agglomerans) with the pronounced biological activities of nitrogen fixation [60]. Similarly, studies also showed that Spirochetes from gut microbiota of termites played important roles in providing carbon, nitrogen, and energy requirements of termite nutrition via acetogenesis and nitrogen fixation [20]. As the symbiotic nitrogen fixation rates were negatively correlated with dietary N, it was suggested that high concentrations of dietary N suppressed symbiotic nitrogen fixation in termites [61]. Also, gut microbiota of dung beetles feed on dung particles with different C/N ratio were characterized and showed that functional gene abundances in larval and adult gut microbiota would reflect differences in their capacities for cellulose digestion and nitrogen metabolism [62].
Vitamin Production
Another benefit from gut microbes is that they can deliver useful metabolites to their host, such as vitamins. A synthetic cycle includes that gut microbiota fix nitrogen into ammonia, which is then assimilated by gut endosymbionts to re-biosynthesize vitamins for insect development [1, 21, 54, 63]. Due to the water-soluble property of B vitamins, they are the major ones produced by gut microbiota in insects. Salem et al. analyzed genes involved in processing of B vitamins through comparative transcriptomic assay. They revealed the differential expression of genes related to the transport and processing of B vitamins, thereby highlighting an important interface for the exchange of symbiont-provided nutritional supplements [64]. Vitamin B deficiency in symbiont-free insects presents that symbiotic gut microbiota undoubtedly play vital role in the supplement of B vitamins for host insects. In addition, research also reported that Wigglesworthia, the mycetocyte symbiont of Glossina brevipalpis requires many vitamins, such as pantothenate (vitamin B5), biotin (vitaminB7), thiamin (vitamin B1), riboflavin and FAD (vitamin B2), pyridoxine (vitamin B6), nicotinamide (vitamin B3) and folate (vitamin B9), as cofactors for its own metabolism [65].
Concluding Remarks
The taxonomic diversity of insects and differences in their habitat and feeding preference cause the diversity of their gut microbiota. At the same time, difference of gut structure also shapes the gut microbiota of insects. The different insect species, such as termites (Isoptera), cockroaches (blatta), bees (Anthophila), black solider fly (Hermetia illucens) etc., encompass different gut microbiota composition due to the habitat and feeding preference of their insect hosts. As a major interface between the host insect and microbes, the gut provides an environment for growth of microbes, which reversely benefit their host insects through providing amino acids, lipid metabolites (SCFAs, PHAs etc.), nitrogen fixation, vitamins, etc.
To dissect the diversity of gut microbiota and their functions in nutritional metabolisms in gut microbiota of insects, culture-dependent methods and culture-independent methods were integrated to analysis. Molecular technologies from the prevailed 16S rRNA profiling of gut communities to newly emerged metagenomic and NGS technologies provide more and more evidence that gut microbiota is critical to the nutritional metabolic process in insects. The increasing knowledge on the composition and functions of gut microbiota in insects and the link between a balanced gut microbiota and insects could prompt the use of gut microorganisms for insect growth, thus benefiting the environment and further application on human health.
Research on functions of gut microbiota in nutritional metabolisms facilitate in-depth understanding of gut microorganisms for insect growth. Under this circumstance, studying the functional genomics of a symbiotic community were focused to profile the symbiotic mechanism of insect gut microbiota. Recently, Frances Blow et al. identified a specialized gut microbiota dominated by the obligate symbiont “Candidatus Erwinia dacicola” in the olive fruit fly Bactrocera oleae. Candidatus, which could supplement dietary nitrogen to the host [66]. Based on this study, they also reconstructed a number of pathways related to nitrogen assimilation within the host. In addition, along with the microbiota identification, functional redundancy between different microbial taxa was observed for genes involved in urea hydrolysis. These studies all indicated that exploring the nutritional metabolism in the insect gut microbiota symbiosis could further understand the symbiotic mechanism of insect gut microbiota. It could be expected that with further understanding the symbiotic relationship between insect and gut microbiota, engineered microbes might be developed to change the insect gut environment to facilitate their growth, metabolism, etc.
