Abstract
Regulatory (Treg) cells are key regulators of inflammation and important for immune tolerance and homeostasis. A major progress has been made in the identification and classification of Treg cells. Due to technological advances, we have gained deep insights in the epigenetic regulation of Treg cells. The use of fate reporter mice allowed addressing the functional consequences of loss of Foxp3 expression. Depending on the environment Treg cells gain effector functions upon loss of Foxp3 expression. However, the traditional view that Treg cells become necessarily pathogenic by gaining effector functions was challenged by recent findings and supports the notion of Treg cell lineage plasticity. Treg cell stability is also a major issue for Treg cell therapies. Clinical trials are designed to use polyclonal Treg cells as therapeutic tools. Here, we summarize the role of Treg cells in selected autoimmune diseases and recent advances in the field of Treg targeted therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Development of regulatory T cells—a look back
More than 50 years ago, it was shown that thymectomy in mice results in a wasting disease and autoimmune phenomena [1, 2] suggesting the presence of immune regulatory cells. In 1995, Sakaguchi described a subset of CD4+ T lymphocytes that express the IL-2Rα (CD25) and suppress autoimmune disease in thymectomized mice and other models of autoimmunity [3]. Isolated CD25+ cells were anergic but suppressed the proliferation of naïve T cells in vitro. Since the 1980s, numerous laboratories confirmed the existence of CD4+ T cell that expresses the IL-2r and is capable of suppressing autoimmunity upon transfer of CD4+CD25+ cells. Mice lacking the IL2Rα or IL2Rβ chain suffer from systemic autoimmunity [4, 5]. The key lineage-defining transcription factor (TF) Forkhead Box P3 (Foxp3), together with other transcriptional regulators, was found to control the expression of gene programs, which define and maintain Treg cell identity and function [6, 7]. The importance of Foxp3 is also illustrated by Foxp3 gene mutations. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked (IPEX) syndrome in humans and scurfy mutant mice, both bearing Foxp3 mutations, develop severe autoimmunity [8, 9] such as inflammatory bowel disease, and allergy accompanying hyperproduction of IgE [9,10,11]. Depletion of Treg cells in adults also leads to similar autoimmune pathology, demonstrating that Treg cells are needed for lifelong maintenance, of immune self-tolerance and homeostasis [12]. Ectopic expression of Foxp3 confers suppressive capacity on conventional T cells [13]. In addition to Foxp3 expression itself, an increasing amount of publications highlight the role of the epigenome for the development and identification of the Treg cell lineage, such as DNA methylation, nucleosome positioning, or histone modifications. Treg cell development requires the establishment of Treg-specific DNA hypomethylation pattern [14]. DNA hypomethylation is linked to transcriptionally permissive states, which enable transcription factors to bind to their target gene loci [15]. Foxp3 has also been shown to function as a transcriptional repressor [16] and contributes to Treg function by exploiting a preexisting enhancer network which is established during Treg cell development [17].
Regulation of Foxp3
Three main post-translational modifications have been described for the Foxp3 protein, namely acetylation, phosphorylation, and ubiquitination, which affects the DNA-binding capacity of Foxp3 and thus Treg function. The binding of Foxp3 expression to its targets and stable Foxp3 expression is promoted by acetylation of lysine residues by lysine acetyltransferases, such as TIP60 and p300 [18]. Phosphorylation is performed at serine and threonine residues by several kinases, including PIM-1, PIM-2, and CDK2 [19,20,21].
Transcriptional regulation of Treg cell development
Foxp3 mainly acts as a transcriptional repressor and thereby contributes to the characteristics of Treg cells. Foxp3 target genes are predominantly activated by TCR stimulation such as Zap70, Ptpn22, and Itk. Foxp3 also represses IL-2 production and also Satb1 to inhibit the expression of pro-inflammatory cytokines [22,23,24,25]. Foxp3 is also involved in the upregulation of key genes, such as IL2Rα, CTLA-4, and TNFRSF18 [25, 26]. Similar to other TFs Foxp3 interacts with a number of other cofactors, which are required for Treg phenotype and function in physiological and pathological conditions [7, 27,28,29,30]. The requirement to interact with cofactors indicates that they also need to be expressed in Treg cells for Foxp3-dependent transcription but are also direct targets of Foxp3 itself which indicates that Foxp3 and some cofactors also positively regulate each other [31,32,33]. Five TF have been shown to cooperate with Foxp3 to generate most of the Treg-type gene expression, namely Eos, Gata1, IRF4, Satb1, and Lef1 [6]. A more global analysis suggests that Foxp3 binding partner build a network of multi-protein complexes that bind to pre-existing DNA enhancers and regulate gene transcription [34, 35]. Foxp3 also interacts with genes that mediate epigenetic modifications, such as TIP60, sirtuins, and HDAC7 and thereby modulate TF binding and gene transcription [36].
Epigenetic regulation of Treg cells
Stable expression of Foxp3 and its cofactors is linked to certain epigenetic modifications, which include, for example, DNA methylation and histone modifications, which are important for stable Foxp3 expression: While methylation of CpG residues interferes with TF binding, demethylation increases the accessibility. Treg specific DNA demethylated regions (TSDRs) are present in Foxp3 gene but also in other Treg signature genes, such as Ikzf2, Ikzf4, Tnfrsf18, and CTLA4 and correlate with Treg cell stability [37, 38]. The FOXP3 gene hast three conserved noncoding enhancer regions, CNS1–3 [39]. CNS2 has binding sites for Runx1-CBF-β TF complexes that are important for Foxp3 stability and prevention of autoimmunity. In line, CNS2 deficient mice develop signs of autoimmunity. CNS2 contains a conserved CpG island (TSDR), which is hypomethylated in Treg cells. The methylation pattern allows distinguishing Treg cells from conventional T cells [40,41,42,43].
Histone modification is another important regulator of gene transcription and cell identity [44]. TF binding depends on the chromatin formation. Determination of histone modifications, such as monomethylation, dimethylation, and trimethylation of histone H3 at Lys4 (H3K4me1, H3K4me2, and H3K4me3, respectively), acetylation, and trimethylation of histone H3 at Lys27 (H3K27ac, H3K27me3), and acetylation of histone H3 at Lys9 (H3K9ac), allows to define permissive and repressive chromatin states. Studying these histone modifications further allows the definition of the status of gene transcription and enhancer activity [44, 45]. Treg cells have a unique pattern of histone modifications. While permissive histone modifications are found in upregulated genes in Treg cells, repressive modifications are associated with downregulated genes, partially controlled in a Foxp3 dependent manner. Foxp3 target genes are characterized by repressive marks [23]. In contrast, promoters of Treg signature genes are marked with permissive histone modifications and correlate with gene expression, such as DNA demethylation at TSDRs, suggesting that Treg cell-specific DNA demethylation and histone modifications have similar roles in the maintenance of Treg cells [44]. This epigenetic landscape needs to be established before or with the expression of lineage-specifying TFs [46]. In addition, cell type-specific super-enhancers have been identified, that define cell identity and lineage specification [47,48,49]. Recent data show that super-enhancers in Treg cells are gradually established and activated in early stages of Treg cell development before the expression of Treg specific DNA demethylation. Treg specific super-enhancers were associated with Treg signature genes, such as Foxp3, CTLA4, and IL2Rα [50].
Types of Treg cells
Different types of Treg cells have been described, which can be classified according to their developmental origin: thymus-derived or formally called naturally occurring Treg cells develop in the thymus as a separate lineage at the stage of CD4+ single-positive thymocytes and are thought to show enrichment for T cell antigen receptors (TCRs) with high affinity for self-peptides [51]. Peripherally derived Treg (pTreg) cells are generated in the periphery upon encounter to antigens in the presence of additional factors such as IL-2 and TGF-beta [36] as compared to in vitro generated Treg (iTreg) cells [52]. One of the most exciting advances in the field of Treg cells was the understanding of tissue-resident Treg cells, which will contribute to the resolution of local inflammation. The most recent findings are reviewed elsewhere [36, 53].
Function of Treg cells
A wide range of Treg cell-mediated suppressive mechanisms have been discussed as mediators for Treg cell function, including CTLA-4, IL-10, TGF-β, ITGb8, IL-35, granzyme, perforin, CD39, CD73, and TIGIT [54], and the overall view in the field is that there is no one universal mechanism. Treg cell can mediate their suppressive function depending on the particular situation [55]. CTLA-4 has been described as a key molecule for Treg function. Loss of CTLA-4 in Treg cells leads to the development of fatal autoimmunity in mice [56,57,58]. Similarly, haploinsufficiency of CTLA-4 leads to severe autoimmune syndrome, similar to the IPEX syndrome [59, 60]. A variety of possible mechanisms have been shown, but the exact role of CTLA-4 in Treg function remains not fully understood. Treg cells may, for example, downregulate CD80 and CD86 on dendritic cells in a CTLA-4 dependent manner to inhibit effector cell activation [61]. CTLA-4 ligation may also lead to expression of indoleamine 2,3-dioxygenase [62]. TGF-β, IL-10, and IL-35 are involved in the direct suppression of effector signaling and are the main regulatory cytokines released by Treg cells [55]. Treg cells may also exert their suppressive function through granzymes and are thereby able to effector functions through apoptosis [63].
Another key feature of Treg cells is their inability to produce IL-2, which is essential for proliferation and differentiation of effector T cells. Binding to the IL-2 receptor of Treg cells leads to IL-2 deprivation from other T cells and therefore represents one mechanism of immune-mediated suppression. Overexpression of CTLA-4 and repression of IL-2 in effector T cells resembles Treg-mediated suppressive features [64].
