Abstract
Candida glycerinogenes, the glycerol producer with excellent multi-stress tolerances, is considered to be a potential biotechnological host used in the production of glycerol and its derivatives under extreme fermentation conditions. In this study, to evaluate the multiple roles of mitogen-activated protein kinase CgHOG1, we constructed a gene disruption system in the diploid C. glycerinogenes to obtain CgHOG1 null mutant. Pseudohyphae generation of the CgHOG1 mutant under non-inducing condition indicated a repressor role in morphological transitions. Disruption of CgHOG1 resulted in increased sensitivities to osmotic, acetic acid, and oxidative stress but not involved in thermotolerance. In the CgHOG1 mutant, NaCl shock failed to stimulate the accumulation of intracellular glycerol and was fatal. In addition, the CgHOG1 mutant displayed a significant prolonged growth lag phase in YPD medium with no decrease in glycerol production, whereas the mutant cannot grow under hyperosmotic condition with no detectable glycerol in broth. These results suggested that CgHOG1 plays important roles in morphogenesis and multi-stress tolerance. The growth and glycerol overproduction under osmotic stress are heavily dependent on CgHOG1 kinase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
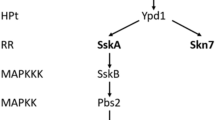
Yeast cells are challenged by the complex and dynamic factors, such as the weak acids, osmotic stress, and oxidative stress when exposed to the culture environment. Some efficient response mechanisms are employed to enable cells survival. The high-osmolarity glycerol (HOG) response pathway mediated by the key component of mitogen-activated protein kinase, HOG1, is demonstrated to involve in tolerance of the osmotic stress [20], even oxidative stress [11], acetic acid stress [17], and cold stress [23]. In Saccharomyces cerevisiae, ScHOG1 kinase is fully active on Thr174 and Tyr176 in the catalytic domain by MAPK signaling cascades, and then the phosphorylated ScHOG1 is transported and accumulated in nucleus to employ the downstream regulatory mechanism [1]. Under hyperosmotic condition, glycerol as the major compatible solute is overproduced and accumulated intracellularly by inducing the expression of glycerol biosynthesis genes and controlling the glycerol flux [9].
Candida glycerinogenes is a multi-stress-tolerant yeast that can survive in 55 % (W/V) glucose or 15 % (W/V) NaCl medium. When C. glycerinogenes is cultured in high-glucose medium, more than 120 g glycerol l−1 can be produced; therefore, it has been successfully used for commercial scale production of glycerol in the last decades [26]. However, there is no evidence that multi-stress tolerance is closely related to glycerol overproduction via the activated HOG pathway. In a previous study, we have cloned HOG1 homologous gene in C. glycerinogenes and characterized the kinase function via complementary expression in a S. cerevisiae HOG1 △null mutant [12]. However, more information is needed to understand the key role of CgHOG1 in stress tolerance and glycerol overproduction. Unlike S. cerevisiae, a genetic study on C. glycerinogenes is more difficult. In a previous study, we have developed the transformation method for C. glycerinogenes using phleomycin as a drug-resistance marker, and the parameters involved in transformation efficiency were optimized [4]. Integrative vectors were also constructed for heterogenous gene expression in C. glycerinogenes [24, 25]. However, more available tools are required for facilitating the genetic engineering studies. Here, we constructed an effective gene disruption system to generate the CgHOG1 mutant, and the characterization may provide more insight into the multi-roles of CgHOG1 in stress tolerance of the industrial yeast C. glycerinogenes.
Materials and Methods
Strains and Media
A uracil auxotrophic mutant of C glycerinogenes WL2002-5 was isolated from 5-fluoroorotic acid (5-FOA) plate and used as a parental strain in this study. The strain was cultured in YPD medium (2 % glucose, 2 % peptone, 1 %yeast extract), or synthetic dextrose (SD) medium (2 % glucose and 0.67 % yeast nitrogen base without amino acid) and SD medium supplemented with 0.1 % 5-FOA and 0.005 % uracil during genetic manipulation. E. coli JM109 was cultured in LB medium (0.5 % yeast extract, 1 % tryptone, and 1 % glucose) to maintain plasmids at 37 °C.
