Abstract
The rise of the AIDS epidemic made the requirement for T cells in our continuous protection from pathogens critically apparent. The striking frequency with which AIDS patients exhibited profound neurological pathologies brought attention to many chronic infections that are latent within the immune-privileged CNS. One of the most common lethal opportunistic infections of these patients was with the protozoan parasite, Toxoplasma gondii. Reactivation of Toxoplasma cysts within the brain causes massive tissue destruction evidenced as multiple ring-enhancing lesions on MRI and is called toxoplasmic encephalitis (TE). TE is not limited to AIDS patients, but rather is a risk for all severely immunocompromised patients, including recipients of chemotherapy or transplant recipients. The lessons learned from these patient populations are supported by T cell depletion studies in mice. Such experiments have demonstrated that CD4+ and CD8+ T cells are required for protection against TE. Although it is clear that these T cell subsets work synergistically to fight infection, much evidence has been generated that suggests CD8+ T cells play a dominant role in protection during chronic toxoplasmosis. In other models of CNS inflammation, such as intracerebral infection with LCMV and experimental autoimmune encephalomyelitis (EAE), infiltration of T cells into the brain is harmful and even fatal. In the brain of the immunocompetent host, the well-regulated T cell response to T. gondii is therefore an ideal model to understand a controlled inflammatory response to CNS infection. This review will examine our current understanding of CD8+ T cells in the CNS during T. gondii infection in regards to the (1) mechanisms governing entry into the brain, (2) cues that dictate behavior within the brain, and (3) the functional and phenotypic properties exhibited by these cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Toxoplasma gondii is an obligate protozoan parasite that can replicate within a wide variety of cell types [1]. The tremendous success of this pathogen rests, in part, in its ability to transition to a cyst form and persist for the lifetime of the host. The effectiveness of our immune response in controlling T. gondii is evidenced by the fact that despite a global seroprevalence of about 30 %, symptomatic disease is a rare event [2]. During the AIDS epidemic, individuals with TE revealed not only the consequences of a suppressed immune response, but also revealed the brain as an important site of infection. Toxoplasma-induced pathology in these patients nearly always occurs in the CNS, though retinitis is another significant pathology [3, 4]. This points at two possibilities. One possibility, supported by several animal studies, suggests that the brain is a primary site of infection and there is continuous requirement for T cells at this site to prevent reactivation of latent cysts [5–8]. On the other hand, the brain is not the only site of infection and the parasite can be found in other tissues such as muscle. Thus, the second possibility is that reactivation at these sites or newly acquired infections lead to parasite dissemination to the brain [9]. However, regardless of the scenario, it is clear that to prevent pathology in the CNS, T cell trafficking and migration into the infected brain is critical.
The mouse, a natural host for Toxoplasma, has provided an excellent system for studying the immune response to this parasite. Using luciferase-expressing parasites the dissemination and location of the parasite can be tracked in a live mouse over time [8]. Such experiments alongside traditional histological techniques demonstrate that during chronic infection Toxoplasma is localized to the brain, where it is observed primarily in the neurons of the frontal cortex [10, 7, 11, 12]. Infiltration into the brain by the immune system is often harmful and the brain is uniquely adapted to regulate this process (for review, see [13]). During chronic Toxoplasma infection, dendritic cells, macrophages, NK cells, as well as both T and B cells have all been reported in the brain [14, 15]. The specific role for each cell population is an ongoing area of inquiry, but mouse studies conducted from the late 1980s to early 1990s demonstrated the absolute requirement for T cells and the cytokine IFN-γ to prevent parasite reactivation [5, 6]. These studies showed that mice treated with IFN-γ depleting antibodies displayed pathology indicative of parasite reactivation, including areas of neural necrosis and the presence of free tachyzoites [5, 6]. These cytokine depleting studies were followed not long after by T cell depletion studies demonstrating 100 % mortality rate when mice were treated simultaneously with anti-CD4 and anti-CD8 antibodies [6]. The crucial requirement for T cells in resistance to T. gondii in the CNS has shaped research on TE for over 20 years. Importantly, depletion of CD4+ T cells alone revealed no effect on mortality, in contrast to an observed 50 % mortality upon depletion of CD8+ T cells alone. This suggests that although CD4+ and CD8+ T cells work synergistically to control infection, CD8+ T cells are critical for protection. Supporting this conclusion, resistance in the mouse, maps to the gene encoding the CD8-restricted MHC class I molecule, H-2L d. Thus, BALB/c mice that express H-2L d are relatively resistant to chronic toxoplasmosis in comparison to the C57BL/6 mouse which lacks this gene and exhibits higher levels of cyst and tachyzoite numbers, along with inflammation and cytokine production [16, 17].
There have been significant advances in our understanding of CD8+ T cells in the context of chronic toxoplasmosis, but many questions still remain. This review will examine areas of ongoing research in three broad categories: entry of CD8+ T cells across the blood-brain barrier and into the brain parenchyma, their behavior and migration once within the tissue, and finally their phenotype and effector capacities for controlling chronic infection. In the majority of CNS inflammatory models, chronic T cell infiltration to the brain is highly pathological [13]. During murine infections with T. gondii, millions of T cells can be harvested from the brain, while the mice appear indistinguishable in terms of sickness behavior from their naïve counterparts. This suggests that the immune response to Toxoplasma in the CNS is governed by distinct mechanisms that distinguish it from a lethal CNS infection model such as cerebral malaria or LCMV [18, 19]. Thus, understanding the T cell response to Toxoplasma infection in the CNS will provide a greater understanding and new insights into the complex immune responses at this site.
Entry into the brain
Although the brain is highly vascularized, the blood-brain barrier (BBB) limits immune cell infiltration. Tight junctions connect the endothelial cells of the capillaries in the brain. This, along with astrocytic endfeet surrounding the vasculature, allows for highly controlled entry of peripheral molecules and immune cells under normal conditions. Arrays of chemokine receptors, selectins, and integrins have been implicated in T cell entry in other models of CNS inflammation [13]. This includes the requirement of adhesion molecules to slow and facilitate rolling of T cells on the endothelium at the blood/brain interface. PECAM-1, ICAM-1, and VCAM-1 are cellular adhesion molecules that are constitutively expressed on the endothelial cells of the brain vasculature. The upregulation of these molecules in the brain during T. gondii infection has been observed as early as the acute stage of infection (days 9–14) [20–22]; implying that their expression precedes parasite or immune cell infiltration of the CNS. Increased expression of VCAM-1 is, at least partially, dependent on IFN-γ signaling [22]; however, the driving force behind the regulation of endothelial cell expression of multiple adhesion molecules and whether these signals are locally derived or due to systemic circulating cytokines is unknown.