References
Engel P, Moran NA (2013) The gut microbiota of insects-diversity in structure and function. FEMS Microbiol Rev 37(5):699–735. https://doi.org/10.1111/1574-6976.12025
Adak A, Khan MR (2018) An insight into gut microbiota and its functionalities. Cell Mol Life Sci 76(3):473–493. https://doi.org/10.1007/s00018-018-2943-4
Klassen JL (2014) Microbial secondary metabolites and their impacts on insect symbioses. Curr Opin Insect Sci 4:15–22. https://doi.org/10.1016/j.cois.2014.08.004
Rohlfs M, Churchill AC (2011) Fungal secondary metabolites as modulators of interactions with insects and other arthropods. Fungal Genet Biol 48(1):23–34. https://doi.org/10.1016/j.fgb.2010.08.008
Douglas AE (1998) Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu Rev Entomol 43:17–37. https://doi.org/10.1146/annurev.ento.43.1.17
Zhang G, Hussain M, O’Neill SL, Asgari S (2013) Wolbachia uses a host microRNA to regulate transcripts of a methyltransferase, contributing to dengue virus inhibition in Aedes aegypti. Proc Natl Acad Sci USA 110(25):10276–10281. https://doi.org/10.1073/pnas.1303603110
Douglas AE (2015) Multiorganismal insects: diversity and function of resident microorganisms. Annu Rev Entomol 60:17–34. https://doi.org/10.1146/annurev-ento-010814-020822
Lee JB, Park K-E, Lee SA, Jang SH, Eo HJ, Am Jang H, Kim C-H, Ohbayashi T, Matsuura Y, Kikuchi Y (2017) Gut symbiotic bacteria stimulate insect growth and egg production by modulating hexamerin and vitellogenin gene expression. Dev Comp Immunol 69:12–22. https://doi.org/10.1016/j.dci.2016.11.019
Liao X, Mao K, Ali E, Zhang X, Wan H, Li J (2017) Temporal variability and resistance correlation of sulfoxaflor susceptibility among Chinese populations of the brown planthopper Nilaparvata lugens (Stål). Crop Protect 102:141–146. https://doi.org/10.1016/j.cropro.2017.08.024
Jones RM, Desai C, Darby TM, Luo L, Wolfarth AA, Scharer CD, Ardita CS, Reedy AR, Keebaugh ES, Neish AS (2015) Lactobacilli modulate epithelial cytoprotection through the Nrf2 pathway. Cell Rep 12(8):1217–1225. https://doi.org/10.1016/j.celrep.2015.07.042
Broderick NA, Lemaitre B (2012) Gut-associated microbes of Drosophila melanogaster. Gut microbes 3(4):307–321. https://doi.org/10.4161/gmic.19896
Krishnan S, Cooper JA (2014) Effect of dietary fatty acid composition on substrate utilization and body weight maintenance in humans. Eur J Nutr 53(3):691–710. https://doi.org/10.1007/s00394-013-0638-z
Moll RM, Romoser WS, Modrakowski MC, Moncayo AC, Lerdthusnee K (2001) Meconial peritrophic membranes and the fate of midgut bacteria during mosquito (Diptera: Culicidae) metamorphosis. J Med Entomol 38(1):29–32. https://doi.org/10.1603/0022-2585-38.1.29
Kaufman MG, Klug MJ, Merritt RW (1989) Growth and food utilization parameters of germ-free house crickets, Acheta domesticus. J Insect Physiol 35(12):957–967. https://doi.org/10.1016/0022-1910(89)90019-X
Kashima T, Nakamura T, Tojo S (2006) Uric acid recycling in the shield bug, Parastrachia japonensis (Hemiptera: Parastrachiidae), during diapause. J Insect Physiol 52(8):816–825. https://doi.org/10.1016/j.jinsphys.2006.05.003
Hongoh Y (2010) Diversity and genomes of uncultured microbial symbionts in the termite gut. Biosci Biotechnol Biochem 74(6):1145–1151. https://doi.org/10.1271/bbb.100094
Douglas AE (2018) Omics and the metabolic function of insect-microbial symbioses. Curr Opin Insect Sci 29:1–6. https://doi.org/10.1016/j.cois.2018.05.012