Construction of CgHOG1 Mutant
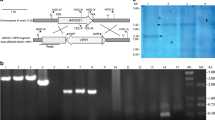
The C. glycerinogenes HOG1 gene was disrupted by a URA-Blaster system with modifications [15]. A 1.6 kb URA5 gene was amplified with genomic DNA of C. glycerinogenes WL2002-5 using primer URA5-R and URA5-F. Two 1.1 kb hisG fragments were amplified with plasmid pCUB6 using two group of primers (hisG-F1, hisG-R1 and hisG-F2, hisG-R2) and then inserted separately into the flank of URA5 gene under the assistance of a clone vector pMD-19T (Takara). Finally, a general vector pMD-HUH containing hisG-URA5-hisG cassette was constructed. Two rounds of disruptions were performed as described previously to obtain the CgHOG1 mutant [15]. DNA transformation of C. glycerinogenes was carried out by the method developed by Chen [4]. The integrative expression vector harboring phleomycin resistance gene as a selective marker and 5.8S ribosomal RNA (rRNA) gene region as a homologous arm was constructed to reintroduce CgHOG1 under the control of PCgGAP promoter in CgHOG1△ mutant. All the primers can be found in Table 1.
Analysis of Cell Survival
The cells of wild-type C. glycerinogenes and CgHOG1△ mutant on exponential phase were collected in 1.5-ml tubes, washed twice with sterile water, and then incubated with 1 ml YPD or YPD supplied with 1 M NaCl for various time points. Tenfold serial dilutions were performed using saline solution after the supernatant was removed by centrifugation at 5000 rpm, and then 100 μl dilutions were spread on YPD plate to produce 20–200 colonies per plate. Colonies were then counted after 48 h of incubation at 30 °C. Five plates were used for each experiment and the average value was calculated. Relative survival (%) of the samples shocked with and without NaCl was expressed as the ratio of counting colonies.
Real-Time Quantitative PCR
Candida glycerinogenes was grown up to the exponential phase in YPD medium at 30 °C for 16 h, and then NaCl was added to the culture. After incubation for the indicated time, the cells were collected by centrifugation. The total RNA extraction and the synthesis of cDNA were performed as described previously [12]. The expression levels of genes were determined on a Bio-Rad CFX96 Real-Time PCR system. Each reaction mixture contained cDNA (10 ng), 2× UltraSYBR Mixture with ROX (CWbiotech, 25 μl), 10 μM forward and reverse primers (1 μl), and RNase-free water (up to 50 μl). The primers RT-GPDr and RT-GPDf were used to study the transcription expression of CgGPD. ACT1 was used as an internal reference with the primers RT-ACTr and RT-ACTf. All the primers are listed in Table 1. The relative transcription levels are analyzed via ΔΔCt method.
GPD Activity Assay
Treated cells were harvested by centrifugation, and cell extracts were prepared as described [6]. The cells were disrupted by agitation with glass beads (Sigama, 425 × 600 mm) in a vortex mixer, alternating 30 s on vortex and 1 min on ice 15 times. GPD activity assay was performed in the reaction solution containing 20 mM imidazole–HCl (pH 7.0), 1 mM MgCl2, 1 mM DTT, 0.67 mM DHAP, and 0.09 mM NADH [5]. One unit (U) of enzyme activity is defined as the amount of enzyme for 1 μmol NADH extinction per minute at 25 °C. Protein content of extracts was determined using bicinchoninic acid method (CWBiotech, BAC protein assay kit).