One of the ligands of VCAM-1 is the integrin α4β1 (VLA-4), which is expressed on activated T cells [23]. Adhesion molecules play a central role in the extravasation of T cells into inflamed tissues [13]. The importance of VLA-4 in T cell recruitment to the brain was first observed in the mouse model of multiple sclerosis (MS), experimental autoimmune encephalitis (EAE) [24]. Indeed, antibody-mediated blockade of VLA-4 is the molecular basis of the MS therapeutic natulizumab [25]. During Toxoplasma infection, treatment of chronically infected mice with anti-VLA-4 antibodies, thereby blocking VLA-4/VCAM-1 interactions, inhibits the recruitment of antigen-specific activated CD8+ T cells into the brain and leads to a significant increase in parasite burden [26]. A similar requirement for VLA-4 on CD8+ T cells is seen in a model of T. gondii infection that mimics ongoing reactivation [27]. Together, these studies have demonstrated the importance of VLA-4 in the recruitment of T cells to the brain during T. gondii infection. The specific roles of PECAM-1 and ICAM-1 remain to be studied, although LFA-1/ICAM-1 interactions have been implicated in both T cell and dendritic cell recruitment to the brain [15, 27]. In addition to these molecules, PSLG-1 and ALCAM have recently been linked to CD8+ T cell entry in other models of CNS inflammation and perhaps are relevant to TE [20, 28–30].
Once cells have slowed and crossed the endothelium, the cells reach the perivascular space. Thus, specific signals that draw cells from this space into the brain parenchyma are of interest. CCR7 signaling could provide necessary signals for T cells to migrate into the brain parenchyma from the perivascular space. In spontaneous autoimmunity, CCR7 is involved in T cell infiltration of peripheral tissues, which has in part guided interest in the role of this chemokine receptor in brain entry during chronic toxoplasmosis [31, 32]. In the Toxoplasma-infected brain, the CCR7 ligands, CCL21 and CCL19, are upregulated during the chronic stage. Despite this, CCL21 appears to be uniquely required for CD4+, and not CD8+, T cells to efficiently migrate into the brain parenchyma. Chronically infected plt mice (mice lacking expression of CCL19 and CCL21) have a significantly higher proportion of CD4+ T cells in the perivascular space compared to wild type [33, 26]. CD8+ T cells do not seem to share this requirement, although CCR7 may play a role in CD8+ T cell migration within the brain.
In addition to CCR7, CXCR3 is a chemokine receptor that has been associated with T cell responses during a variety of neuroinflammatory conditions [34–36]. During West Nile Virus infection, CXCL10 expressed by neurons in the brain parenchyma specifically regulates T cell entry to the CNS [37, 38]. Studies have demonstrated a similar upregulation of CXCR3 ligands in microglia and astrocytes [39, 40] within the brain and CXCR3 expression on invading T cells during chronic toxoplasmosis [41]. Treatment with anti-CXCL10 antibodies, leads to a significant reduction (∼40 %) in CD8+ T cells in the infected brain [41]. This strongly indicates that in contrast to CCR7, CXCR3 is necessary to maintain the CD8+ T cell population in the CNS. Importantly, one limitation of this data is that it does not definitively establish whether CXCR3 is required for entry from the perivascular space and/or meninges into the parenchyma or is needed for the retention of these cells. Intravital imaging allows for T cells to be visualized as they cross the BBB and thus will be critical for addressing the key steps involved in the infiltration of T cells into the CNS during TE.
In addition to the requirement for VLA-4 and CXCR3, studies in injury and infectious models of brain inflammation suggest that CD8+ T cell accumulation is antigen-dependent [18, 42]. The requirement for antigen-specific interactions during Toxoplasma infection was tested using parasites expressing the model antigen ovalbumin. Infection with parasites that secrete OVA protein led to the accumulation of OVA-specific CD8+ T cells in the infected brain [26]. However, activation of OVA-specific T cells in vitro or in vivo using OVA protein did not lead to CD8+ T cell accumulation in the brains of mice that were not infected with OVA-secreting parasites. Thus, similar to the LCMV model, where blockade of MHC class I inhibited T cell contacts with meningeal vasculature, this data provides compelling evidence for antigen-specific interactions in generating a CD8+ T cell population within the brain [18]. However, LCMV-specific CD8+ T cells have been shown to enter the CNS during chronic toxoplasmosis, but these cells do not persist, offering support for antigen specificity as a condition for retention and/or survival rather than for entry into the parenchyma [41]. Further studies in the LCMV model shows that antigen presentation to CD8+ T cells leads to their local proliferation in the brain [43]. During Toxoplasma infection, T cell proliferation has also been observed within the brain parenchyma [26]. As this was a rare event, it is clearly not the primary cause of the expansion of the CD8+ T cell population in the CNS. These differences between a controlled protective CD8+ response to Toxoplasma and a pathological one during viral encephalitis may point to the amount of antigen and the degree to which it is presented as an important limiting factor for CD8 expansion in the brain.
In addition to the LCMV model of viral encephalitis, during EAE, there is evidence that T cells interact with meningeal and/or vessel-associated phagocytes and this interaction presumably regulates entry, retention, and/or survival [43–45]. The identity of cells responsible for presenting Toxoplasma antigen at this site remains unknown. Imaging studies have revealed that OVA-specific CD8+ T cells interact with CD11c-expressing cells in the CNS [15]. In addition, numerous APCs, including macrophages and dendritic cells are recruited to the CNS during infection. In the absence of inflammation, the expression of MHC class I in the CNS is minimal, but is upregulated following infection [46]. Furthermore, it is possible that the astrocytes forming the glia limitans could present parasite antigens at this site [47, 48].