Plasticity vs stability of Treg cells
Loss of Foxp3 expression in tTreg cells has been observed under various conditions in vitro as well as in different settings in vivo and has been shown to contribute to autoimmunity and inflammation [65,66,67,68,69,70,71,72,73]. Treg cells that lost Foxp3 expression during the development of experimental autoimmune encephalitis (EAE) and diabetes gained effector functions and could induce EAE and could transfer diabetes similar to effector T cells [68, 74]. Under arthritic conditions, exFoxp3 cells transdifferentiate into Th17 cells with an osteoclastogenic potential [75]. However, in order to regulate immune responses, Treg cells also need to adapt to their local environment which requires a certain degree of plasticity. Treg cells also need to upregulate certain TF to control inflammatory responses, while Foxp3 expression is maintained. Expression of the TF T-bet is necessary to control Th1-mediated inflammation [76]. Development of Tbet+Treg cells is dependent on the TF STAT1 and occurred in response to IFN-γ [77]. Loss of Tbet+Treg cells results in severe Th1 autoimmunity [78]. In addition to T-bet expression, Th1-like Treg cells upregulate the chemokine receptor CCR5 and CXCR3. Increased frequencies of IFN-γ producing Treg cells have also been observed in autoimmune diseases such as type 1 diabetes [79], multiple sclerosis [80], and autoimmune hepatitis [81]. STAT3 expression has been shown to be important for the regulation for Th17-mediated diseases [82]. RORC expressing Th17-like Treg cells have also been described in healthy subjects, which secrete IL-17, express CCR4, and CCR6 and maintain suppressive function [83, 84]. The role in human autoimmune diseases remains controversial, since beneficial as well as pathogenic aspects have been described depending on the disease setting [75, 85,86,87,88,89]. Expression of the TF IRF4 or GATA3 has been shown to be a key element in Treg-mediated suppression of Th2-mediated inflammation [90, 91]. Th2 like Treg cells, upregulate IRF-4, and GATA3 and secrete Th2-associated cytokines such as IL-4 and IL-13 and have been observed in mice susceptible to allergy and in patients with food allergy and systemic sclerosis [92, 93]. T helper cell-like Treg cells have a demethylated TSDR in the Foxp3 locus, which suggests a reversible phenotype [79].
Identifications of Treg cells in humans
Human Treg cells were identified in the thymus and the peripheral blood as CD4+ T cells with the highest expression of CD25. Since CD25 and Foxp3 are also expressed by activated CD4+ T cells, much research has been focused on the identification of further markers to precisely distinguish Treg cells from recently activated T cells. Foxp3 as an intracellular protein cannot be used for Treg isolation and functional characterization. However, CD127, the IL-7 receptor a-chain, in combination with CD25 can be used to identify and isolate Treg cells [94]. Foxp3 expression and suppressive capacity are enriched in T cells with low expression levels of CD127. However, also CD127 has its limitation as a marker for Treg cells. It is also downregulated in activated T cells and a high percentage of CD127+ cells express Foxp3 and reciprocally cells with low expression of CD127 did not express Foxp3 [95]. A variety of additional markers were described for the identification of Treg cells, including GITR, CTLA-4, and CD49d [56, 96, 97]. Depletion of CD49d removes effector T cells from CD25+ Treg cells and allows purification of Foxp3+ cells in combination with CD127. Miyara et al. divided the Treg cell compartment into three subpopulations according to the expression of CD45RA, namely suppressive CD45RA+Foxp3low (resting Treg cells), CD45RA−Foxp3high (activated Treg cells), and non-suppressive cytokine secreting CD45RA−Foxp3low non-Treg cells [98].
Treg cells in human autoimmune diseases
Studies from monogenic conditions reveal the importance of Treg cells for human immune homeostasis. Patients with CD25 deficiency suffer from autoimmune phenomena and immunodeficiency, similar to the already mentioned IPEX syndrome. Although CD25 deficiency does not affect Treg numbers, impaired suppressive activity has been observed due to decreased IL-10 production [99]. Besides CD25, mutations in other crucial genes have been reported, which are associated with autoimmune phenotypes, such as STAT5B, CTLA4, or LRBA [60, 100,101,102]. Human studies on Treg cells clearly suffer from the limitation of a definition and the clear identification of Treg cells. In the last decade, an increasing amount of papers have been published, which address numbers and functions of Treg cells in a variety of autoimmune diseases. Due to the evolving numbers of different Treg markers and the lack of a clear definition of a human Treg cells, partly conflicting results in numbers and or function of Treg cells, isolated from the peripheral blood (ref. Miyara) have been reported in a variety of autoimmune diseases, including type 1 diabetes, multiple sclerosis, systemic lupus erythematosus (SLE), myasthenia gravis, rheumatoid arthritis (RA), and others. We will only summarize the more recent findings of selected systemic and organ-specific autoimmune diseases. An extensive summary of the publications of systemic autoimmune diseases can be found in Table 1.
Systemic lupus erythematosus
SLE represents a systemic autoimmune disease, which can affect multiple organs. In patients with SLE quantitative as well as functional deficiencies of Treg cells have been described. A more recent publication reports low levels of STAT5 phosphorylation upon IL-2 which suggests an inherent Treg cell defect [195]. Major reasons for the discrepancy of these findings are not only the lack of a reliable Treg specific marker but also the heterogeneity of the disease, the small size of the studied cohorts and different methods used for the isolation of Treg cells. In addition, a subset of Foxp3+ cells that lack the expression of CD25, have been described in SLE patients, which was later on also described in patients with RA [196] and MS [197]. While our own group observed phenotypic and functional characteristics of Treg cells within this newly described cell population [103], others report that CD25−Foxp3+ Treg cells in SLE patients are activated T cells, rather than a distinct Treg cell population [198]. In addition, we could show that CD4+CD25−Foxp3+ T cells are elevated in SLE patients with renal involvement. CD4+CD25−Foxp3+ T cells were also detected in urine sediment samples of patients with active glomerulonephritis and correlated with the extent of proteinuria [104]. A recent study confirmed the regulatory features of this cell population, including demethylation of the Foxp3 TSDR and constitutive expression of the TF HELIOS in the majority of the cells and an inability to produce IL-2 [105]. Interestingly treatment of SLE patients with low dose IL-2 led to a twofold to threefold increase in the expression levels of CD25 in Treg cells and a dramatic expansion of CD25high Treg cells among Foxp3+CD127low Treg cells [199].
Rheumatoid arthritis
RA is a systemic autoimmune disease which leads to chronic inflammation and tissue destruction in the joint. Conflicting results have been reported for numbers of Treg cells in the peripheral blood and the synovial fluid [161, 165,166,167,168,169,170,171, 176,177,178, 192,193,194, 200, 201]. In addition to quantitative defects, functional deficiencies have been reported in patients with RA [192,193,194]. Treatment with anti-TNF and anti-IL6 restored the balance of Treg and Th17 cells in RA patients and can affect Treg function [170, 194, 201].
Ankylosis spondylitis
AS represents an inflammatory autoimmune disease, associated with HLA-B27. Similar to other autoimmune diseases, data on Treg numbers and function in AS patients remain controversial [184,185,186, 202]. Similar to RA, recent data indicate that anti-TNF treatment might affect the Th17/Treg ratio [203, 204].
Systemic sclerosis (SSc)
SSc is a connective tissue disease, which is characterized by immune abnormalities, microvascular injury, and fibrosis of the skin and internal organs [205]. Several reports describe defects in the number of Treg cells, although conflicting results exist [116, 145,146,147,148,149, 151,152,153,154,155,156,157,158,159,160, 206, 207]. Similarly, impaired as well as normal Treg function has been reported in SSc patients [145, 146, 152,153,154, 159]. Mathian et al. report that activated and resting Treg cells are not functionally impaired in SSc patients. Numbers of activated Treg cells are decreased in early and resting Treg cells in late stages of the disease [154].
Treg-based therapies
The contribution of Tregs in various human autoimmune diseases has opened up a new therapeutic avenue, which included Treg-based cellular therapies or therapies which aim to restore the balance of Treg and Teff cells such as IL-2 therapies. The concept to use Treg cells as a cell-based therapeutic approach was first demonstrated in murine models, such as EAE or CIA, in which Treg cells has been shown to be involved in the pathogenesis. Transfer of Treg cells could ameliorate the disease [208, 209]. The strategy of Treg cell transfer cannot simply be translated into humans due to the low number of Treg cells. Thus, manufacturing protocols were developed to expand Treg cells in vitro to produce large numbers of polyclonal Treg cells. Phenotypic and functional characterization showed that expanded Treg cells are highly suppressive with high expression levels of Treg-associated markers such as CTLA4, CD25, and Foxp3 and demethylation of the TSDR [210, 211]. Interestingly, Treg cells from RA patients, which were expanded in the presence of rapamycin, maintained their suppressive capacity and Foxp3 demethylation and were more effective in suppressing conventional T cell proliferation compared with their ex vivo counterparts [212]. Several phase I and phase II clinical trials were designed which use ex vivo expanded Treg cells as an approach for the treatment of autoimmune diseases, such as autoimmune hepatitis, GvHD, type I diabetes, SLE, kidney, and liver transplantation [213,214,215,216].
Beside the development of Treg cell-based therapies, a lot of existing therapies target Treg cells as well as Teff cells, such as rapamycin, anti-CD3, CTLA-4Ig, or anti-CD25 [217,218,219]. Rapamycin, an inhibitor of PI3Kakt-mTORC1 signaling, promotes the expansion and survival of Treg cells, while suppressing the proliferation of Th1 and Th17 cells [220,221,222]. Treatment with CD3 antibody increased Treg number and stabilized Treg function in type 1 diabetic mouse model [223]. Anti-CD3 treatment preserved residual beta-cell function in patients with type 1 diabetes [224, 225].