Hegedus D, Erlandson M, Gillott C, Toprak U (2009) New insights into peritrophic matrix synthesis, architecture, and function. Annu Rev Entomol 54:285–302. https://doi.org/10.1146/annurev.ento.54.110807.090559
Yun J-H, Roh SW, Whon TW, Jung M-J, Kim M-S, Park D-S, Yoon C, Nam Y-D, Kim Y-J, Choi J-H, Kim J-Y, Shin N-R, Kim S-H, Lee W-J, Bae J-W (2014) Insect gut bacterial diversity determined by environmental habitat, diet, developmental stage, and phylogeny of host. Appl Environ Microbiol 80(17):5254–5264. https://doi.org/10.1128/AEM.01226-14
Dillon RJ, Dillon VM (2004) The gut bacteria of insects: nonpathogenic interactions. Annu Rev Entomol 49:71–92. https://doi.org/10.1146/annurev.ento.49.061802.123416
Paniagua Voirol LR, Frago E, Kaltenpoth M, Hilker M, Fatouros NE (2018) Bacterial symbionts in lepidoptera: their diversity, transmission, and impact on the host. Front Microbiol 9:556. https://doi.org/10.3389/fmicb.2018.00556
Hongoh Y, Ekpornprasit L, Inoue T, Moriya S, Trakulnaleamsai S, Ohkuma M, Noparatnaraporn N, Kudo T (2006) Intracolony variation of bacterial gut microbiota among castes and ages in the fungus-growing termite Macrotermes gilvus. Mol Ecol 15(2):505–516. https://doi.org/10.1111/j.1365-294X.2005.02795.x
Vargas-Asensio G, Pinto-Tomas A, Rivera B, Hernandez M, Hernandez C, Soto-Montero S, Murillo C, Sherman DH, Tamayo-Castillo G (2014) Uncovering the cultivable microbial diversity of costa rican beetles and its ability to break down plant cell wall components. PLoS ONE 9(11):e113303. https://doi.org/10.1371/journal.pone.0113303
Samoilova ES, Kostina NV, Striganova BR (2016) Microbial population of the digestive tract of click beetle larvae (Elateridae, Coleoptera). Izv Akad Nauk Ser Biol 5:532–543. https://doi.org/10.1134/S1062359016050083
Lund JB, List M, Baumbach J (2017) Interactive microbial distribution analysis using BioAtlas. Nucleic Acids Res 45(W1):W509–w513. https://doi.org/10.1093/nar/gkx304
Edgar R (2018) Taxonomy annotation and guide tree errors in 16S rRNA databases. PeerJ 6:e5030. https://doi.org/10.7717/peerj.5030
Anderson MJ, Crist TO, Chase JM, Vellend M, Inouye BD, Freestone AL, Sanders NJ, Cornell HV, Comita LS, Davies KF, Harrison SP, Kraft NJB, Stegen JC, Swenson NG (2011) Navigating the multiple meanings of β diversity: a roadmap for the practicing ecologist. Ecol Lett 14(1):19–28. https://doi.org/10.1111/j.1461-0248.2010.01552.x
Maynard C, Weinkove D (2018) The gut microbiota and ageing. Subcell Biochem 90:351–371. https://doi.org/10.1007/978-981-13-2835-0_12
Clark RI, Salazar A, Yamada R, Fitz-Gibbon S, Morselli M, Alcaraz J, Rana A, Rera M, Pellegrini M, Ja WW, Walker DW (2015) Distinct shifts in microbiota composition during Drosophila aging impair intestinal function and drive mortality. Cell Rep 12(10):1656–1667. https://doi.org/10.1016/j.celrep.2015.08.004
Borrelli L, Coretti L, Dipineto L, Bovera F, Menna F, Chiariotti L, Nizza A, Lembo F, Fioretti A (2017) Insect-based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens. Sci Rep 7(1):16269. https://doi.org/10.1038/s41598-017-16560-6
Anderson KE, Ricigliano VA, Mott BM, Copeland DC, Floyd AS, Maes P (2018) The queen's gut refines with age: longevity phenotypes in a social insect model. Microbiome 6(1):108. https://doi.org/10.1186/s40168-018-0489-1
Remolina SC, Hughes KA (2008) Evolution and mechanisms of long life and high fertility in queen honey bees. Age (Dordr) 30(2–3):177–185. https://doi.org/10.1007/s11357-008-9061-4
Kwong WK, Medina LA, Koch H, Sing K-W, Soh EJY, Ascher JS, Jaffé R, Moran NA (2017) Dynamic microbiome evolution in social bees. Sci Adv 3(3):e1600513. https://doi.org/10.1126/sciadv.1600513