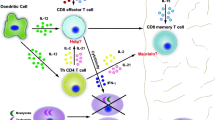
A compelling model for CD8+ T cell entry into the brain during T. gondii infection posits a three-step process where VLA-4 allows slowing of T cells within the blood vessel, adherence to endothelium and extravasation into the perivascular space. Perivascular APCs ensure retention within the CNS, and expression of CXCL9/10 by microglia and astrocytes and CXCR3 by CD8+ T cells regulates entry into the parenchyma (Fig. 1a). However this model is incomplete and requires further investigation. For example, knockout and depletion methods for these targets do not completely abolish entry into the brain; therefore it is likely that additional molecules are involved. In addition, detailed two-photon microscopy experiments in conjunction with traditional flow cytometric and histological techniques will be required to determine if these signals are required for migration across the BBB or retention of cells once within the infected brain.
a VLA-4 and VCAM-1 interactions are required for recruitment of CD8+ T cells to the brain. Whether interaction between CXCR3 and its ligands governs entry into the parenchyma during chronic toxoplasmosis has not been established but has been observed in West Nile Virus. Astrocytes and microglia appear to be sources of CXCL9 and CXCL10 during chronic toxoplasmosis. b Upon entry into the brain CXCR3 ligands help increase the velocity of Lévy walks. Interaction between CCR7 and ligands may also govern migration within the parenchyma. For example, CCL21 is expressed on linear networks that appear in the brain parenchyma at the chronic stage of infection. c Antigen recognition within the tissue may also govern long-term survival/entry. Microglia, astrocytes, and neurons all express MHC I, but CD8+ T cells have been observed to contact infiltrating DCs during Toxoplasma gondii infection. A percentage of DCs may present antigen through cross presentation or may be directly infected d CD8+ T cells contribute to control of reactivation through the actions of IFN-γ, and control of cyst burden through perforin-mediated cell lysis
Behavior within the brain
Once cells reach the brain parenchyma, less is known about the signals they receive that may direct their behavior and their ability to control infection. T cells could be directed by infection-induced cues or behave using a random search strategy [41]. The ability to monitor the behavior of T cells in live tissue using multi-photon microscopy has dramatically changed the field of immunology, allowing the CD8+ T cell response to Toxoplasma to be observed in real time. Imaging of Toxoplasma infection in the lymph nodes revealed rapid and significant changes in neutrophil and T cell behavior within the lymph nodes [49, 50]. The first study to look at CD8+ T cells in the CNS during chronic Toxoplasma infection in the brain revealed a highly heterogeneous population of cells [26], some of which seemed to interact with cysts and each other. In terms of general migration patterns, the average velocity, displacement, and meandering index of a transferred population of CD8 T cells varied over the course of chronic infection. These parameters were examined in a transferred population of antigen-specific CD8+GFP+ T cells and found that the average velocity of migrating cells ranged between 4 and 8 μm/min with some reaching up to 25 μm/min, with peak velocities reached between 1 and 2 weeks post transfer [26]. Such speeds are similar to those measured in CD8+ T cells in naïve lymph nodes. As discussed in this study, cells exhibited behaviors ranging and transitioning between constrained to motile migration patterns. Cells also clustered, proliferated, and exhibited repetitive or circling behaviors [26]. A further study imaging interactions of T cells, parasites and antigen presenting cells suggest that CD8+ T cells made very few close contacts with intact cysts or CNS resident cells, however, formed significant clusters with CD11b+ cells especially in areas of free parasites. The reduced cell velocity at these sites supports the concept that these are areas containing parasite antigen that is being presented to CD8+ T cells. In chronic toxoplasmosis, there is an extensive network of dendritic cells recruited to the brain, along with activated resident microglia [15]. The role for infiltrating dendritic cells has not been defined, but their association with both the parasite and CD8+ T cells does suggest they may play a role in the cytotoxic response. One possibility is that these dendritic cells serve as a source of soluble chemokine that guides migration; however, their potential role as APC’s will be discussed in the following section. In addition to dendritic cells, there are multiple potential sources of chemokines in the brain including neurons, microglia, astrocytes, and infiltrating dendritic cells (Fig. 1b). All of these cell populations have been shown to express both chemokines and/or their receptors during CNS inflammation [51, 52].
Of the chemokines that are upregulated during infection, CCL21 appears to be present in fibers that were detected by immunofluorescent staining [26]. Importantly, this in situ staining also reveals that CD8+ cells associate with these fibers. Second harmonic signals as seen by two-photon microscopy imaging have revealed a fibrous network upon which CD8+ T cells appear to migrate. Although it has not been definitively established, these networks could represent the same linear structures as seen by CCL21 staining. These structures, their regulation and whether or not chemokines actively bind to such extracellular networks are ongoing areas of study; however, such presentation of chemotactic molecules may provide efficient migratory signals to T cells in the CNS.
Such roles for chemokine signaling within the infected brain parenchyma have now been established for the CXCR3/CXCL10 system [41]. In addition to its role in recruitment or retention of effector CD8+ T cells, CXCL10 is required to increase the speed of CD8 T cell migration within the brain [41]. CD8 T cells in mice treated with anti-CXCL10 antibodies migrated at a significantly slower velocity than their untreated counterparts [41]. Blocking all Gαi-coupled receptor signaling with pertussis toxin further reduced the average T cell velocity. These data suggest two things; firstly, that although CXCL10 contributes to T cell velocity, other chemokines are also involved to maintain optimal speed. Secondly, non-chemokine (Gαi-independent) signaling also contributes to T cell migration in the CNS. Rigorous statistical analysis in these studies also revealed that CD8+ T cell migration does not appear to be directed over the observed timescales, suggesting that chemokine gradients may only be present over very short distances [41]. In addition, in contrast to preliminary studies examining the migration patterns of T cells in the lymph node, CD8 T cell migration in the brain was not well described by a simple random walk or Brownian motion. Instead, a generalized Lévy walk (GLW) described the T cell migration pattern. In this model, CD8 T cells alternate between runs and pauses and the length of the runs and pauses is random and drawn from a Lévy distribution. Thus, the movements and pauses by T cells are typically short, with rare long runs and pauses. Surprisingly, CD8+ T cells maintained the GLW behavior in the absence of CXCL10 and chemokine signals, suggesting that chemokines increase the speed, but not the directionality or pattern of migration. Moreover, mathematical modeling of CD8+ T cell migration suggested that chemokines are import for the control of T. gondii in the brain, as they shorten the distance that T cells must travel before encountering parasites. This study also raises the question of whether this pattern of migration is characteristic of all T cells. Recent analysis of CD8+ T cells in the uninflamed lymph node revealed that cells do not exhibit GLW behavior [53] suggesting that the activation status of the CD8 T cells during infection or the brain environment may influence the GLW behavior.