Glycerol Quantification
Exponentially growing cells were collected, washed twice, and shifted to the fresh YPD medium with or without NaCl. The supernatant and the cells were separated and then used for intracellular glycerol quantification. For intracellular glycerol quantification, the cells were resuspended in 1 ml water and disrupted by glass beads. Glycerol content was determined by the method of Lambert and expressed as mg of glycerol per mg of dry cell weight [26].
Results and Discussion
Disruption of CgHOG1 by Homologous Recombination
C. glycerinogenes was treated with ultraviolet light to generate uracil auxotrophic mutant for applying in further genetic manipulation. One uracil auxotrophic mutant was obtained on the positive selection using 5-FOA medium. We cloned genes in the UMP biosynthesis via de novo pathway and sequencing indicated that the unique URA5 (Orotate phosphoribosyltransferase 1) gene was broken on the orotate binding site in the mutant. Then the uracil auxotrophic mutant was employed as a host to construct the CgHOG1 null mutant. Since C. glycerinogenes has been confirmed to be diploid with no sexual regeneration [22], the URA-Blaster disruption system widely applied in diploid yeast such as Candida albicans was used here with necessary modifications. Although the homologous recombination efficiency is relatively low, eight CgHOG1△ mutant clones were obtained on the selective medium after performing two rounds of homologous recombination. We have long been difficult on the genetic manipulation in the diploid C. glycerinogenes, this study provided a feasible procedure for gene disruption that might be applied in genetical engineering of the potential biotechnological host C. glycerinogenes.
CgHOG1 Functions as a Repressor in Morphogenesis
When CgHOG1 was disrupted, visible changes of cell morphogenesis can be observed under the microscope. Pseudohyphae generated under non hyphae-inducing conditions (Fig. 1). This morphological transition was also observed in the fungal pathogen Candida albicans in the absence of HOG1 [3]. HOG1 kinase is considered to participate in the morphological transitions, and it prevents the yeast-to-hypha switch independent of any morphogenetic signal [3, 7, 8]. When the CgHOG1 mutant was rescued by complementation of wild-type CgHOG1, the pseudohyphae generation was prevented. These results suggest that CgHOG1 might also function as a repressor of morphogenetic switching in C. glycerinogenes.
The CgHOG1△ mutant generates pseudohyphae under non-inducing conditions. Micrographs illustrating the morphology of wild-type (WT) C. glycerinogenes, CgHOG1△ mutant, and CgHOG1△ rescued by wild-type CgHOG1 gene with strains grown in YPD medium at 30 °C and pH 5.5. The figure shows the result of a representative experiment
CgHOG1 Plays Important Roles in Stress Tolerance
Candida glycerinogenes can tolerate hyperosmotic stress, high temperature, and low pH. In addition, it has been reported to have excellent tolerance to 2-phenylethanol [16]. To investigate the potential roles of CgHOG1 in stress signal transduction, the growth of mutant under various stress conditions was monitored. In agreement with the expected function in osmoadaptation, the CgHOG1 mutant completely failed to grow on YPD plates containing 1 M NaCl or 1.2 M glucose, and showed impaired growth on 1 M sorbitol (Fig. 2). These indicate that the mutant displayed different tolerance to various osmolytes, which implies that the active CgHOG1 differs depending on the osmolyte type, and this profile was also found in the halotolerant Hortaea werneckii [14]. CgHOG1 mutant displayed increased sensitivity to 10 mM hydrogen peroxide and 70 mM acetic acid (Fig. 2). In contrast, the CgHOG1 null mutation had no significant effect on growth at higher temperature, which was in agreement with the result observed in C. albicans HOG1 mutant [13] and the thermotolerant yeast Kluyveromyces marxianus [19]. The results suggest that CgHOG1 is involved in multi-stress tolerance except thermotolerance.