One of the most exiting concepts, which evolved during the last couple of years is based on the expression of the IL-2 receptor in Treg cells, which allows a preferential expansion of Treg cells with low amounts of IL-2. Since IL-2 is also a known growth factor for effector cells, the use of IL-2 in autoimmune diseases was thought to be contraindicated. However, murine studies, in which IL-2 signaling was impaired but not abrogated, showed that IL-2R-signaling promoted only the development of Treg cells but not effector T cells, which lead to the investigation of low dose IL-2 as a treatment strategy for autoimmune diseases [226,227,228,229]. Treatment with IL-2 was shown to block the differentiation of naïve CD4+ T cells into effector T cells [230, 231]. Indeed, promising data were already reported for a variety of diseases such as type I diabetes, GvHD, alopecia areata, hepatitis C virus-induced vasculitis, and SLE [199, 226, 232,233,234,235]. In patients with alopecia areata, low dose IL-2 treatment expanded Treg cells in the blood and the hair follicles and led to an impressive hair regrowth [234]. In SLE patients, a dysbalance between Treg and effector T cells have been reported, and a recent paper described Treg defects as hallmarks of IL-2 deficiencies. Lack of IL-2 production by CD4+ T cells thereby accounts for the loss of CD25 expression which could be reversed by IL-2 [199]. Treatment with low dose IL-2 was anticipated as a potentially effective treatment. Indeed, numbers of circulating Treg cells were significantly increased [199] along with a reduction in disease activity [235]. A more recent study by Klatzmann et al. investigated the potential of low dose IL-2 therapy as a new therapeutic approach in 11 different autoimmune diseases, including RA, AS, SLE, psoriasis, Behcet’s disease, granulomatosis with polyangiitis (GPA), Takayasu’s disease, Crohn’s disease (CD), ulcerative colitis, autoimmune hepatitis, and sclerosing cholangitis. In general, low dose IL-2 was well tolerated and led to a Treg specific expansion and activation. These data certainly highlight the potential use of this treatment strategy for various additional autoimmune and inflammatory diseases [236].
Conclusion
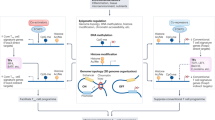
Technological progress in the recent years allowed to define and characterize murine Treg cells and helped to understand the balance between Treg plasticity, which is necessary for proper Treg function but also Treg instability, which can drive autoimmunity (Fig. 1). On the other hand, data from the human system, especially in patients with autoimmune or inflammatory conditions are still conflicting and misleading due to a lack of a reliable Treg cell-specific marker. The progress that has been made in the murine system has to be translated in the human system. It will be important to determine the physiological relevance and the mechanism that drive plasticity and stability of Treg cells in patients with autoimmune diseases and the contribution of the epigenetic signature to disease pathology. This is ultimately necessary for the new and exciting treatment approaches, which target Treg cells as a direct Treg-based therapy, using polyclonal Treg cells or as a Treg cell-targeted therapy. Clear identification of patients with functional or numerical Treg deficiencies will be necessary for future successful treatment and monitoring of patients with Treg targeted therapies. A more detailed understanding of the exact role of Treg cells under various inflammatory conditions will help to develop a personalized treatment approach within the next years.
References
MCintire KR, Sell S, Miller JF (1964) Pathogenesis of the post-neonatal thymectomy wasting syndrome. Nature 204:151–155
Nishizuka Y, Sakakura T (1969) Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science. 166:753–755
Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M (1995) Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 155:1151–1164
Malek TR, Yu A, Vincek V, Scibelli P, Kong L (2002) CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity 17:167–178
Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ (2002) Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med 196:851–857
Fu W, Ergun A, Lu T, Hill JA, Haxhinasto S, Fassett MS et al (2012) A multiply redundant genetic switch “locks in” the transcriptional signature of regulatory T cells. Nat Immunol 13:972–980. https://doi.org/10.1038/ni.2420
Rudra D, deRoos P, Chaudhry A, Niec RE, Arvey A, Samstein RM et al (2012) Transcription factor Foxp3 and its protein partners form a complex regulatory network. Nat Immunol 13:1010–1019. https://doi.org/10.1038/ni.2402
Fontenot JD, Gavin MA, Rudensky AY (2003) Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4:330–336. https://doi.org/10.1038/ni904
Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L et al (2001) The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 27:20–21. https://doi.org/10.1038/83713
Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA et al (2001) Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet 27:68–73. https://doi.org/10.1038/83784
Wildin RS, Smyk-Pearson S, Filipovich AH (2002) Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet 39:537–545
Kim JM, Rasmussen JP, Rudensky AY (2007) Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol 8:191–197. https://doi.org/10.1038/ni1428
Hori S (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057–1061. https://doi.org/10.1126/science.1079490
Ohkura N, Kitagawa Y, Sakaguchi S (2013) Development and maintenance of regulatory T cells. Immunity 38:414–423. https://doi.org/10.1016/j.immuni.2013.03.002
Thurman RE, Rynes E, Humbert R, Vierstra J, Maurano MT, Haugen E et al (2012) The accessible chromatin landscape of the human genome. Nature 489:75–82. https://doi.org/10.1038/nature11232
Schubert LA, Jeffery E, Zhang Y, Ramsdell F, Ziegler SF (2001) Scurfin (FOXP3) acts as a repressor of transcription and regulates T cell activation. J Biol Chem 276:37672–37679
Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY (2012) Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell 150:29–38. https://doi.org/10.1016/j.cell.2012.05.031
van Loosdregt J, Vercoulen Y, Guichelaar T, Gent YYJ, Beekman JM, van Beekum O et al (2010) Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood 115:965–974. https://doi.org/10.1182/blood-2009-02-207118
Deng G, Nagai Y, Xiao Y, Li Z, Dai S, Ohtani T et al (2015) Pim-2 kinase influences regulatory T cell function and stability by mediating Foxp3 protein N-terminal phosphorylation. J Biol Chem 290:20211–20220. https://doi.org/10.1074/jbc.M115.638221
Li Z, Lin F, Zhuo C, Deng G, Chen Z, Yin S et al (2014) PIM1 kinase phosphorylates the human transcription factor FOXP3 at serine 422 to negatively regulate its activity under inflammation. J Biol Chem 289:26872–26881. https://doi.org/10.1074/jbc.M114.586651
Morawski PA, Mehra P, Chen C, Bhatti T, Wells AD (2013) Foxp3 protein stability is regulated by cyclin-dependent kinase 2. J Biol Chem 288:24494–24502. https://doi.org/10.1074/jbc.M113.467704
Morikawa H, Ohkura N, Vandenbon A, Itoh M, Nagao-Sato S, Kawaji H et al (2014) Differential roles of epigenetic changes and Foxp3 expression in regulatory T cell-specific transcriptional regulation. Proc Natl Acad Sci 111:5289–5294. https://doi.org/10.1073/pnas.1312717110
Arvey A, van der Veeken J, Samstein RM, Feng Y, Stamatoyannopoulos JA, Rudensky AY (2014) Inflammation-induced repression of chromatin bound by the transcription factor Foxp3 in regulatory T cells. Nat Publ Group 15:580–587. https://doi.org/10.1038/ni.2868
Beyer M, Thabet Y, Müller R-U, Sadlon T, Classen S, Lahl K et al (2011) Repression of the genome organizer SATB1 in regulatory T cells is required for suppressive function and inhibition of effector differentiation. Nat Immunol 12:898–907. https://doi.org/10.1038/ni.2084
Marson A, Kretschmer K, Frampton GM, Jacobsen ES, Polansky JK, MacIsaac KD et al (2007) Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature 445:931–935. https://doi.org/10.1038/nature05478
Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA et al (2007) Foxp3-dependent programme of regulatory T-cell differentiation. Nature 445:771–775. https://doi.org/10.1038/nature05543
Darce J, Rudra D, Li L, Nishio J, Cipolletta D et al (2012) An N-terminal mutation of the Foxp3 transcription factor alleviates arthritis but exacerbates diabetes. Immunity 36:731–741. https://doi.org/10.1016/j.immuni.2012.04.007
Bettelli E, Dastrange M, Oukka M (2005) Foxp3 interacts with nuclear factor of activated T cells and NF-kappa B to repress cytokine gene expression and effector functions of T helper cells. Proc Natl Acad Sci 102:5138–5143. https://doi.org/10.1073/pnas.0501675102
Xiao Y, Nagai Y, Deng G, Ohtani T, Zhu Z, Zhou Z et al (2014) Dynamic interactions between TIP60 and p300 regulate FOXP3 function through a structural switch defined by a single lysine on TIP60. Cell Rep 7:1471–1480. https://doi.org/10.1016/j.celrep.2014.04.021
Pan F, Yu H, Dang EV, Barbi J, Pan X, Grosso JF et al (2009) Eos mediates Foxp3-dependent gene silencing in CD4+ regulatory T cells. Science 325:1142–1146. https://doi.org/10.1126/science.1176077
Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M (2008) Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol 9:194–202. https://doi.org/10.1038/ni1549
Bruno L, Mazzarella L, Hoogenkamp M, Hertweck A, Cobb BS, Sauer S et al (2009) Runx proteins regulate Foxp3 expression. J Exp Med 206:2329–2337. https://doi.org/10.1084/jem.20090226
Vanvalkenburgh J, Albu DI, Bapanpally C, Casanova S, Califano D, Jones DM et al (2011) Critical role of Bcl11b in suppressor function of T regulatory cells and prevention of inflammatory bowel disease. J Exp Med 208:2069–2081. https://doi.org/10.1084/jem.20102683
Samstein RM, Arvey A, Josefowicz SZ, Peng X, Reynolds A, Sandstrom R et al (2012) Foxp3 exploits a pre-existent enhancer landscape for regulatory T cell lineage specification. Cell 151:153–166. https://doi.org/10.1016/j.cell.2012.06.053
Kwon H-K, Chen H-M, Mathis D, Benoist C (2017) Different molecular complexes that mediate transcriptional induction and repression by FoxP3. Nat Immunol 18:1238–1248. https://doi.org/10.1038/ni.3835
Dominguez-Villar M, Hafler DA (2018) Regulatory T cells in autoimmune disease. Nat Immunol 1–9. https://doi.org/10.1038/s41590-018-0120-4.