Despite the fact that there is no evidence that CD8+ T cells within the brain in the chronic toxoplasmosis model are guided by chemokine gradients or towards an antigenic target in vivo, more research is required to investigate the role of additional chemokines and chemokine receptors. It is likely that these molecules contribute to a protective immune response in diverse ways, as may be expected for the control of a complex infection.
Phenotypic and functional characteristics
Previous work has made clear that CD8+ T cells play a crucial role in control of chronic toxoplasmosis through the actions of perforin and IFN-γ [5, 6, 54–56]. In support of the H-2L d data, adoptive transfer of the CD8+ subset of a major IFN-γ-producing population, TCR variant Vβ8, conferred resistance to chronic infection in nude mice [16, 54, 56]. In addition to the requirement for IFN-γ, perforin is required for the control of cyst burden [57, 55]. Importantly, perforin is sufficient for control of cyst burden in IFN-γ−/− mice, suggesting that although IFN-γ is required for protection against reactivation, control of cyst burden is uniquely the function of perforin secreted by CD8+ T cells [16]. Nonetheless, there are several details that remain unanswered. For example, the targets of perforin and IFN-γ remain unknown and furthermore, CD8+ T cells rarely directly associate with a cyst [58].
Perhaps the most crucial question regarding CD8 T cells during chronic toxoplasmosis is how they exert effector functions to control infection. Early studies suggested that CD8+ T cells confer protection through perforin secretion [57]. Perforin knockout (PKO) mice succumbed to chronic infection earlier than their wild-type counterparts and exhibited significantly increased parasite burden. However, PKO mice that were vaccinated with ts4 strain parasites were still resistant to challenge with the virulent RH strain. This suggests that perforin is uniquely required for control of cyst burden within the brain (Fig. 1c).
More recently, it has been shown that when CD8 T cells from chronically infected BALB/c mice are transferred to their SCID counterparts, there was a significant reduction in cyst burden compared to pre-transfer levels [55]. Importantly, this was still seen in mice receiving CD8 T cells from IFN-γ−/− donors, suggesting that IFN-γ is not the primary mediator of cyst burden control. Furthermore, recipients given CD8 T cells from PKO mice showed equivalent cyst burdens to pre-transfer numbers [55]. This is compelling evidence suggesting that during chronic infection perforin mediated cell lysis is one of the crucial mediators for the control of cyst burden and parasite reactivation [59].
A recurrent question underlying the discussion of each aspect of the CD8 response during chronic infection is antigen source. It is not currently clear if parasite antigen escapes from infected cells into the brain parenchyma and the periphery or if CD8+ T cells recognize reactivating cysts. The use of parasite-specific MHC I tetramers has provided an important piece to this puzzle by demonstrating that ROP7, but not GRA-4 antigen-specific CD8+ T cells are present in the brain during the chronic stage [60]. ROP7 is expressed by both the bradyzoite and tachyzoites, whereas GRA4 is uniquely expressed by tachyzoites. This supports the possibility that CD8+ T cells are responding to both bradyzoites and tachyzoites within the brain, which is supportive of continuous antigen exposure and presentation.
Live imaging of infiltrating DCs within the infected brain parenchyma demonstrated that these cells both associate with the parasite and maintain prolonged contacts with CD8+ T cells, certainly supporting the idea that DCs are acting as local APCs [15]. Perhaps DCs process and present secreted antigen from infected neurons to CD8+ T cells via cross presentation, although this is by no means the only possibility (Fig. 1d). An elegant study in acute toxoplasmosis using a combination of mCherry labeled parasite reporter and a pH sensitive dye showed that the proportion of dendritic cells directly infected by the parasite were roughly comparable to the proportion that had taken up and processed parasite antigen by phagocytosis [61]. This suggests that during chronic infection, a population of infiltrating dendritic cells could be infected with tachyzoites from reactivating cysts. In this case CD8+ T cells could recognize antigen via the endogenous MHC I pathway, versus cross presentation.
Related to antigen presentation and targeting is the question of where CD8+ T cell priming occurs and whether continuous recruitment/turnover of CD8+ T cells occurs. CD8+ T cells isolated from the brain during chronic toxoplasmosis express high levels of CD44 and low levels of CD62L, characteristic of an activated phenotype [62]. Furthermore, T cells from the brain fail to take up BrdU upon restimulation, suggesting that continuous recruitment may be required to replenish this population [62]. If this is true, then it remains to be defined whether priming occurs in the periphery or perhaps through the actions of perivascular APCs in the CNS. Combining imaging methodologies used for documenting cell migration with readouts of cell function, i.e., NFAT translocation reporter [44], will prove highly useful to visualize these interactions during chronic toxoplasmosis.
Regardless of the mechanism by which this critical CD8+ T cell population recognizes antigen within the parenchyma and exert their effector functions, the response appears dampened or impaired. Notably, T cells in the Toxoplasma-infected brain exhibit an exhausted phenotype that has been observed in other chronic infections [63]. The polyfunctional nature of a single CD8+ T cell means it can exert a variety of effector responses against infected cells, for example, secretion of granzyme B, IFN-γ, IL-2, and TNF-α [64]. In an exhaustion phenotype, this polyfunctionality is lost. Receptors such as PD-1 are upregulated in exhausted CD8+ T cells as a result of persistent engagement of the TCR with antigen [65]. During chronic toxoplasmosis, a proportion of CD8+ T cells in the brain express PD-1 along with reduced proliferative capacity and reduced expression of effector molecules such as IFN-γ and granzyme B [26, 66]. Although this can be temporarily recovered either through blockade of PD-1 or transfer of CD8+ T cells from acute infection, ultimately this exhaustion phenotype persists. [67]. Taken together, this suggests that the CD8+ T cells during chronic infection can acquire progressive loss of effector function that perhaps contributes to the persistence of the parasite within the brain parenchyma.
T cell exhaustion can be considered a direct result of the persistent nature of chronic toxoplasmosis. This persistence also means that the role of memory populations in controlling the parasite, along with the signals that maintain such a population, is currently unknown. As discussed, the T cells present during chronic toxoplasmosis display unique phenotypes comparable to other models of chronic infection, suggesting that they are functionally distinct from the T cells responding to cleared infections [68, 26, 65]. Memory T cells have been described in the CNS, including immune surveillance that occurs in the human CSF predominantly by a circulating central memory phenotype (CD4+CCR7+) [36]. The discovery of brain resident memory CD8+ T cells in the context of viral infection suggests a potential role for memory in the form of parenchymal surveillance [69].