Osmoadaptation Response Depending on CgHOG1
In S. cerevisiae, hyperosmotic shock causes a rapid loss of intracellular water and then the cells shrink lethally. To further confirm the role of CgHOG1 in response to osmotic stress, the main mechanism of accumulation of intracellular glycerol was investigated in C. glycerinogenes. When incubated under hyperosmotic stress, the mutant lost the controlling of HOG pathway displayed poor survival to compare with the wild type (Fig. 3a). The production and intracellular accumulation of compatible solute is the main rapid response strategy to counteract the loss of turgor stress, this process is mediated by HOG pathway [9, 10]. In wild-type C. glycerinogenes, the amount of glycerol accumulated intracellularly rapidly and peaked soon after hyperosmotic shock for 15 min; meanwhile, in the CgHOG1 mutant, intracellular glycerol remained at low levels and no obvious changes can be observed (Fig. 3b).
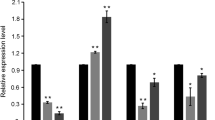
Effect of CgHOG1 mutation on the adaptive response to the hyperosmotic shock. Relative survival (a), intracellular glycerol accumulation (b), and activity of glycerol 3-phosphate dehydrogenase (c) of C. glycerinogenes or CgHOG1△ mutant were measured after shocked with 1 M NaCl for indicated time. d Relative expression levels of CgGPD in the presence of NaCl were reported. The expression levels of specific transcripts were normalized against their expression without osmotic shock. All data from three independent replicates were used to calculate the mean and standard deviation
The transient increase in the intracellular glycerol can be caused by the effective modulation of enzymatic activity and gene expression of the key enzyme glycerol-3-phosphate dehydrogenase [2]. In C. glycerinogenes, a unique CgGPD gene encoding glycerol-3-phosphate dehydrogenase homologous to GPD1/GPD2 from S. cerevisiae has been cloned and characterized in a previous study [17]. The specific activity of CgGPD was induced transiently by hyperosmotic shock with a significant increase in the first 15 min (Fig. 3c). The transcription level of CgGPD also increased greatly with NaCl incubation, as well as higher osmolarity made stronger induction (Fig. 3d). However, the enzyme modulation and gene transcription regulation mediated by HOG pathway were deactivated when CgHOG1 is disrupted.
In traditional yeast S. cerevisiae, control of the glycerol flux is another important mechanism for the rapid accumulation of glycerol upon hyperosmotic stress. A member of the aquaporin family, transmembrane channel FPS1, closes to maintain intracellular glycerol in response to hyperosmotic stress [18]. It is considered to be the fastest mechanism to alter glycerol concentration [21]. We scanned the whole genome of Pichia kudriavzevii (synonyms for C. glycerinogenes); however, no FPS1 homologous gene was found, which implies an untraditional mechanism of glycerol export in C. glycerinogenes. In conclusion, the results highlighted the conserved role of CgHOG1 kinase in the osmotic stress response.
Effects of CgHOG1 on the Cell Growth and Glycerol Overproduction in Broth
CgHOG1 mutant also showed a marked growth defect in the moderate YPD without any additional osmolytes. A further time course of growth assay indicated that the CgHOG1 mutant displayed a significant prolonged growth lag phase than the wild type on YPD liquid medium (Fig. 4); however, finally the biomass is not influenced significantly. The data on transcription profiling in C. albicans revealed that the deletion of HOG1 has significant effects on transcriptome in the absence of any stress [8]. Hence, a subset of growth-related genes in the absence of operation by HOG pathway in the mutant might contribute to the prolonged growth lag phase under basal conditions.