Polansky JK, Schreiber L, Thelemann C, Ludwig L, Krüger M, Baumgrass R et al (2010) Methylation matters: binding of Ets-1 to the demethylated Foxp3 gene contributes to the stabilization of Foxp3 expression in regulatory T cells. J Mol Med 88:1029–1040. https://doi.org/10.1007/s00109-010-0642-1
Kim H-P, Leonard WJ (2007) CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. J Exp Med 204:1543–1551. https://doi.org/10.1084/jem.20070109
Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY (2010) Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 463:808–812. https://doi.org/10.1038/nature08750
Feng Y, Arvey A, Chinen T, van der Veeken J, Gasteiger G, Rudensky AY (2014) Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell 158:749–763. https://doi.org/10.1016/j.cell.2014.07.031
Li X, Liang Y, LeBlanc M, Benner C, Zheng Y (2014) Function of a Foxp3 cis-element in protecting regulatory T cell identity. Cell 158:734–748. https://doi.org/10.1016/j.cell.2014.07.030
Baron U, Floess S, Wieczorek G, Baumann K, Grutzkau A, Dong J et al (2007) DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3(+) conventional T cells. Eur J Immunol 37:2378–2389. https://doi.org/10.1002/eji.200737594
Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J et al (2007) Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol 5:e38. https://doi.org/10.1371/journal.pbio.0050038
Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z et al (2009) Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30:155–167. https://doi.org/10.1016/j.immuni.2008.12.009
Bannister AJ, Kouzarides T (2011) Regulation of chromatin by histone modifications. Cell Res 21:381–395. https://doi.org/10.1038/cr.2011.22
Arner E, Daub CO, Vitting-Seerup K, Andersson R, Lilje B, Drablos F et al (2015) Transcribed enhancers lead waves of coordinated transcription in transitioning mammalian cells. Science 347:1010–1014. https://doi.org/10.1126/science.1259418
Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH et al (2013) Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153:307–319. https://doi.org/10.1016/j.cell.2013.03.035
Hnisz D, Abraham BJ, Lee TI, Lau A, Saint-André V, Sigova AA et al (2013) Super-enhancers in the control of cell identity and disease. Cell. 155:934–947. https://doi.org/10.1016/j.cell.2013.09.053
Vahedi G, Kanno Y, Furumoto Y, Jiang K, Parker SCJ, Erdos MR et al (2015) Super-enhancers delineate disease-associated regulatory nodes in T cells. Nature 520:558–562. https://doi.org/10.1038/nature14154
Kitagawa Y, Ohkura N, Kidani Y, Vandenbon A, Hirota K, Kawakami R et al (2016) Guidance of regulatory T cell development by Satb1-dependent super-enhancer establishment. Nat Immunol 18:173–183. https://doi.org/10.1038/ni.2590
Josefowicz SZ, Lu L-F, Rudensky AY (2012) Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 30:531–564. https://doi.org/10.1146/annurev.immunol.25.022106.141623
Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S et al (2013) Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol 14:307–308. https://doi.org/10.1038/ni.2554
Burzyn D, Benoist C, Mathis D (2013) Regulatory T cells in nonlymphoid tissues. Nat Immunol 14:1007–1013. https://doi.org/10.1038/ni.2683
Yamaguchi T, Wing JB, Sakaguchi S (2011) Two modes of immune suppression by Foxp3(+) regulatory T cells under inflammatory or non-inflammatory conditions. Semin Immunol 23:424–430. https://doi.org/10.1016/j.smim.2011.10.002
Shevach EM (2018) Foxp3+ T regulatory cells: still many unanswered questions—a perspective after 20 years of study. Front Immun 9:389. https://doi.org/10.1056/NEJMoa1105143
Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z et al (2008) CTLA-4 control over Foxp3+ regulatory T cell function. Science 322:271–275. https://doi.org/10.1126/science.1160062
Wing JB, Ise W, Kurosaki T, Sakaguchi S (2014) Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity 41:1013–1025. https://doi.org/10.1016/j.immuni.2014.12.006
Sage PT, Paterson AM, Lovitch SB, Sharpe AH (2014) The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity 41:1026–1039. https://doi.org/10.1016/j.immuni.2014.12.005
Kuehn HS, Ouyang W, Lo B, Deenick EK, Niemela JE, Avery DT et al (2014) Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science 345:1623–1627. https://doi.org/10.1126/science.1255904
Schubert D, Bode C, Kenefeck R, Hou TZ, Wing JB, Kennedy A et al (2014) Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med 20:1410–1416. https://doi.org/10.1038/nm.3746
Oderup C, Cederbom L, Makowska A, Cilio CM, Ivars F (2006) Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology 118:240–249. https://doi.org/10.1111/j.1365-2567.2006.02362.x
Schmidt A, Oberle N, Krammer PH (2012) Molecular mechanisms of Treg-mediated T cell suppression. Front Immun 3:51. https://doi.org/10.3389/fimmu.2012.00051
Gondek DC, Devries V, Nowak EC, Lu L-F, Bennett KA, Scott ZA et al (2008) Transplantation survival is maintained by granzyme B+ regulatory cells and adaptive regulatory T cells. J Immunol 181:4752–4760
Yamaguchi T, Kishi A, Osaki M, Morikawa H, Prieto-Martin P, Wing K et al (2013) Construction of self-recognizing regulatory T cells from conventional T cells by controlling CTLA-4 and IL-2 expression. Proc Natl Acad Sci 110:E2116–E2125. https://doi.org/10.1073/pnas.1307185110
Williams LM, Rudensky AY (2007) Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol 8:277–284. https://doi.org/10.1038/ni1437
Komatsu N, Mariotti-Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S (2009) Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci 106:1903–1908. https://doi.org/10.1073/pnas.0811556106
Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D et al (2010) Stability of the regulatory T cell lineage in vivo. Science 329:1667–1671. https://doi.org/10.1126/science.1191996
Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, Ashby M et al (2009) Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol 10:1000–1007. https://doi.org/10.1038/ni.1774
Hoffmann P, Boeld TJ, Eder R, Huehn J, Floess S, Wieczorek G et al (2018) Regulatory T cells in the treatment of disease. Nat Publ Group 17:823–844. https://doi.org/10.1038/nrd.2018.148
Koenen HJPM, Smeets RL, Vink PM, van Rijssen E, Boots AMH, Joosten I (2008) Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood 112:2340–2352. https://doi.org/10.1182/blood-2008-01-133967
Laurence A, Amarnath S, Mariotti J, Kim YC, Foley J, Eckhaus M et al (2012) STAT3 transcription factor promotes instability of nTreg cells and limits generation of iTreg cells during acute murine graft-versus-host disease. Immunity 37:209–222. https://doi.org/10.1016/j.immuni.2012.05.027
Duarte JH, Zelenay S, Bergman M-L, Martins AC, Demengeot J (2009) Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur J Immunol 39:948–955. https://doi.org/10.1002/eji.200839196
Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, Dos Santos L et al (2009) Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity 31:772–786. https://doi.org/10.1016/j.immuni.2009.10.001
Bailey-Bucktrout SL, Martinez-Llordella M, Zhou X, Anthony B, Rosenthal W, Luche H et al (2013) Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity 39:949–962. https://doi.org/10.1016/j.immuni.2013.10.016
Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T et al (2013) Pathogenic conversion of Foxp3. Nat Med 20:62–68. https://doi.org/10.1038/nm.3432
Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ (2009) The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol 10:595–602. https://doi.org/10.1038/ni.1731
Koch MA, Thomas KR, Perdue NR, Smigiel KS, Srivastava S, Campbell DJ (2012) T-bet+ Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor β2. Immunity 37:501–510. https://doi.org/10.1016/j.immuni.2012.05.031
Levine AG, Medoza A, Hemmers S, Moltedo B, Niec RE, Schizas M et al (2017) Stability and function of regulatory T cells expressing the transcription factor T-bet. Nature 546:421–425. https://doi.org/10.1038/528S132a
McClymont SA, Putnam AL, Lee MR, Esensten JH, Liu W, Hulme MA et al (2011) Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J Immunol 186:3918–3926. https://doi.org/10.4049/jimmunol.1003099
Kitz A, de Marcken M, Gautron A-S, Mitrovic M, Hafler DA, Dominguez-Villar M (2016) AKT isoforms modulate Th1-like Treg generation and function in human autoimmune disease. EMBO Rep 17:1169–1183. https://doi.org/10.15252/embr.201541905
Arterbery AS, Osafo-Addo A, Avitzur Y, Ciarleglio M, Deng Y, Lobritto SJ et al (2016) Production of proinflammatory cytokines by monocytes in liver-transplanted recipients with de novo autoimmune hepatitis is enhanced and induces TH1-like regulatory T cells. J Immunol 196:4040–4051. https://doi.org/10.4049/jimmunol.1502276
Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A et al (2009) CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 326:986–991. https://doi.org/10.1126/science.1172702
Beriou G, Costantino CM, Ashley CW, Yang L, Kuchroo VK, Baecher-Allan C et al (2009) IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood 113:4240–4249. https://doi.org/10.1182/blood-2008-10-183251
Ayyoub M, Deknuydt F, Raimbaud I, Dousset C, Leveque L, Bioley G et al (2009) Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc Natl Acad Sci 106:8635–8640. https://doi.org/10.1073/pnas.0900621106