Regarding the signals that maintain memory CD8+ T cells, the classical view is that IL-7R is expressed by both memory and naïve T cells and helps maintain long-term survival in a quiescent state [70, 71]. The chronic nature of T. gondii infection suggests that the signals that maintain memory T cells, as well as the nature of immunological memory itself, may differ from infections that are cleared. Mice immunized with an attenuated strain of Toxoplasma, ts4 and treated with IL-15 (a ligand for IL-7R), then challenged with the PLK strain of the parasite showed enhanced protection relative to their untreated counterparts [72]. However, a subsequent study revealed that following vaccination with the ts-4 strain IL-15 is not necessary for protection during a virulent challenge [73]. As ts4 parasites are cleared, this is not reflective of the environment of the chronically inflamed brain, where there is persistent antigen. Additionally, IL-7 may compensate for the function of IL-15. In response to this, IL-15−/− mice were treated with IL-7 depleting antibodies. This study revealed a defect in the ability of CD8+ T cells with a memory phenotype to develop [74]. This suggests that IL-7R is required for the generation of memory CD8+ T cells during chronic toxoplasmosis; however, the role for memory populations in conferring protection at this stage is unknown. Furthermore, the requirement for IL-7R during chronic infection to maintain an effective CD8+ T cell population within the brain has not been tested.
Indeed, it is possible that IL-7R is not required for maintenance of memory populations in the Toxoplasma-infected brain at all. Studies of chronic viral infection suggest that the memory CD8+ T cells generated in this context become dependent on their cognate antigen for long-term survival instead of IL-15 or IL-7 [68]. The network of dendritic cells that has been discussed in previous sections could support this hypothesis. However in addition to dendritic cells, CD8+ T cells could encounter antigen presented by microglia, astrocytes, and neurons, as all these cell populations express MHC I (Table 1) [75, 47, 15]. In other models of brain inflammation, astrocytes have been shown to form synapses with CD8+ T cells as well as to serve as a source of cytokines, and thus they form a compelling candidate to play multiple roles at the site of infection [47, 48]. Experiments similar to those done in acute LCMV infection, visualizing CD8+ T cell interaction with APCs and subsequent activation as measured by proliferation could be useful to establish the targets of CD8+ T cells within the brain. Additionally, studies confirming the survival signals and roles of memory CD8+ T cells during chronic toxoplasmosis have yet to be conducted. The specific role that CD8+ T cell memory populations play in control of any chronic infection has been largely unexplored and T. gondii may prove a useful model to answer this question.
Conclusion
As a model of chronic inflammation in the CNS, T. gondii infection can both inform and be informed by other models of CNS inflammation. The evidence examined in this review suggests that the CD8+ T cell response at the chronic stage of toxoplasmosis is essential to control of infection, yet there are many questions that remain to be explored. CD8+ T cells are required for control of toxoplasmosis, yet they can exhibit an exhausted phenotype indicating suppression of function. Although blockade of PD-1 can temporarily restore CD8+ T cell function, permanent recovery has not yet been achieved. It is useful to consider whether recovery will enhance the immune response to the parasite, potentially to the point of clearance, or whether this suppression is actually required along with regulatory cytokines to prevent inflammation-related pathology. A robust CD8+ T cell response is a strong correlate of protection in other diseases, and it seems that this is the case in chronic T. gondii infection as well, yet the targets and the signals that enhance target location are not fully understood. The infrequency with which CD8+ T cells are observed to contact cyst-infected neurons suggests their response is predominantly against parasite reactivation. The current evidence suggests that whatever the target, chemokines increase the speed at which CD8+ T cells search for said target. However, without a broader understanding of what CD8+ T cells are responding to and how they find what they are responding to, it is important to be cautious in enhancing CD8+ T cell function and behavior towards a therapeutic end. The context within which these CD8+ T cells operate is important, and may define what is protective in one context and pathogenic in another. The study of the CD8+ T cell response during chronic Toxoplasma infection within the brain is clearly a useful and informative tool for understanding the generation of protective immunity in the brain during ongoing inflammation—relevant to a number of infectious and non-infectious immune responses in the CNS.
References
Carruthers V, Boothroyd JC (2007) Pulling together: an integrated model of Toxoplasma cell invasion. Curr Opin Microbiol 10(1):83–89. doi:10.1016/j.mib.2006.06.017
Pappas G, Roussos N, Falagas ME (2009) Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol 39(12):1385–1394. doi:10.1016/j.ijpara.2009.04.003
Luft BJ, Brooks RG, Conley FK, McCabe RE, Remington JS (1984) Toxoplasmic encephalitis in patients with acquired immune deficiency syndrome. JAMA 252(7):913–917
Luft BJ, Remington JS (1988) AIDS commentary. Toxoplasmic encephalitis. J Infect Dis 157(1):1–6
Suzuki Y, Conley FK, Remington JS (1989) Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J Immunol 143(6):2045–2050
Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A (1992) Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol 149(1):175–180
Dellacasa-Lindberg I, Hitziger N, Barragan A (2007) Localized recrudescence of Toxoplasma infections in the central nervous system of immunocompromised mice assessed by in vivo bioluminescence imaging. Microbes Infect 9(11):1291–1298. doi:10.1016/j.micinf.2007.06.003
Saeij JP, Boyle JP, Grigg ME, Arrizabalaga G, Boothroyd JC (2005) Bioluminescence imaging of Toxoplasma gondii infection in living mice reveals dramatic differences between strains. Infect Immun 73(2):695–702. doi:10.1128/IAI. 73.2.695-702.2005
Barcan LA, Dallurzo ML, Clara LO, Valledor A, Macias S, Zorkin E, Gerona S, Livellara B (2002) Toxoplasma gondii pneumonia in liver transplantation: survival after a severe case of reactivation. Transpl Infect Dis 4(2):93–96
Hermes G, Ajioka JW, Kelly KA, Mui E, Roberts F, Kasza K, Mayr T, Kirisits MJ, Wollmann R, Ferguson DJ, Roberts CW, Hwang JH, Trendler T, Kennan RP, Suzuki Y, Reardon C, Hickey WF, Chen L, McLeod R (2008) Neurological and behavioral abnormalities, ventricular dilatation, altered cellular functions, inflammation, and neuronal injury in brains of mice due to common, persistent, parasitic infection. J Neuroinflammation 5:48. doi:10.1186/1742-2094-5-48
Koshy AA, Fouts AE, Lodoen MB, Alkan O, Blau HM, Boothroyd JC (2010) Toxoplasma secreting Cre recombinase for analysis of host-parasite interactions. Nat Methods 7 (4):307–309. doi: 10.1038/nmeth.1438
Melzer TC, Cranston HJ, Weiss LM, Halonen SK (2010) Host cell preference of Toxoplasma gondii cysts in murine brain: a confocal study. J Neuroparasitology 1. doi:10.4303/jnp/N100505
Wilson EH, Weninger W, Hunter CA (2010) Trafficking of immune cells in the central nervous system. J Clin Invest 120(5):1368–1379. doi:10.1172/JCI41911
Schluter D, Hein A, Dorries R, Deckert-Schluter M (1995) Different subsets of T cells in conjunction with natural killer cells, macrophages, and activated microglia participate in the intracerebral immune response to Toxoplasma gondii in athymic nude and immunocompetent rats. Am J Pathol 146(4):999–1007
John B, Ricart B, Tait Wojno ED, Harris TH, Randall LM, Christian DA, Gregg B, De Almeida DM, Weninger W, Hammer DA, Hunter CA (2011) Analysis of behavior and trafficking of dendritic cells within the brain during toxoplasmic encephalitis. PLoS Pathog 7(9):e1002246. doi:10.1371/journal.ppat.1002246
Suzuki Y, Sa Q, Gehman M, Ochiai E (2011) Interferon-gamma- and perforin-mediated immune responses for resistance against Toxoplasma gondii in the brain. Expert Rev Mol Med 13:e31. doi:10.1017/S1462399411002018
Brown CR, Hunter CA, Estes RG, Beckmann E, Forman J, David C, Remington JS, McLeod R (1995) Definitive identification of a gene that confers resistance against Toxoplasma cyst burden and encephalitis. Immunology 85(3):419–428
Kim JV, Kang SS, Dustin ML, McGavern DB (2009) Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature 457(7226):191–195. doi:10.1038/nature07591
Frevert U, Nacer A (2014) Fatal cerebral malaria: a venous efflux problem. Front Cell Infect Microbiol 4:155. doi:10.3389/fcimb.2014.00155
Silva NM, Manzan RM, Carneiro WP, Milanezi CM, Silva JS, Ferro EA, Mineo JR (2010) Toxoplasma gondii: the severity of toxoplasmic encephalitis in C57BL/6 mice is associated with increased ALCAM and VCAM-1 expression in the central nervous system and higher blood–brain barrier permeability. Exp Parasitol 126 (2):167–177. doi:10.1016/j.exppara.2010.04.019
Deckert-Schluter M, Schluter D, Hof H, Wiestler OD, Lassmann H (1994) Differential expression of ICAM-1, VCAM-1 and their ligands LFA-1, Mac-1, CD43, VLA-4, and MHC class II antigens in murine Toxoplasma encephalitis: a light microscopic and ultrastructural immunohistochemical study. J Neuropathol Exp Neurol 53(5):457–468
Wang X, Michie SA, Xu B, Suzuki Y (2007) Importance of IFN-gamma-mediated expression of endothelial VCAM-1 on recruitment of CD8+ T cells into the brain during chronic infection with Toxoplasma gondii. J Interferon Cytokine Res 27(4):329–338. doi:10.1089/jir.2006.0154
Carrithers MD, Visintin I, Kang SJ, Janeway CA Jr (2000) Differential adhesion molecule requirements for immune surveillance and inflammatory recruitment. Brain 123(Pt 6):1092–1101
Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N (1992) Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature 356(6364):63–66. doi:10.1038/356063a0
Engelhardt B, Kappos L (2008) Natalizumab: targeting alpha4-integrins in multiple sclerosis. Neurodegener Dis 5(1):16–22. doi:10.1159/000109933
Wilson EH, Harris TH, Mrass P, John B, Tait ED, Wu GF, Pepper M, Wherry EJ, Dzierzinski F, Roos D, Haydon PG, Laufer TM, Weninger W, Hunter CA (2009) Behavior of parasite-specific effector CD8+ T cells in the brain and visualization of a kinesis-associated system of reticular fibers. Immunity 30(2):300–311. doi:10.1016/j.immuni.2008.12.013
Sa Q, Ochiai E, Sengoku T, Wilson ME, Brogli M, Crutcher S, Michie SA, Xu B, Payne L, Wang X, Suzuki Y (2014) VCAM-1/alpha4beta1 integrin interaction is crucial for prompt recruitment of immune T cells into the brain during the early stage of reactivation of chronic infection with Toxoplasma gondii to prevent toxoplasmic encephalitis. Infect Immun 82(7):2826–2839. doi:10.1128/IAI. 01494-13
Williams DW, Calderon TM, Lopez L, Carvallo-Torres L, Gaskill PJ, Eugenin EA, Morgello S, Berman JW (2013) Mechanisms of HIV entry into the CNS: increased sensitivity of HIV infected CD14+CD16+ monocytes to CCL2 and key roles of CCR2, JAM-A, and ALCAM in diapedesis. PLoS One 8 (7):e69270. doi:10.1371/journal.pone.0069270
Sathiyanadan K, Coisne C, Enzmann G, Deutsch U, Engelhardt B (2014) PSGL-1 and E/P-selectins are essential for T-cell rolling in inflamed CNS microvessels but dispensable for initiation of EAE. Eur J Immunol 44 (8):2287–2294. doi:10.1002/eji.201344214