Wang X, Gao Q, Wang W, Wang X, Lei C, Zhu F (2018) The gut bacteria across life stages in the synanthropic fly Chrysomya megacephala. BMC Microbiol 18(1):131. https://doi.org/10.1186/s12866-018-1272-y
McCutcheon JP, Boyd BM, Dale C (2019) The life of an insect endosymbiont from the cradle to the grave. Curr Biol 29(11):R485–r495. https://doi.org/10.1016/j.cub.2019.03.032
Romero S, Nastasa A, Chapman A, Kwong WK, Foster LJ (2019) The honey bee gut microbiota: strategies for study and characterization. Insect Mol Biol 28(4):455–472. https://doi.org/10.1111/imb.12567
Gong J, Yang C (2012) Advances in the methods for studying gut microbiota and their relevance to the research of dietary fiber functions. Food Res Int 48(2):916–929. https://doi.org/10.1016/j.foodres.2011.12.027
Sarangi AN, Goel A, Aggarwal R (2019) Methods for studying gut microbiota: a primer for physicians. J Clin Exp Hepatol 9(1):62–73. https://doi.org/10.1016/j.jceh.2018.04.016
Rouzé R, Moné A, Delbac F, Belzunces L, Blot N (2019) The honeybee gut microbiota is altered after chronic exposure to different families of insecticides and infection by Nosema ceranae. Microbes Environ 34(3):226–233. https://doi.org/10.1264/jsme2.ME18169
De Palma G, Nadal I, Collado MC, Sanz Y (2009) Effects of a gluten-free diet on gut microbiota and immune function in healthy adult human subjects. Br J Nutr 102(8):1154–1160. https://doi.org/10.1017/S0007114509371767
Ni J, Tokuda G (2013) Lignocellulose-degrading enzymes from termites and their symbiotic microbiota. Biotechnol Adv 31(6):838–850. https://doi.org/10.1016/j.biotechadv.2013.04.005
Chen MX, Wang S-Y, Kuo C-H, Tsai IL (2019) Metabolome analysis for investigating host-gut microbiota interactions. J Formos Med Assoc 118(Suppl 1):S10–S22. https://doi.org/10.1016/j.jfma.2018.09.007
Ogué-Bon E, Khoo C, McCartney AL, Gibson GR, Rastall RA (2010) In vitro effects of synbiotic fermentation on the canine faecal microbiota. FEMS Microbiol Ecol 73(3):587–600. https://doi.org/10.1111/j.1574-6941.2010.00915.x
Liu N, Zhang L, Zhou H, Zhang M, Yan X, Wang Q, Long Y, Xie L, Wang S, Huang Y, Zhou Z (2013) Metagenomic insights into metabolic capacities of the gut microbiota in a fungus-cultivating termite (Odontotermes yunnanensis). PLoS ONE 8(7):e69184. https://doi.org/10.1371/journal.pone.0069184
Savijoki K, Ingmer H, Varmanen P (2006) Proteolytic systems of lactic acid bacteria. Appl Microbiol Biotechnol 71(4):394–406. https://doi.org/10.1007/s00253-006-0427-1
Liu M, Bayjanov JR, Renckens B, Nauta A, Siezen RJ (2010) The proteolytic system of lactic acid bacteria revisited: a genomic comparison. BMC Genomics 11:36. https://doi.org/10.1186/1471-2164-11-36
Pessione E (2012) Lactic acid bacteria contribution to gut microbiota complexity: lights and shadows. Front Cell Infect Microbiol 2:86. https://doi.org/10.3389/fcimb.2012.00086
Birkle LM, Minto LB, Douglas AE (2002) Relating genotype and phenotype for tryptophan synthesis in an aphid–bacterial symbiosis. Physiol Entomol 27(4):302–306. https://doi.org/10.1046/j.1365-3032.2002.00301.x
Scully ED, Geib SM, Carlson JE, Tien M, Mckenna DD, Hoover K (2014) Functional genomics and microbiome profiling of the Asian longhorned beetle (Anoplophora glabripennis) reveal insights into the digestive physiology and nutritional ecology of wood feeding beetles. BMC Genomics 15(1):1096–1096. https://doi.org/10.1186/1471-2164-15-1096