Cell growth and glycerol production of C. glycerinogenes CgHOG1△ mutants. Wild-type (WT) or CgHOG1△ mutants were inoculated in YPD or YPD containing 1 M NaCl and incubated on a rotary shaker at 32 °C. The biomass and glycerol production was measured at the indicated time point; closed circles WT/YPD; open circles WT/1 M NaCl; closed squares CgHOG1△/YPD; open squares CgHOG1△/1 M NaCl. All data are the average values from three independent replicates
Although the CgHOG1 mutant displayed a prolonged growth lag phase in moderate YPD medium, finally the glycerol production was not affected, indicating that glycerol production constitutively under non-osmotic stress is independent of HOG pathway. The glycerol production significantly increased when C. glycerinogenes was cultured in YPD with additional 1 M NaCl; meanwhile, no glycerol can be detected in the broth of mutant because the hyperosmotic stress is fatal in the absence of CgHOG1. C. glycerinogenes produces more than 120 g glycerol l−1 in high-glucose medium with 250 g glucose l−1 [26], in which the CgHOG1 mutant cannot survive under this osmotic stress condition. In contrast, the deletion of HOG1 in S. cerevisiae resulted in a slight decrease in growth rate and only a 20 % decrease in glycerol production; hence, the results of this study demonstrate a different role of CgHOG1 in the control of glycerol production [20]. In conclusion, the HOG pathway mediated by CgHOG1 in C. glycerinogenes makes a significant contribution to cell growth and glycerol overproduction under hyperosmotic stress.
References
Alepuz PM, Jovanovic A, Reiser V, Ammerer G (2001) Stress-induced map kinase Hog1 is part of transcription activation complexes. Mol Cell 7(4):767–777
Bouwman J, Kiewiet J, Lindenbergh A, van Eunen K, Siderius M, Bakker BM (2011) Metabolic regulation rather than de novo enzyme synthesis dominates the osmo-adaptation of yeast. Yeast 28(1):43–53
Cheetham J, MacCallum DM, Doris KS, da Silva Dantas A, Scorfield S, Odds F, Smith DA, Quinn J (2011) MAPKKK-independent regulation of the Hog1 stress-activated protein kinase in Candida albicans. J Biol Chem 286(49):42002–42016
Chen X, Fang H, Rao Z, Shen W, Zhuge B, Wang Z, Zhuge J (2008) An efficient genetic transformation method for glycerol producer Candida glycerinogenes. Microbiol Res 163(5):531–537
Chen X, Fang H, Rao Z, Shen W, Zhuge B, Wang Z, Zhuge J (2008) Cloning and characterization of a NAD+ -dependent glycerol-3-phosphate dehydrogenase gene from Candida glycerinogenes, an industrial glycerol producer. FEMS Yeast Res 8(5):725–734
Chen XZ, Fang HY, Rao ZM, Shen W, Zhuge B, Wang ZX, Zhuge J (2009) Comparative characterization of genes encoding glycerol 3-phosphate dehydrogenase from Candida glycerinogenes and Saccharomyces cerevisiae. Prog Biochem Biophys 36(2):198–205
Eisman B, Alonso-Monge R, Roman E, Arana D, Nombela C, Pla J (2006) The Cek1 and Hog1 mitogen-activated protein kinases play complementary roles in cell wall biogenesis and chlamydospore formation in the fungal pathogen Candida albicans. Eukaryot Cell 5(2):347–358
Enjalbert B, Smith DA, Cornell MJ, Alam I, Nicholls S, Brown AJ, Quinn J (2006) Role of the Hog1 stress-activated protein kinase in the global transcriptional response to stress in the fungal pathogen Candida albicans. Mol Biol Cell 17(2):1018–1032
Hohmann S (2009) Control of high osmolarity signalling in the yeast Saccharomyces cerevisiae. FEBS Lett 583(24):4025–4029
Hohmann S (2015) An integrated view on a eukaryotic osmoregulation system. Curr Genet 61(3):373–382
Ikner A, Shiozaki K (2005) Yeast signaling pathways in the oxidative stress response. Mutat Res 569(1):13–27