Schneider-Hohendorf T, Rossaint J, Mohan H, Boning D, Breuer J, Kuhlmann T, Gross CC, Flanagan K, Sorokin L, Vestweber D, Zarbock A, Schwab N, Wiendl H (2014) VLA-4 blockade promotes differential routes into human CNS involving PSGL-1 rolling of T cells and MCAM-adhesion of TH17 cells. J Exp Med 211 (9):1833–1846. doi:10.1084/jem.20140540
Forster R, Davalos-Misslitz AC, Rot A (2008) CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol 8(5):362–371. doi:10.1038/nri2297
Noor S, Habashy AS, Nance JP, Clark RT, Nemati K, Carson MJ, Wilson EH (2010) CCR7-dependent immunity during acute Toxoplasma gondii infection. Infect Immun 78(5):2257–2263. doi:10.1128/IAI. 01314-09
Ploix CC, Noor S, Crane J, Masek K, Carter W, Lo DD, Wilson EH, Carson MJ (2011) CNS-derived CCL21 is both sufficient to drive homeostatic CD4+ T cell proliferation and necessary for efficient CD4+ T cell migration into the CNS parenchyma following Toxoplasma gondii infection. Brain Behav Immun 25(5):883–896. doi:10.1016/j.bbi.2010.09.014
Christensen JE, Nansen A, Moos T, Lu B, Gerard C, Christensen JP, Thomsen AR (2004) Efficient T-cell surveillance of the CNS requires expression of the CXC chemokine receptor 3. J Neurosci 24(20):4849–4858. doi:10.1523/JNEUROSCI. 0123-04.2004
Muller M, Carter SL, Hofer MJ, Manders P, Getts DR, Getts MT, Dreykluft A, Lu B, Gerard C, King NJ, Campbell IL (2007) CXCR3 signaling reduces the severity of experimental autoimmune encephalomyelitis by controlling the parenchymal distribution of effector and regulatory T cells in the central nervous system. J Immunol 179(5):2774–2786
Kivisakk P, Trebst C, Liu Z, Tucky BH, Sorensen TL, Rudick RA, Mack M, Ransohoff RM (2002) T-cells in the cerebrospinal fluid express a similar repertoire of inflammatory chemokine receptors in the absence or presence of CNS inflammation: implications for CNS trafficking. Clin Exp Immunol 129(3):510–518
Zhang B, Chan YK, Lu B, Diamond MS, Klein RS (2008) CXCR3 mediates region-specific antiviral T cell trafficking within the central nervous system during West Nile virus encephalitis. J Immunol 180(4):2641–2649
Klein RS, Lin E, Zhang B, Luster AD, Tollett J, Samuel MA, Engle M, Diamond MS (2005) Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J Virol 79(17):11457–11466. doi:10.1128/JVI. 79.17.11457-11466.2005
Brunn A, Montesinos-Rongen M, Strack A, Reifenberger G, Mawrin C, Schaller C, Deckert M (2007) Expression pattern and cellular sources of chemokines in primary central nervous system lymphoma. Acta Neuropathol 114(3):271–276. doi:10.1007/s00401-007-0258-x
Strack A, Schluter D, Asensio VC, Campbell IL, Deckert M (2002) Regulation of the kinetics of intracerebral chemokine gene expression in murine Toxoplasma encephalitis: impact of host genetic factors. Glia 40(3):372–377. doi:10.1002/glia.10104
Harris TH, Banigan EJ, Christian DA, Konradt C, Tait Wojno ED, Norose K, Wilson EH, John B, Weninger W, Luster AD, Liu AJ, Hunter CA (2012) Generalized Levy walks and the role of chemokines in migration of effector CD8+ T cells. Nature 486(7404):545–548. doi:10.1038/nature11098
Galea I, Bernardes-Silva M, Forse PA, van Rooijen N, Liblau RS, Perry VH (2007) An antigen-specific pathway for CD8 T cells across the blood–brain barrier. J Exp Med 204(9):2023–2030. doi:10.1084/jem.20070064
Kang SS, Herz J, Kim JV, Nayak D, Stewart-Hutchinson P, Dustin ML, McGavern DB (2011) Migration of cytotoxic lymphocytes in cell cycle permits local MHC I-dependent control of division at sites of viral infection. J Exp Med 208(4):747–759. doi:10.1084/jem.20101295
Lodygin D, Odoardi F, Schlager C, Korner H, Kitz A, Nosov M, van den Brandt J, Reichardt HM, Haberl M, Flugel A (2013) A combination of fluorescent NFAT and H2B sensors uncovers dynamics of T cell activation in real time during CNS autoimmunity. Nat Med 19(6):784–790. doi:10.1038/nm.3182
Bartholomaus I, Kawakami N, Odoardi F, Schlager C, Miljkovic D, Ellwart JW, Klinkert WE, Flugel-Koch C, Issekutz TB, Wekerle H, Flugel A (2009) Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 462(7269):94–98. doi:10.1038/nature08478
Truong P, Heydari S, Garidou L, McGavern DB (2009) Persistent viral infection elevates central nervous system MHC class I through chronic production of interferons. J Immunol 183(6):3895–3905. doi:10.4049/jimmunol.0803085
Barcia C, Sanderson NS, Barrett RJ, Wawrowsky K, Kroeger KM, Puntel M, Liu C, Castro MG, Lowenstein PR (2008) T cells’ immunological synapses induce polarization of brain astrocytes in vivo and in vitro: a novel astrocyte response mechanism to cellular injury. PLoS One 3(8):e2977. doi:10.1371/journal.pone.0002977
Barcia C, Wawrowsky K, Barrett RJ, Liu C, Castro MG, Lowenstein PR (2008) In vivo polarization of IFN-gamma at Kupfer and non-Kupfer immunological synapses during the clearance of virally infected brain cells. J Immunol 180(3):1344–1352
Chtanova T, Schaeffer M, Han SJ, van Dooren GG, Nollmann M, Herzmark P, Chan SW, Satija H, Camfield K, Aaron H, Striepen B, Robey EA (2008) Dynamics of neutrophil migration in lymph nodes during infection. Immunity 29(3):487–496. doi:10.1016/j.immuni.2008.07.012
Schaeffer M, Han SJ, Chtanova T, van Dooren GG, Herzmark P, Chen Y, Roysam B, Striepen B, Robey EA (2009) Dynamic imaging of T cell-parasite interactions in the brains of mice chronically infected with Toxoplasma gondii. J Immunol 182(10):6379–6393. doi:10.4049/jimmunol.0804307
Nair A, Frederick TJ, Miller SD (2008) Astrocytes in multiple sclerosis: a product of their environment. Cell Mol Life Sci 65(17):2702–2720. doi:10.1007/s00018-008-8059-5
Lalor SJ, Segal BM (2010) Lymphoid chemokines in the CNS. J Neuroimmunol 224(1–2):56–61. doi:10.1016/j.jneuroim.2010.05.017