Ayayee PA, Larsen T, Rosa C, Felton GW, Ferry JG, Hoover K (2016) Essential amino acid supplementation by gut microbes of a wood-feeding cerambycid. Environ Entomol 45(1):66–73. https://doi.org/10.1093/ee/nvv153
Zheng H, Powell JE, Steele MI, Dietrich C, Moran NA (2017) Honeybee gut microbiota promotes host weight gain via bacterial metabolism and hormonal signaling. Proc Natl Acad Sci USA 114(18):4775–4780. https://doi.org/10.1073/pnas.1701819114
Ong SY, Kho HP, Riedel SL, Kim SW, Gan CY, Taylor TD, Sudesh K (2018) An integrative study on biologically recovered polyhydroxyalkanoates (PHAs) and simultaneous assessment of gut microbiome in yellow mealworm. J Biotechnol 265:31–39. https://doi.org/10.1016/j.jbiotec.2017.10.017
Shin B, Park SH, Kim B-Y, Jo S-I, Lee SK, Shin J, Oh D-C (2017) Deinococcucins A-D, aminoglycolipids from Deinococcus sp., a gut bacterium of the carpenter ant Camponotus japonicus. J Nat Prod 80(11):2910–2916. https://doi.org/10.1021/acs.jnatprod.7b00426
Anand AA, Vennison SJ, Sankar SG, Prabhu DI, Vasan PT, Raghuraman T, Geoffrey CJ, Vendan SE (2010) Isolation and characterization of bacteria from the gut of Bombyx mori that degrade cellulose, xylan, pectin and starch and their impact on digestion. J Insect Sci 10:107. https://doi.org/10.1673/031.010.10701
Gales A, Chatellard L, Abadie M, Bonnafous A, Auer L, Carrere H, Godon JJ, Hernandez-Raquet G, Dumas C (2018) Screening of phytophagous and xylophagous insects guts microbiota abilities to degrade lignocellulose in bioreactor. Front Microbiol 9:2222. https://doi.org/10.3389/fmicb.2018.02222
Warnecke F, Luginbuhl P, Ivanova N, Ghassemian M, Richardson TH, Stege JT, Cayouette M, McHardy AC, Djordjevic G, Aboushadi N, Sorek R, Tringe SG, Podar M, Martin HG, Kunin V, Dalevi D, Madejska J, Kirton E, Platt D, Szeto E, Salamov A, Barry K, Mikhailova N, Kyrpides NC, Matson EG, Ottesen EA, Zhang X, Hernandez M, Murillo C, Acosta LG, Rigoutsos I, Tamayo G, Green BD, Chang C, Rubin EM, Mathur EJ, Robertson DE, Hugenholtz P, Leadbetter JR (2007) Metagenomic and functional analysis of hindgut microbiota of a wood-feeding higher termite. Nature 450(7169):560–565. https://doi.org/10.1038/nature06269
Breznak JA, Brill WJ, Mertins JW, Coppel HC (1973) Nitrogen fixation in termites. Nature 244(5418):577. https://doi.org/10.1038/244577a0
Benemann JR (1973) Nitrogen fixation in termites. Science 181(4095):164–165. https://doi.org/10.1126/science.181.4095.164
Yamada A, Inoue T, Wiwatwitaya D, Ohkuma M, Kudo T, Sugimoto A (2006) Nitrogen fixation by termites in tropical forests, Thailand. Ecosystem 9(1):75–83. https://doi.org/10.1007/S10021-005-0024-7
Zhou J, Duan J, Gao M, Wang Y, Wang X, Zhao K (2019) Diversity, roles, and biotechnological applications of symbiotic microorganisms in the gut of termite. Curr Microbiol 76(6):755–761. https://doi.org/10.1007/s00284-018-1502-4
Meuti ME, Jones SC, Curtis PS (2010) 15N discrimination and the sensitivity of nitrogen fixation to changes in dietary nitrogen in Reticulitermes flavipes (Isoptera: Rhinotermitidae). Environ Entomol 39(6):1810–1815. https://doi.org/10.1603/EN10082
Shukla SP, Sanders JG, Byrne MJ, Pierce NE (2016) Gut microbiota of dung beetles correspond to dietary specializations of adults and larvae. Mol Ecol 25(24):6092–6106. https://doi.org/10.1111/mec.13901
Salem H, Florez L, Gerardo N, Kaltenpoth M (2015) An out-of-body experience: the extracellular dimension for the transmission of mutualistic bacteria in insects. Proc Biol Sci 282(1804):20142957. https://doi.org/10.1098/rspb.2014.2957