Singh K, Gatzka M, Peters T, Borkner L, Hainzl A, Wang H et al (2013) Reduced CD18 levels drive regulatory T cell conversion into Th17 cells in the CD18hypo PL/J mouse model of psoriasis. J Immunol 190:2544–2553. https://doi.org/10.4049/jimmunol.1202399
Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM et al (2015) Individual intestinal symbionts induce a distinct population of RORγ<sup>+</sup> regulatory T cells. Science 349:993
Yang B-H, Hagemann S, Mamareli P, Lauer U, Hoffmann U, Beckstette M et al (2016) Foxp3(+) T cells expressing RORgammat represent a stable regulatory T-cell effector lineage with enhanced suppressive capacity during intestinal inflammation. Mucosal Immunol 9:444–457. https://doi.org/10.1038/mi.2015.74
Kluger MA, Luig M, Wegscheid C, Goerke B, Paust H-J, Brix SR et al (2014) Stat3 programs Th17-specific regulatory T cells to control GN. J Am Soc Nephrol 25:1291–1302. https://doi.org/10.1681/ASN.2013080904
Kluger MA, Melderis S, Nosko A, Goerke B, Luig M, Meyer MC et al (2016) Treg17 cells are programmed by Stat3 to suppress Th17 responses in systemic lupus. Kidney Int 89:158–166. https://doi.org/10.1038/ki.2015.296
Zheng Y, Chaudhry A, Kas A, de Roos P, Kim JM, Chu T-T et al (2009) Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control TH2 responses. Nature 458:351–356. https://doi.org/10.1038/nature07674
Wohlfert EA, Grainger JR, Bouladoux N, Konkel JE, Oldenhove G, Ribeiro CH et al (2011) GATA3 controls Foxp3+ regulatory T cell fate during inflammation in mice. J Clin Invest 121:4503–4515. https://doi.org/10.1172/JCI57456DS1
Rivas MN, Burton OT, Wise P, Charbonnier L-M, Georgiev P, Oettgen HC et al (2015) Regulatory T cell reprogramming toward a Th2-cell- like lineage impairs oral tolerance and promotes food allergy. Immunity 42:512–523. https://doi.org/10.1016/j.immuni.2015.02.004
KG MD, Dawson NA, Huang Q, Dunne JV, Levings MK, Broady R (2015) Regulatory T cells produce profibrotic cytokines in the skin of patients with systemic sclerosis. J Allergy Clin Immunol 135:946–955.e9. https://doi.org/10.1016/j.jaci.2014.12.1932
Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S et al (2006) CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 203:1701–1711. https://doi.org/10.1084/jem.20060772
Klein S, Kretz CC, Krammer PH, Kuhn A (2010) CD127(low/−) and FoxP3(+) expression levels characterize different regulatory T-cell populations in human peripheral blood. J Investig Dermatol 130:492–499. https://doi.org/10.1038/jid.2009.313
Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S (2002) Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol 3:135–142
Piconese S, Valzasina B, Colombo MP (2008) OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med 205:825–839. https://doi.org/10.1084/jem.20071341
Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A et al (2009) Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30:899–911. https://doi.org/10.1016/j.immuni.2009.03.019
Malek TR (2008) The biology of interleukin-2. Annu Rev Immunol 26:453–479. https://doi.org/10.1146/annurev.immunol.26.021607.090357
Bernasconi A, Marino R, Ribas A, Rossi J, Ciaccio M, Oleastro M et al (2006) Characterization of immunodeficiency in a patient with growth hormone insensitivity secondary to a novel STAT5b gene mutation. Pediatrics 118:e1584–e1592. https://doi.org/10.1542/peds.2005-2882
Nadeau K, Hwa V, Rosenfeld RG (2011) STAT5b deficiency: an unsuspected cause of growth failure, immunodeficiency, and severe pulmonary disease. J Pediatr 158:701–708. https://doi.org/10.1016/j.jpeds.2010.12.042
Charbonnier L-M, Janssen E, Chou J, Ohsumi TK, Keles S, Hsu JT et al (2015) Regulatory T-cell deficiency and immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like disorder caused by loss-of-function mutations in LRBA. J Allergy Clin Immunol 135:217–227. https://doi.org/10.1016/j.jaci.2014.10.019
Bonelli M, Savitskaya A, Steiner CW, Rath E, Smolen JS, Scheinecker C (2009) Phenotypic and functional analysis of CD4+CD25-Foxp3+ T cells in patients with systemic lupus erythematosus. J Immunol 182:1689–1695. https://doi.org/10.4049/jimmunol.182.3.1689
Bonelli M, Schl LG, Blüml S, Karonitsch T, Steiner C-W, Steiner GN et al (2014) T cells: a marker for lupus nephritis? 16:1–11. https://doi.org/10.1186/ar4553
Ferreira RC, Simons HZ, Thompson WS, Rainbow DB, Yang X, Cutler AJ et al (2017) Cells with Treg-specific FOXP3 demethylation but low CD25 are prevalent in autoimmunity. J Autoimmun 84:75–86. https://doi.org/10.1016/j.jaut.2017.07.009
Mesquita D, Kirsztajn GM, Franco MF, Reis LA, Perazzio SF, Mesquita FV et al (2018) CD4+ T helper cells and regulatory T cells in active lupus nephritis: an imbalance towards a predominant Th1 response? Clin Exp Immunol 191:50–59. https://doi.org/10.1111/cei.13050
Singla S, Wenderfer SE, Muscal E, Sagcal-Gironella ACP, Orange JS, Makedonas G (2017) Changes in frequency and activation status of major CD4(+) T-cell subsets after initiation of immunosuppressive therapy in a patient with new diagnosis childhood-onset systemic lupus erythematosus. Front Pediatr 5:104. https://doi.org/10.3389/fped.2017.00104
Eltayeb AA, Sayed DM, Afifi NA, Ibrahim MA, Sheref TM (2014) Regulatory T cell subsets in children with systemic lupus erythematosus. Clin Rheumatol 33:1085–1091. https://doi.org/10.1007/s10067-014-2636-9
Prado C, de Paz B, López P, Gomez J, Rodríguez-Carrio J, Suárez A (2013) Relationship between FOXP3 positive populations and cytokine production in systemic lupus erythematosus. Cytokine 61:90–96. https://doi.org/10.1016/j.cyto.2012.08.033
Yan B, Ye S, Chen G, Kuang M, Shen N, Chen S (2008) Dysfunctional CD4+,CD25+ regulatory T cells in untreated active systemic lupus erythematosus secondary to interferon-α–producing antigen-presenting cells. Arthritis Rheum 58:801–812. https://doi.org/10.1002/art.23268
Azab NA, Bassyouni IH, Emad Y, Abd El-Wahab GA, Hamdy G, Mashahit MA (2008) CD4+CD25+ regulatory T cells (TREG) in systemic lupus erythematosus (SLE) patients: the possible influence of treatment with corticosteroids. Clin Immunol 127:151–157. https://doi.org/10.1016/j.clim.2007.12.010
Suarez A, Lopez P, Gomez J, Gutierrez C (2006) Enrichment of CD4+ CD25high T cell population in patients with systemic lupus erythematosus treated with glucocorticoids. Ann Rheum Dis 65:1512–1517. https://doi.org/10.1136/ard.2005.049924
Rodriguez-Reyna TS, Furuzawa-Carballeda J, Cabiedes J, Fajardo-Hermosillo LD, Martinez-Reyes C, Diaz-Zamudio M et al (2012) Th17 peripheral cells are increased in diffuse cutaneous systemic sclerosis compared with limited illness: a cross-sectional study. Rheumatol Int 32:2653–2660. https://doi.org/10.1007/s00296-011-2056-y
Pan X, Yuan X, Zheng Y, Wang W, Shan J, Lin F et al (2012) Increased CD45RA+ FoxP3(low) regulatory T cells with impaired suppressive function in patients with systemic lupus erythematosus. PLoS One 7:e34662
Mesquita D, de Melo Cruvinel W, Araujo J, Pucci F, Salmazi K, Kallas E et al (2011) Systemic lupus erythematosus exhibits a dynamic and continuum spectrum of effector/regulatory T cells. Scand J Rheumatol 40:41–50. https://doi.org/10.3109/03009742.2010.489229
Banica L, Besliu A, Pistol G, Stavaru C, Ionescu R, Forsea A-M et al (2009) Quantification and molecular characterization of regulatory T cells in connective tissue diseases. Autoimmunity 42:41–49. https://doi.org/10.1080/08916930802282651
Atfy M, Amr GE, Elnaggar AM, Labib HA, Esh A, Elokely AM (2009) Impact of CD4+CD25high regulatory T-cells and FoxP3 expression in the peripheral blood of patients with systemic lupus erythematosus. Egypt J Immunol 16:117–126
Lee H-Y, Hong Y-K, Yun H-J, Kim Y-M, Kim J-R, Yoo W-H (2008) Altered frequency and migration capacity of CD4+CD25+ regulatory T cells in systemic lupus erythematosus. Rheumatology (Oxford) 47:789–794. https://doi.org/10.1093/rheumatology/ken108
Hu S, Xiao W, Kong F, Ke D, Qin R, Su M (2008) Regulatory T cells and their molecular markers in peripheral blood of the patients with systemic lupus erythematosus. J Huazhong Univ Sci Technolog Med Sci 28:549–552. https://doi.org/10.1007/s11596-008-0513-y
Zhao S-S, Li X-M, Li X-P, Zhai Z-M, Chen Z, Ma Y et al (2008) Expression of CD4+ CD25+ CD127(low/−) T cells in patients with systemic lupus erythematosus. Zhonghua Yi Xue Za Zhi 88:453–456
Hahn BH, Anderson M, Le E, La Cava A (2008) Anti-DNA Ig peptides promote Treg cell activity in systemic lupus erythematosus patients. Arthritis Rheum 58:2488–2497. https://doi.org/10.1002/art.23609
Barath S, Aleksza M, Tarr T, Sipka S, Szegedi G, Kiss E (2007) Measurement of natural (CD4+CD25high) and inducible (CD4+IL-10+) regulatory T cells in patients with systemic lupus erythematosus. Lupus 16:489–496. https://doi.org/10.1177/0961203307080226
Lyssuk EY, Torgashina AV, Soloviev SK, Nassonov EL, Bykovskaia SN (2007) Reduced number and function of CD4+CD25highFoxP3+ regulatory T cells in patients with systemic lupus erythematosus. Adv Exp Med Biol 601:113–119
Crispín JC, Martinez A, Alcocer-Varela J (2003) Quantification of regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun 21:273–276