Banigan EJ, Harris TH, Christian DA, Hunter CA, Liu AJ (2015) Heterogeneous CD8+ T cell migration in the lymph node in the absence of inflammation revealed by quantitative migration analysis. PLoS Comput Biol 11 (2):e1004058. doi:10.1371/journal.pcbi.1004058
Kang H, Liesenfeld O, Remington JS, Claflin J, Wang X, Suzuki Y (2003) TCR V beta 8+ T cells prevent development of toxoplasmic encephalitis in BALB/c mice genetically resistant to the disease. J Immunol 170(8):4254–4259
Suzuki Y, Wang X, Jortner BS, Payne L, Ni Y, Michie SA, Xu B, Kudo T, Perkins S (2010) Removal of Toxoplasma gondii cysts from the brain by perforin-mediated activity of CD8+ T cells. Am J Pathol 176(4):1607–1613. doi:10.2353/ajpath.2010.090825
Wang X, Claflin J, Kang H, Suzuki Y (2005) Importance of CD8(+)Vbeta8(+) T cells in IFN-gamma-mediated prevention of toxoplasmic encephalitis in genetically resistant BALB/c mice. J Interferon Cytokine Res 25(6):338–344. doi:10.1089/jir.2005.25.338
Denkers EY, Yap G, Scharton-Kersten T, Charest H, Butcher BA, Caspar P, Heiny S, Sher A (1997) Perforin-mediated cytolysis plays a limited role in host resistance to Toxoplasma gondii. J Immunol 159(4):1903–1908
Coombes JL, Robey EA (2010) Dynamic imaging of host-pathogen interactions in vivo. Nat Rev Immunol 10 (5):353–364. doi:10.1038/nri2746
Nance JP, Vannella KM, Worth D, David C, Carter D, Noor S, Hubeau C, Fitz L, Lane TE, Wynn TA, Wilson EH (2012) Chitinase dependent control of protozoan cyst burden in the brain. PLoS Pathog 8 (11):e1002990. doi:10.1371/journal.ppat.1002990
Frickel EM, Sahoo N, Hopp J, Gubbels MJ, Craver MP, Knoll LJ, Ploegh HL, Grotenbreg GM (2008) Parasite stage-specific recognition of endogenous Toxoplasma gondii-derived CD8+ T cell epitopes. J Infect Dis 198(11):1625–1633. doi:10.1086/593019
Dupont CD, Christian DA, Selleck EM, Pepper M, Leney-Greene M, Harms Pritchard G, Koshy AA, Wagage S, Reuter MA, Sibley LD, Betts MR, Hunter CA (2014) Parasite fate and involvement of infected cells in the induction of CD4+ and CD8+ T cell responses to Toxoplasma gondii. PLoS Pathog 10(4):e1004047. doi:10.1371/journal.ppat.1004047
Schluter D, Meyer T, Kwok LY, Montesinos-Rongen M, Lutjen S, Strack A, Schmitz ML, Deckert M (2002) Phenotype and regulation of persistent intracerebral T cells in murine Toxoplasma encephalitis. J Immunol 169(1):315–322
Zinselmeyer BH, Heydari S, Sacristan C, Nayak D, Cammer M, Herz J, Cheng X, Davis SJ, Dustin ML, McGavern DB (2013) PD-1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J Exp Med 210(4):757–774. doi:10.1084/jem.20121416
Bhadra R, Khan IA (2012) Redefining chronic toxoplasmosis—a T cell exhaustion perspective. PLoS Pathog 8(10):e1002903. doi:10.1371/journal.ppat.1002903
Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R (2007) Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27(4):670–684. doi:10.1016/j.immuni.2007.09.006
Bhadra R, Gigley JP, Weiss LM, Khan IA (2011) Control of Toxoplasma reactivation by rescue of dysfunctional CD8+ T-cell response via PD-1-PDL-1 blockade. Proc Natl Acad Sci U S A 108(22):9196–9201. doi:10.1073/pnas.1015298108
Bhadra R, Cobb DA, Khan IA (2013) Donor CD8+ T cells prevent Toxoplasma gondii de-encystation but fail to rescue the exhausted endogenous CD8+ T cell population. Infect Immun 81(9):3414–3425. doi:10.1128/IAI. 00784-12
Shin H, Blackburn SD, Blattman JN, Wherry EJ (2007) Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J Exp Med 204(4):941–949. doi:10.1084/jem.20061937
Wakim LM, Woodward-Davis A, Bevan MJ Memory T (2010) cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci U S A 107 (42):17872–17879. doi: 10.1073/pnas.1010201107
Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R (2002) Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med 195(12):1541–1548
Schluns KS, Kieper WC, Jameson SC, Lefrancois L (2000) Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol 1(5):426–432. doi:10.1038/80868
Khan IA, Casciotti L (1999) IL-15 prolongs the duration of CD8+ T cell-mediated immunity in mice infected with a vaccine strain of Toxoplasma gondii. J Immunol 163(8):4503–4509
Lieberman LA, Villegas EN, Hunter CA (2004) Interleukin-15-deficient mice develop protective immunity to Toxoplasma gondii. Infect Immun 72(11):6729–6732. doi:10.1128/IAI. 72.11.6729-6732.2004
Bhadra R, Guan H, Khan IA (2010) Absence of both IL-7 and IL-15 severely impairs the development of CD8 T cell response against Toxoplasma gondii. PLoS One 5(5):e10842. doi:10.1371/journal.pone.0010842
Sauer BM, Schmalstieg WF, Howe CL (2013) Axons are injured by antigen-specific CD8(+) T cells through a MHC class I- and granzyme B-dependent mechanism. Neurobiol Dis 59:194–205. doi:10.1016/j.nbd.2013.07.010
Drogemuller K, Helmuth U, Brunn A, Sakowicz-Burkiewicz M, Gutmann DH, Mueller W, Deckert M, Schluter D (2008) Astrocyte gp130 expression is critical for the control of Toxoplasma encephalitis. J Immunol 181(4):2683–2693
Sosa RA, Murphey C, Ji N, Cardona AE, Forsthuber TG (2013) The kinetics of myelin antigen uptake by myeloid cells in the central nervous system during experimental autoimmune encephalomyelitis. J Immunol 191 (12):5848–5857. doi: 10.4049/jimmunol.1300771
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is a contribution to the special issue on CD8+ T-cell Responses Against Non-viral Pathogens - Guest Editors: Fidel Zavala and Imtiaz Kahn
Rights and permissions
About this article
Cite this article
Landrith, T.A., Harris, T.H. & Wilson, E.H. Characteristics and critical function of CD8+ T cells in the Toxoplasma-infected brain. Semin Immunopathol 37, 261–270 (2015). https://doi.org/10.1007/s00281-015-0487-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-015-0487-3