Salem H, Bauer E, Strauss AS, Vogel H, Marz M, Kaltenpoth M (2014) Vitamin supplementation by gut symbionts ensures metabolic homeostasis in an insect host. Proc Biol Sci 281(1796):20141838. https://doi.org/10.1098/rspb.2014.1838
Douglas AE (2009) The microbial dimension in insect nutritional ecology. Funct Ecol 23(1):38–47. https://doi.org/10.1111/j.1365-2435.2008.01442.x
Blow F, Gioti A, Goodhead IB, Kalyva M, Kampouraki A, Vontas J, Darby AC (2020) Functional genomics of a symbiotic community: shared traits in the olive fruit fly gut microbiota. Genome Biol Evol 12(2):3778–3791. https://doi.org/10.1093/gbe/evz258
Nakajima H, Hongoh Y, Usami R, Kudo T, Ohkuma M (2005) Spatial distribution of bacterial phylotypes in the gut of the termite Reticulitermes speratus and the bacterial community colonizing the gut epithelium. FEMS Microbiol Ecol 54(2):247–255. https://doi.org/10.1016/j.femsec.2005.03.010
Shinzato N, Muramatsu M, Matsui T, Watanabe Y (2007) Phylogenetic analysis of the gut bacterial microflora of the fungus-growing termite Odontotermes formosanus. Biosci Biotechnol Biochem 71(4):906–915. https://doi.org/10.1271/bbb.60540
Thongaram T, Hongoh Y, Kosono S, Ohkuma M, Trakulnaleamsai S, Noparatnaraporn N, Kudo T (2005) Comparison of bacterial communities in the alkaline gut segment among various species of higher termites. Extremophiles 9(3):229–238. https://doi.org/10.1007/s00792-005-0440-9
Mackenzie LM, Muigai AT, Osir EO, Lwande W, Keller M, Toledo G, Boga HI (2007) Bacterial diversity in the intestinal tract of the funguscultivating termite Macrotermes michaelseni (Sjöstedt). Afr J Biotechnol 6(6):658–667. https://doi.org/10.4314/ajb.v6i6.56873
Cruden D, Markovetz A (1984) Microbial aspects of the cockroach hindgut. Arch Microbiol 138(2):131–139. https://doi.org/10.1007/bf00413013
Cruden D, Markovetz A (1987) Microbial ecology of the cockroach gut. Annu Rev Microbiol 41(1):617–643. https://doi.org/10.1146/annurev.mi.41.100187.003153
Kakumanu ML, Maritz JM, Carlton JM, Schal C (2018) Overlapping community compositions of gut and fecal microbiomes in lab-reared and field-collected german cockroaches. Appl Environ Microbiol. https://doi.org/10.1128/AEM.01037-18
Garofalo C, Osimani A, Milanović V, Taccari M, Cardinali F, Aquilanti L, Riolo P, Ruschioni S, Isidoro N, Clementi F (2017) The microbiota of marketed processed edible insects as revealed by high-throughput sequencing. Food Microbiol 62:15–22. https://doi.org/10.1016/j.fm.2016.09.012
Waite DWDM, Biswas K, Ward DF, Deines P, Taylor MW (2015) Microbial community structure in the gut of the New Zealand insect Auckland tree weta (Hemideina thoracica). Arch Microbiol 197(4):603–612. https://doi.org/10.1007/s00203-015-1094-3
Jiang CL, Jin WZ, Tao XH, Zhang Q, Zhu J, Feng SY, Xu XH, Li HY, Wang ZH, Zhang ZJ (2019) Black soldier fly larvae (Hermetia illucens) strengthen the metabolic function of food waste biodegradation by gut microbiome. Microb Biotechnol 12(3):528–543. https://doi.org/10.1111/1751-7915.13393
Liu C, Wang C, Yao H (2019) Comprehensive resource utilization of waste using the black soldier fly (Hermetia illucens (L.)) (Diptera: Stratiomyidae). Animals (Basel). https://doi.org/10.3390/ani9060349
Khamesipour F, Lankarani KB, Honarvar B, Kwenti TE (2018) A systematic review of human pathogens carried by the housefly (Musca domestica L.). BMC Public Health 18(1):1049. https://doi.org/10.1186/s12889-018-5934-3
Remolina SC, Hughes KA (2008) Evolution and mechanisms of long life and high fertility in queen honey bees. Age (Dordr) 30(2–3):177–185. https://doi.org/10.1007/s11357-008-9061-4