Wang X, Qiao Y, Yang L, Song S, Han Y, Tian Y et al (2017) Leptin levels in patients with systemic lupus erythematosus inversely correlate with regulatory T cell frequency. Lupus 26:1401–1406. https://doi.org/10.1177/0961203317703497
Margiotta D, Navarini L, Vadacca M, Basta F, Lo Vullo M, Pignataro F et al (2016) Relationship between leptin and regulatory T cells in systemic lupus erythematosus: preliminary results. Eur Rev Med Pharmacol Sci 20:636–641
Zabinska M, Krajewska M, Koscielska-Kasprzak K, Jakuszko K, Bartoszek D, Myszka M et al (2016) CD4(+)CD25(+)CD127(−) and CD4(+)CD25(+)Foxp3(+) regulatory T cell subsets in mediating autoimmune reactivity in systemic lupus erythematosus patients. Arch Immunol Ther Exp 64:399–407. https://doi.org/10.1007/s00005-016-0399-5
Legorreta-Haquet MV, Chavez-Rueda K, Chavez-Sanchez L, Cervera-Castillo H, Zenteno-Galindo E, Barile-Fabris L et al (2016) Function of Treg cells decreased in patients with systemic lupus erythematosus due to the effect of prolactin. Medicine (Baltimore) 95:e2384
Dal Ben ERR, do Prado CH, Baptista TSA, Bauer ME, Staub HL (2014) Patients with systemic lupus erythematosus and secondary antiphospholipid syndrome have decreased numbers of circulating CD4(+)CD25(+)Foxp3(+) Treg and CD3(−)CD19(+) B cells. Rev Bras Reumatol 54:241–246
Tselios K, Sarantopoulos A, Gkougkourelas I, Boura P (2014) CD4+CD25highFOXP3+ T regulatory cells as a biomarker of disease activity in systemic lupus erythematosus: a prospective study. Clin Exp Rheumatol 32:630–639
Szmyrka-Kaczmarek M, Kosmaczewska A, Ciszak L, Szteblich A, Wiland P (2014) Peripheral blood Th17/Treg imbalance in patients with low-active systemic lupus erythematosus. Postepy Hig Med Dosw (Online) 68:893–898
Longhi MS, Ma Y, Grant CR, Samyn M, Gordon P, Mieli-Vergani G et al (2013) T-regs in autoimmune hepatitis-systemic lupus erythematosus/mixed connective tissue disease overlap syndrome are functionally defective and display a Th1 cytokine profile. J Autoimmun 41:146–151. https://doi.org/10.1016/j.jaut.2012.12.003
Kim J-R, Chae J-N, Kim S-H, Ha J-S (2012) Subpopulations of regulatory T cells in rheumatoid arthritis, systemic lupus erythematosus, and Behcet's disease. J Korean Med Sci 27:1009–1013. https://doi.org/10.3346/jkms.2012.27.9.1009
Xing Q, Su H, Cui J, Wang B (2012) Role of Treg cells and TGF-beta1 in patients with systemic lupus erythematosus: a possible relation with lupus nephritis. Immunol Investig 41:15–27. https://doi.org/10.3109/08820139.2011.578189
Xing Q, Wang B, Su H, Cui J, Li J (2012) Elevated Th17 cells are accompanied by FoxP3+ Treg cells decrease in patients with lupus nephritis. Rheumatol Int 32:949–958. https://doi.org/10.1007/s00296-010-1771-0
Henriques A, Ines L, Couto M, Pedreiro S, Santos C, Magalhaes M et al (2010) Frequency and functional activity of Th17, Tc17 and other T-cell subsets in systemic lupus erythematosus. Cell Immunol 264:97–103. https://doi.org/10.1016/j.cellimm.2010.05.004
Suen J-L, Li H-T, Jong Y-J, Chiang B-L, Yen J-H (2009) Altered homeostasis of CD4 FoxP3 regulatory T-cell subpopulations in systemic lupus erythematosus. Immunology 127:196–205. https://doi.org/10.1111/j.1365-2567.2008.02937.x
Lee J-H, Wang L-C, Lin Y-T, Yang Y-H, Lin D-T, Chiang B-L (2006) Inverse correlation between CD4+ regulatory T-cell population and autoantibody levels in paediatric patients with systemic lupus erythematosus. Immunology 117:280–286. https://doi.org/10.1111/j.1365-2567.2005.02306.x
Miyara M, Amoura Z, Parizot C, Badoual C, Dorgham K, Trad S et al (2005) Global natural regulatory T cell depletion in active systemic lupus erythematosus. J Immunol 175:8392–8400
C. Jacquemin, J.-F. Augusto, M. Scherlinger, N. Gensous, E. Forcade, I. Douchet et al (2018) OX40L/OX40 axis impairs follicular and natural Treg function in human SLE. JCI Insight 3. https://doi.org/10.1172/jci.insight.122167.
Vitales-Noyola M, Layseca-Espinosa E, Baranda L, Abud-Mendoza C, Nino-Moreno P, Monsiváis-Urenda A et al (2018) Analysis of sodium chloride intake and Treg/Th17 lymphocytes in healthy individuals and patients with rheumatoid arthritis or systemic lupus erythematosus. J Immunol Res 2018:9627806. https://doi.org/10.1155/2018/9627806
V. Kailashiya, U. Singh, R. Rana, N.K. Singh, D. Dash, J. Kailashiya (2018) Regulatory T cells and their association with serum markers and symptoms in systemic lupus erythematosus and rheumatoid arthritis, Immunol Investig 1–15. https://doi.org/10.1080/08820139.2018.1527852.
Venigalla RKC, Tretter T, Krienke S, Max R, Eckstein V, Blank N et al (2008) Reduced CD4+,CD25- T cell sensitivity to the suppressive function of CD4+,CD25high,CD127 −/low regulatory T cells in patients with active systemic lupus erythematosus. Arthritis Rheum 58:2120–2130. https://doi.org/10.1002/art.23556
Kleczynska W, Jakiela B, Plutecka H, Milewski M, Sanak M, Musial J (2011) Imbalance between Th17 and regulatory T-cells in systemic lupus erythematosus. Folia Histochem Cytobiol 49:646–653
Liu X, Gao N, Li M, Xu D, Hou Y, Wang Q et al (2013) Elevated levels of CD4(+)CD25(+)FoxP3(+) T cells in systemic sclerosis patients contribute to the secretion of IL-17 and immunosuppression dysfunction. PLoS One 8:e64531
Radstake TRDJ, van Bon L, Broen J, Wenink M, Santegoets K, Deng Y et al (2009) Increased frequency and compromised function of T regulatory cells in systemic sclerosis (SSc) is related to a diminished CD69 and TGFbeta expression. PLoS One 4:e5981. https://doi.org/10.1371/journal.pone.0005981
Giovannetti A, Rosato E, Renzi C, Maselli A, Gambardella L, Giammarioli AM et al (2010) Analyses of T cell phenotype and function reveal an altered T cell homeostasis in systemic sclerosis. Correlations with disease severity and phenotypes. Clin Immunol 137:122–133. https://doi.org/10.1016/j.clim.2010.06.004
Jiang N, Li M, Zeng X (2014) Correlation of Th17 cells and CD4(+)CD25(+) regulatory T cells with clinical parameters in patients with systemic sclerosis. Chin Med J 127:3557–3561
Ugor E, Simon D, Almanzar G, Pap R, Najbauer J, Nemeth P et al (2017) Increased proportions of functionally impaired regulatory T cell subsets in systemic sclerosis. Clin Immunol 184:54–62. https://doi.org/10.1016/j.clim.2017.05.013
Slobodin G, Ahmad MS, Rosner I, Peri R, Rozenbaum M, Kessel A et al (2010) Regulatory T cells (CD4(+)CD25(bright)FoxP3(+)) expansion in systemic sclerosis correlates with disease activity and severity. Cell Immunol 261:77–80. https://doi.org/10.1016/j.cellimm.2009.12.009
Antiga E, Quaglino P, Bellandi S, Volpi W, Del Bianco E, Comessatti A et al (2010) Regulatory T cells in the skin lesions and blood of patients with systemic sclerosis and morphoea. Br J Dermatol 162:1056–1063. https://doi.org/10.1111/j.1365-2133.2010.09633.x
Papp G, Horvath IF, Barath S, Gyimesi E, Sipka S, Szodoray P et al (2011) Altered T-cell and regulatory cell repertoire in patients with diffuse cutaneous systemic sclerosis. Scand J Rheumatol 40:205–210. https://doi.org/10.3109/03009742.2010.528021
Fenoglio D, Battaglia F, Parodi A, Stringara S, Negrini S, Panico N et al (2011) Alteration of Th17 and Treg cell subpopulations co-exist in patients affected with systemic sclerosis. Clin Immunol 139:249–257. https://doi.org/10.1016/j.clim.2011.01.013
Mathian A, Parizot C, Dorgham K, Trad S, Arnaud L, Larsen M et al (2012) Activated and resting regulatory T cell exhaustion concurs with high levels of interleukin-22 expression in systemic sclerosis lesions. Ann Rheum Dis 71:1227–1234. https://doi.org/10.1136/annrheumdis-2011-200709
Cordiali-Fei P, Mussi A, D'Agosto G, Trento E, Bordignon V, Trincone S et al (2013) Assessment of T regulatory cells and expanded profiling of autoantibodies may offer novel biomarkers for the clinical management of systemic sclerosis and undifferentiated connective tissue disease. Clin Dev Immunol 2013:390563. https://doi.org/10.1155/2013/390563
Wang YY, Wang Q, Sun XH, Liu RZ, Shu Y, Kanekura T et al (2014) DNA hypermethylation of the forkhead box protein 3 (FOXP3) promoter in CD4+ T cells of patients with systemic sclerosis. Br J Dermatol 171:39–47. https://doi.org/10.1111/bjd.12913
Baraut J, Grigore EI, Jean-Louis F, Khelifa SH, Durand C, Verrecchia F et al (2014) Peripheral blood regulatory T cells in patients with diffuse systemic sclerosis (SSc) before and after autologous hematopoietic SCT: a pilot study. Bone Marrow Transplant 49:349–354. https://doi.org/10.1038/bmt.2013.202
Kataoka H, Yasuda S, Fukaya S, Oku K, Horita T, Atsumi T et al (2015) Decreased expression of Runx1 and lowered proportion of Foxp3(+) CD25(+) CD4(+) regulatory T cells in systemic sclerosis. Mod Rheumatol 25:90–95. https://doi.org/10.3109/14397595.2014.899736
Klein S, Kretz CC, Ruland V, Stumpf C, Haust M, Hartschuh W et al (2011) Reduction of regulatory T cells in skin lesions but not in peripheral blood of patients with systemic scleroderma. Ann Rheum Dis 70:1475–1481. https://doi.org/10.1136/ard.2009.116525
Krasimirova E, Velikova T, Ivanova-Todorova E, Tumangelova-Yuzeir K, Kalinova D, Boyadzhieva V et al (2017) Treg/Th17 cell balance and phytohaemagglutinin activation of T lymphocytes in peripheral blood of systemic sclerosis patients. World J Exp Med 7:84–96. https://doi.org/10.5493/wjem.v7.i3.84