Ji H, Lu X, Wang C, Zong H, Fang H, Sun J, Zhuge J, Zhuge B (2014) Identification of a novel HOG1 homologue from an industrial glycerol producer Candida glycerinogenes. Curr Microbiol 69(6):909–914
Kayingo G, Wong B (2005) The MAP kinase Hog1p differentially regulates stress-induced production and accumulation of glycerol and D-arabitol in Candida albicans. Microbiology 151(9):2987–2999
Kejzar A, Cibic M, Grotli M, Plemenitas A, Lenassi M (2015) The unique characteristics of HOG pathway MAPKs in the extremely halotolerant Hortaea werneckii. FEMS Microbiol Lett 362(8):fnv046
Ko BS, Kim J, Kim JH (2006) Production of xylitol from D-xylose by Da xylitol dehydrogenase gene-disrupted mutant of Candida tropicalis. Appl Environ Microbiol 72(6):4207–4213
Lu X, Wang Y, Zong H, Ji H, Zhuge B, Dong Z (2016) Bioconversion of l-phenylalanine to 2-phenylethanol by the novel stress-tolerant yeast Candida glycerinogenes WL2002-5. Bioengineering. doi:10.1080/21655979.2016.1171437
Mollapour M, Piper PW (2006) Hog1p mitogen-activated protein kinase determines acetic acid resistance in Saccharomyces cerevisiae. FEMS Yeast Res 6(8):1274–1280
Oliveira R, Lages F, Silva-Graça M, Lucas C (2003) Fps1p channel is the mediator of the major part of glycerol passive diffusion in Saccharomyces cerevisiae: artefacts and re-definitions. BBA-Biomembranes 1613(1–2):57–71
Qian J, Qin X, Yin Q, Chu J, Wang Y (2011) Cloning and characterization of Kluyveromyces marxianus Hog1 gene. Biotechnol Lett 33(3):571–575
Remize F, Cambon B, Bamavon L, Dequin S (2003) Glycerol formation during wine fermentation is mainly linked to Gpdlp and is only partially controlled by the the HOG pathway. Yeast 20:1243–1253
Saito H, Posas F (2012) Response to hyperosmotic stress. Genetics 192(2):289–318
Song B, Zhuge B, Fang H, Zhuge J (2011) Analysis of the chromosome ploidy of Candida glycerinogenes. Wei Sheng Wu Xue Bao 51(3):326–331
Tulha J, Lima A, Lucas C, Ferreira C (2010) Saccharomyces cerevisiae glycerol/H+ symporter Stl1p is essential for cold/near-freeze and freeze stress adaptation. A simple recipe with high biotechnological potential is given. Microb Cell Fact 9:82–89
Zhang C, Zong H, Zhuge B, Lu XY, Fang HY, Zhuge J (2015) Integrative expression vectors for overexpression of xylitol dehydrogenase (XYL2) in osmotolerant yeast, Candida glycerinogenes WL2002-5. J Ind Microbiol Biot 42(1):113–124
Zhang C, Zong H, Zhuge B, Lu X, Fang H, Zhuge J (2015) Production of Xylitol from D-xylose by overexpression of xylose reductase in osmotolerant yeast Candida glycerinogenes WL2002-5. Appl Biochem Biotechnol 176(5):1511–1527
Zhuge J, Fang HY, Wang ZX, Chen DZ, Jin HR, Gu HL (2001) Glycerol production by a novel osmotolerant yeast Candida glycerinogenes. Appl Microbiol Biotechnol 55(6):686–692
Acknowledgments
This work was funded by China National “863” High-Tech Program (No. 2012AA021201) and supported by the National Natural Science Foundation of China (Nos. 31570052, 31601456), the Natural Science Foundation of Jiangsu Province (Nos. BK20140134, BK20140138), the Six Talent Peaks Project in Jiangsu Province (No. 2014-XCL-017), and the Fundamental Research Funds for the Central Universities (JUSRP11431). We thank Dr. Jiangye Chen (Chinese Academy of Sciences) for plasmid pCUB6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ji, H., Zhuge, B., Zong, H. et al. Role of CgHOG1 in Stress Responses and Glycerol Overproduction of Candida glycerinogenes . Curr Microbiol 73, 827–833 (2016). https://doi.org/10.1007/s00284-016-1132-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-016-1132-7