Martinson VG, Moy J, Moran NA (2012) Establishment of characteristic gut bacteria during development of the honeybee worker. Appl Environ Microbiol 78(8):2830–2840. https://doi.org/10.1128/aem.07810-11
Richter MR (2000) Social wasp (Hymenoptera: Vespidae) foraging behavior. Annu Rev Entomol 45:121–150. https://doi.org/10.1146/annurev.ento.45.1.121
Suenami S, Konishi Nobu M, Miyazaki R (2019) Community analysis of gut microbiota in hornets, the largest eusocial wasps, Vespa mandarinia and V. simillima. Sci Rep 9(1):9830. https://doi.org/10.1038/s41598-019-46388-1
Grigorescu AS, Renoz F, Sabri A, Foray V, Hance T, Thonart P (2018) Accessing the hidden microbial diversity of aphids: an illustration of how culture-dependent methods can be used to decipher the insect microbiota. Microb Ecol 75(4):1035–1048. https://doi.org/10.1007/s00248-017-1092-x
Vivero RJ, Jaramillo NG, Cadavid-Restrepo G, Soto SI, Herrera CX (2016) Structural differences in gut bacteria communities in developmental stages of natural populations of Lutzomyia evansi from Colombia's Caribbean coast. Parasit Vectors 9:496. https://doi.org/10.1186/s13071-016-1766-0
Lagier JC, Khelaifia S, Alou MT et al (2016) Culture of previously uncultured members of the human gut microbiota by culturomics. Nat Microbiol 1:16203. https://doi.org/10.1038/nmicrobiol.2016.203
Browne HP, Forster SC, Anonye BO, Kumar N, Neville BA, Stares MD, Goulding D, Lawley TD (2016) Culturing of 'unculturable' human microbiota reveals novel taxa and extensive sporulation. Nature 533(7604):543–546. https://doi.org/10.1038/nature17645
Lagier JC, Hugon P, Khelaifia S, Fournier PE, La Scola B, Raoult D (2015) The rebirth of culture in microbiology through the example of culturomics to study human gut microbiota. Clin Microbiol Rev 28(1):237–264. https://doi.org/10.1128/cmr.00014-14
Lau JT, Whelan FJ, Herath I, Lee CH, Collins SM, Bercik P, Surette MG (2016) Capturing the diversity of the human gut microbiota through culture-enriched molecular profiling. Genome Med 8(1):72. https://doi.org/10.1186/s13073-016-0327-7
Costes B, Girodon E, Ghanem N, Chassignol M, Thuong NT, Dupret D, Goossens M (1993) Psoralen-modified oligonucleotide primers improve detection of mutations by denaturing gradient gel electrophoresis and provide an alternative to GC-clamping. Hum Mol Genet 2(4):393–397. https://doi.org/10.1093/hmg/2.4.393
Wang Y, Hammes F, De Roy K, Verstraete W, Boon N (2010) Past, present and future applications of flow cytometry in aquatic microbiology. Trends Biotechnol 28(8):416–424. https://doi.org/10.1016/j.tibtech.2010.04.006
Metzker ML (2005) Emerging technologies in DNA sequencing. Genome Res 15(12):1767–1776. https://doi.org/10.1101/gr.3770505
Acknowledgements
This work was supported by the National Key Technology R & D Program of China (2018YFD0500203 to LY, CY, and 2018YFD0500204 to LF). LY and CY also acknowledge the support from State Key Laboratory of Biocatalysis and Enzyme Engineering.
Author information
Authors and Affiliations
Contributions
Conceptualization: SW, LY; Investigation: SW, LW, XF, CY; Writing-original draft preparation: SW; Formal analysis: LY, LF.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, S., Wang, L., Fan, X. et al. An Insight into Diversity and Functionalities of Gut Microbiota in Insects. Curr Microbiol 77, 1976–1986 (2020). https://doi.org/10.1007/s00284-020-02084-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02084-2