Han GM, O'Neil-Andersen NJ, Zurier RB, Lawrence DA (2008) CD4+CD25high T cell numbers are enriched in the peripheral blood of patients with rheumatoid arthritis. Cell Immunol 253:92–101. https://doi.org/10.1016/j.cellimm.2008.05.007
Dombrecht EJ, Aerts NE, Schuerwegh AJ, Hagendorens MM, Ebo DG, Van Offel JF et al (2006) Influence of anti-tumor necrosis factor therapy (adalimumab) on regulatory T cells and dendritic cells in rheumatoid arthritis. Clin Exp Rheumatol 24:31–37
van Amelsfort JMR, Jacobs KMG, Bijlsma JWJ, Lafeber FPJG, Taams LS (2004) CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum 50:2775–2785. https://doi.org/10.1002/art.20499
Dejaco C, Duftner C, Klauser A, Schirmer M (2010) Altered T-cell subtypes in spondyloarthritis, rheumatoid arthritis and polymyalgia rheumatica. Rheumatol Int 30:297–303. https://doi.org/10.1007/s00296-009-0949-9
Cao D, van Vollenhoven R, Klareskog L, Trollmo C, Malmström V (2004) CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther 6:R335–R346
Jiao Z, Wang W, Jia R, Li J, You H, Chen L et al (2007) Accumulation of FoxP3-expressing CD4+CD25+ T cells with distinct chemokine receptors in synovial fluid of patients with active rheumatoid arthritis. Scand J Rheumatol 36:428–433. https://doi.org/10.1080/03009740701482800
Sempere-Ortells JM, Perez-Garcia V, Marin-Alberca G, Peris-Pertusa A, Benito JM, Marco FM et al (2009) Quantification and phenotype of regulatory T cells in rheumatoid arthritis according to disease activity score-28. Autoimmunity 42:636–645. https://doi.org/10.3109/08916930903061491
Kawashiri S-Y, Kawakami A, Okada A, Koga T, Tamai M, Yamasaki S et al (2011) CD4+CD25(high)CD127(low/−) Treg cell frequency from peripheral blood correlates with disease activity in patients with rheumatoid arthritis. J Rheumatol 38:2517–2521. https://doi.org/10.3899/jrheum.110283
Niu Q, Cai B, Huang Z-C, Shi Y-Y, Wang L-L (2012) Disturbed Th17/Treg balance in patients with rheumatoid arthritis. Rheumatol Int 32:2731–2736. https://doi.org/10.1007/s00296-011-1984-x
Samson M, Audia S, Janikashvili N, Ciudad M, Trad M, Fraszczak J et al (2012) Brief report: inhibition of interleukin-6 function corrects Th17/Treg cell imbalance in patients with rheumatoid arthritis. Arthritis Rheum 64:2499–2503. https://doi.org/10.1002/art.34477
Lina C, Conghua W, Nan L, Ping Z (2011) Combined treatment of etanercept and MTX reverses Th1/Th2, Th17/Treg imbalance in patients with rheumatoid arthritis. J Clin Immunol 31:596–605. https://doi.org/10.1007/s10875-011-9542-6
Zhang X, Zhang X, Zhuang L, Xu C, Li T, Zhang G et al (2018) Decreased regulatory T-cell frequency and interleukin-35 levels in patients with rheumatoid arthritis. Exp Ther Med 16:5366–5372
Wang L, Wang C, Jia X, Yu J (2018) Circulating exosomal miR-17 inhibits the induction of regulatory T cells via suppressing TGFBR II expression in rheumatoid arthritis. Cell Physiol Biochem 50:1754–1763. https://doi.org/10.1159/000494793
Hashemi V, Farrokhi AS, Tanomand A, Babaloo Z, Hojjat-Farsangi M, Anvari E et al (2018) Polymorphism of Foxp3 gene affects the frequency of regulatory T cells and disease activity in patients with rheumatoid arthritis in Iranian population. Immunol Lett 204:16–22. https://doi.org/10.1016/j.imlet.2018.10.001
Sun H, Gao W, Pan W, Zhang Q, Wang G, Feng D et al (2017) Tim3(+) Foxp3 (+) Treg cells are potent inhibitors of effector T cells and are suppressed in rheumatoid arthritis. Inflammation 40:1342–1350. https://doi.org/10.1007/s10753-017-0577-6
Cao D, Malmström V, Baecher-Allan C, Hafler D, Klareskog L, Trollmo C (2003) Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol 33:215–223. https://doi.org/10.1002/immu.200390024
Mottonen M, Heikkinen J, Mustonen L, Isomaki P, Luukkainen R, Lassila O (2005) CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol 140:360–367. https://doi.org/10.1111/j.1365-2249.2005.02754.x
Liu M-F, Wang C-R, Fung L-L, Lin L-H, Tsai C-N (2005) The presence of cytokine-suppressive CD4+CD25+ T cells in the peripheral blood and synovial fluid of patients with rheumatoid arthritis. Scand J Immunol 62:312–317. https://doi.org/10.1111/j.1365-3083.2005.01656.x
Walter GJ, Evans HG, Menon B, Gullick NJ, Kirkham BW, Cope AP et al (2013) Interaction with activated monocytes enhances cytokine expression and suppressive activity of human CD4+CD45ro+CD25+CD127(low) regulatory T cells. Arthritis Rheum 65:627–638. https://doi.org/10.1002/art.37832
Jandus C, Bioley G, Rivals J-P, Dudler J, Speiser D, Romero P (2008) Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum 58:2307–2317. https://doi.org/10.1002/art.23655
Ji L, Geng Y, Zhou W, Zhang Z (2016) A study on relationship among apoptosis rates, number of peripheral T cell subtypes and disease activity in rheumatoid arthritis. Int J Rheum Dis 19:167–171. https://doi.org/10.1111/1756-185X.12211
Wang M, Liu C, Bond A, Yang J, Zhou X, Wang J et al (2018) Dysfunction of regulatory T cells in patients with ankylosing spondylitis is associated with a loss of Tim-3. Int Immunopharmacol 59:53–60. https://doi.org/10.1016/j.intimp.2018.03.032
Ciccia F, Accardo-Palumbo A, Giardina A, Di Maggio P, Principato A, Bombardieri M et al (2010) Expansion of intestinal CD4+CD25(high) Treg cells in patients with ankylosing spondylitis: a putative role for interleukin-10 in preventing intestinal Th17 response. Arthritis Rheum 62:3625–3634. https://doi.org/10.1002/art.27699
Zhao S-S, Hu J-W, Wang J, Lou X-J, Zhou L-L (2011) Inverse correlation between CD4+ CD25high CD127low/− regulatory T-cells and serum immunoglobulin A in patients with new-onset ankylosing spondylitis. J Int Med Res 39:1968–1974. https://doi.org/10.1177/147323001103900543
Guo H, Zheng M, Zhang K, Yang F, Zhang X, Han Q et al (2016) Functional defects in CD4(+) CD25(high) FoxP3(+) regulatory cells in ankylosing spondylitis. Sci Rep 6:37559. https://doi.org/10.1038/srep37559
Wang C, Liao Q, Hu Y, Zhong DA (2015) T lymphocyte subset imbalances in patients contribute to ankylosing spondylitis. Exp Ther Med 9:250–256
Ye L, Goodall JC, Zhang L, Putintseva EV, Lam B, Jiang L et al (2016) TCR usage, gene expression and function of two distinct FOXP3(+)Treg subsets within CD4(+)CD25(hi) T cells identified by expression of CD39 and CD45RO. Immunol Cell Biol 94:293–305. https://doi.org/10.1038/icb.2015.90
Fattahi MJ, Ahmadi H, Jafarnezhad-Ansariha F, Mortazavi-Jahromi SS, Rehm BHA, Cuzzocrea S et al (2018) Oral administration effects of beta-d-mannuronic acid (M2000) on Th17 and regulatory T cells in patients with ankylosing spondylitis. Biomed Pharmacother 100:495–500. https://doi.org/10.1016/j.biopha.2018.02.059
Yan B, Ye S, Chen G, Kuang M, Shen N, Chen S (2008) Dysfunctional CD4+,CD25+ regulatory T cells in untreated active systemic lupus erythematosus secondary to interferon-alpha-producing antigen-presenting cells. Arthritis Rheum 58:801–812. https://doi.org/10.1002/art.23268
Valencia X, Yarboro C, Illei G, Lipsky PE (2007) Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol 178:2579–2588
Schaier M, Gottschalk C, Uhlmann L, Speer C, Kalble F, Eckstein V et al (2018) Immunosuppressive therapy influences the accelerated age-dependent T-helper cell differentiation in systemic lupus erythematosus remission patients. Arthritis Research & Therapy. 20:278. https://doi.org/10.1186/s13075-018-1778-6
Rapetti L, Chavele K-M, Evans CM, Ehrenstein MR (2015) B cell resistance to Fas-mediated apoptosis contributes to their ineffective control by regulatory T cells in rheumatoid arthritis. Ann Rheum Dis 74:294–302. https://doi.org/10.1136/annrheumdis-2013-204049
Flores-Borja F, Jury EC, Mauri C, Ehrenstein MR (2008) Defects in CTLA-4 are associated with abnormal regulatory T cell function in rheumatoid arthritis. Proc Natl Acad Sci 105:19396–19401. https://doi.org/10.1073/pnas.0806855105
Nadkarni S, Mauri C, Ehrenstein MR (2007) Anti-TNF-alpha therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-beta. J Exp Med 204:33–39. https://doi.org/10.1084/jem.20061531
Comte D, Karampetsou MP, Kis-Toth K, Yoshida N, Bradley SJ, Kyttaris VC et al (2016) CD4+ T cells from SLE patients respond poorly to exogenous IL-2. Arthritis Rheumatol. https://doi.org/10.1002/art.40014
de Paz B, Prado C, Alperi-Lopez M, Ballina-Garcia FJ, Rodriguez-Carrio J, Lopez P et al (2012) Effects of glucocorticoid treatment on CD25-FOXP3+ population and cytokine-producing cells in rheumatoid arthritis. Rheumatology 51:1198–1207. https://doi.org/10.1093/rheumatology/kes039
Fransson M, Burman J, Lindqvist C, Atterby C, Fagius J, Loskog A (2010) T regulatory cells lacking CD25 are increased in MS during relapse. Autoimmunity 43:590–597. https://doi.org/10.3109/08916930903541190
Yang H-X, Zhang W, Zhao L-D, Li Y, Zhang F-C, Tang F-L et al (2009) Are CD4+CD25-Foxp3+ cells in untreated new-onset lupus patients regulatory T cells? Arthritis Res Ther 11:R153. https://doi.org/10.1186/ar2829
C. von Spee-Mayer, E. Siegert, D. Abdirama, A. Rose, A. Klaus, T. Alexander et al (2015) Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus, annals of the rheumatic diseases. Annrheumdis–2015–207776. https://doi.org/10.1136/annrheumdis-2015-207776.
Moradi B, Schnatzer P, Hagmann S, Rosshirt N, Gotterbarm T, Kretzer JP et al (2014) CD4(+)CD25(+)/highCD127low/(−) regulatory T cells are enriched in rheumatoid arthritis and osteoarthritis joints--analysis of frequency and phenotype in synovial membrane, synovial fluid and peripheral blood. Arthritis Res Ther 16:R97. https://doi.org/10.1186/ar4545
Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA et al (2004) Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med 200:277–285
Wu Y, Ren M, Yang R, Liang X, Ma Y, Tang Y et al (2011) Reduced immunomodulation potential of bone marrow-derived mesenchymal stem cells induced CCR4+CCR6+ Th/Treg cell subset imbalance in ankylosing spondylitis. Arthritis Res Ther 13:R29. https://doi.org/10.1186/ar3257
Dulic S, Vasarhelyi Z, Bajnok A, Szalay B, Toldi G, Kovacs L et al (2018) The impact of anti-TNF therapy on CD4+ and CD8+ cell subsets in ankylosing spondylitis. Pathobiology 85:201–210. https://doi.org/10.1159/000484250
Liao H-T, Lin Y-F, Tsai C-Y, Chou C-T (2015) Regulatory T cells in ankylosing spondylitis and the response after adalimumab treatment. Joint Bone Spine 82:423–427. https://doi.org/10.1016/j.jbspin.2015.03.003
Frantz C, Auffray C, Avouac J, Allanore Y (2018) Regulatory T cells in systemic sclerosis. Front Immun 9:490. https://doi.org/10.1182/blood-2015-06-649145
Antiga E, Fabbri P, Caproni M (2010) Immunosuppressive therapy may affect the number of circulating regulatory cells in systemic sclerosis: pay attention to the patient selection criteria. Cell Immunol 264:186. https://doi.org/10.1016/j.cellimm.2010.06.007
Huertas A, Phan C, Bordenave J, Tu L, Thuillet R, Le Hiress M et al (2016) Regulatory T cell dysfunction in idiopathic, heritable and connective tissue-associated pulmonary arterial hypertension. Chest 149:1482–1493. https://doi.org/10.1016/j.chest.2016.01.004
Danikowski KM, Jayaraman S, Prabhakar BS (2017) Regulatory T cells in multiple sclerosis and myasthenia gravis. J Neuroinflammation 14:117. https://doi.org/10.1186/s12974-017-0892-8
Morgan ME, Flierman R, van Duivenvoorde LM, Witteveen HJ, van Ewijk W, van Laar JM et al (2005) Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum 52:2212–2221. https://doi.org/10.1002/art.21195
Putnam AL, Brusko TM, Lee MR, Liu W, Szot GL, Ghosh T et al (2009) Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes 58:652–662. https://doi.org/10.2337/db08-1168
Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK et al (2015) Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med 7:315ra189. https://doi.org/10.1126/scitranslmed.aad4134
Rossetti M, Spreafico R, Saidin S, Chua C, Moshref M, Leong JY et al (2015) Ex vivo-expanded but not in vitro-induced human regulatory T cells are candidates for cell therapy in autoimmune diseases thanks to stable demethylation of the FOXP3 regulatory T cell-specific demethylated region. J Immunol 194:113–124. https://doi.org/10.4049/jimmunol.1401145
Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E et al (2011) Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 117:3921–3928. https://doi.org/10.1182/blood-2010-10-311894
Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J et al (2011) Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 117:1061–1070. https://doi.org/10.1182/blood-2010-07-293795
Martelli MF, Di Ianni M, Ruggeri L, Falzetti F, Carotti A, Terenzi A et al (2014) HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood 124:638–644. https://doi.org/10.1182/blood-2014-03-564401
Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Derkowska I, Juscinska J et al (2014) Therapy of type 1 diabetes with CD4(+)CD25(high)CD127-regulatory T cells prolongs survival of pancreatic islets - results of one year follow-up. Clin Immunol 153:23–30. https://doi.org/10.1016/j.clim.2014.03.016
Kuhn C, Weiner HL (2016) Therapeutic anti-CD3 monoclonal antibodies: from bench to bedside. Immunotherapy. 8:889–906. https://doi.org/10.2217/imt-2016-0049
Spence A, Klementowicz JE, Bluestone JA, Tang Q (2015) Targeting Treg signaling for the treatment of autoimmune diseases. Curr Opin Immunol 37:11–20. https://doi.org/10.1016/j.coi.2015.09.002
Becker MO, Bruckner C, Scherer HU, Wassermann N, Humrich JY, Hanitsch LG et al (2011) The monoclonal anti-CD25 antibody basiliximab for the treatment of progressive systemic sclerosis: an open-label study. Ann Rheum Dis 70:1340–1341. https://doi.org/10.1136/ard.2010.137935
Powell JD, Delgoffe GM (2010) The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity 33:301–311. https://doi.org/10.1016/j.immuni.2010.09.002
Battaglia M, Stabilini A, Roncarolo MG (2005) Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 105:4743–4748. https://doi.org/10.1182/blood-2004-10-3932.
Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG (2006) Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol 177:8338–8347
Chatenoud L, Thervet E, Primo J, Bach JF (1994) Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci 91:123–127
Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D et al (2002) Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 346:1692–1698
Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G et al (2005) Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 352:2598–2608. https://doi.org/10.1056/NEJMoa043980
Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V et al (2011) Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med 365:2067–2077. https://doi.org/10.1056/NEJMoa1105143
Klatzmann D, Abbas AK (2015) The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol 15:283–294. https://doi.org/10.1038/nri3823
Yu A, Zhu L, Altman NH, Malek TR (2009) A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity 30:204–217. https://doi.org/10.1016/j.immuni.2008.11.014
Castro I, Yu A, Dee MJ, Malek TR (2011) The basis of distinctive IL-2- and IL-15-dependent signaling: weak CD122-dependent signaling favors CD8+ T central-memory cell survival but not T effector-memory cell development. J Immunol 187:5170–5182. https://doi.org/10.4049/jimmunol.1003961
Ballesteros-Tato A, Leon B, Graf BA, Moquin A, Adams PS, Lund FE et al (2012) Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity 36:847–856. https://doi.org/10.1016/j.immuni.2012.02.012
Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z et al (2007) Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 26:371–381. https://doi.org/10.1016/j.immuni.2007.02.009
Spence A, Tang Q (2016) Restoring regulatory T cells in type 1 diabetes. Curr Diab Rep 16:110. https://doi.org/10.1007/s11892-016-0807-6
Matsuoka K-I, Koreth J, Kim HT, Bascug G, McDonough S, Kawano Y et al (2013) Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med 5:179ra43. https://doi.org/10.1126/scitranslmed.3005265
Castela E, Le Duff F, Butori C, Ticchioni M, Hofman P, Bahadoran P et al (2014) Effects of low-dose recombinant interleukin 2 to promote T-regulatory cells in alopecia areata. JAMA Dermatol 150:748–751. https://doi.org/10.1001/jamadermatol.2014.504
He J, Zhang X, Wei Y, Sun X, Chen Y, Deng J et al (2016) Low-dose interleukin-2 treatment selectively modulates CD4. Nat Med 22:991–993. https://doi.org/10.1038/nm.4148
Rosenzwajg M, Lorenzon R, Cacoub P, Pham HP, Pitoiset F, El Soufi K et al (2019) Immunological and clinical effects of low-dose interleukin-2 across 11 autoimmune diseases in a single, open clinical trial. Ann Rheum Dis 78:209–217. https://doi.org/10.1136/annrheumdis-2018-214229
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This article is a contribution to the special issue on The Pathogenicity of Acquired Immunity in Human Diseases - Guest Editor: Kiyoshi Hirahara
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Göschl, L., Scheinecker, C. & Bonelli, M. Treg cells in autoimmunity: from identification to Treg-based therapies. Semin Immunopathol 41, 301–314 (2019). https://doi.org/10.1007/s00281-019-00741-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-019-00741-8