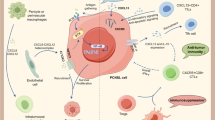
Abstract
The expression pattern of a subset of chemokines and their corresponding receptors was investigated in primary central nervous system lymphomas (PCNSL). The tumor cells consistently expressed CXCR4, CXCL12, CXCR5, and CXCL13, both at mRNA and protein levels. Cerebral endothelial cells were positive for CXCL12 and CXCL13, while reactive astrocytes and microglial cells expressed CXCL12, CCR5, and CCR6. Inflammatory T cells in PCNSL were characterized by CCR5 and CCR6 positivity. Taken together, our data indicate a cell type-specific repertoire of chemokine and chemokine receptor expression in PCNSL suggesting that chemokine-mediated interactions facilitate crossing of the blood-brain barrier as well as intracerebral dissemination of PCNSL cells. In addition, chemokines expressed by tumor cells may contribute to induction of reactive glial changes and influence the composition of inflammatory infiltrates in PCNSL. Therefore, cell type specific expression of distinct chemokine profiles likely plays a role in the pathogenesis of PCNSL and may contribute to their characteristic histological appearance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary central nervous system lymphomas (PCNSL) are high-grade non-Hodgkin’s lymphomas of the diffuse large B cell type (DLBCL) confined to the CNS [26]. PCNSL carry a worse prognosis as compared to systemic DLBCL [29]. The pathogenesis of PCNSL is essentially unknown. In particular, it is still unclear why these neoplasms exclusively manifest in the immunoprivileged brain and often show multifocal CNS dissemination in the absence of any systemic spread.
With respect to the regulation of homing and spread of lymphocytes within the CNS, chemokines may play a pivotal role [8]. Chemokines represent a family of small chemotactic cytokines critically involved in homing of lymphocytes to specific organs, including secondary lymphoid organs [7, 24]. Lymphoid chemokines are involved in sustaining homeostatic leukocyte traffic and cell compartmentalization within these organs [41]. CXCR4, the receptor of CXCL12, is constitutively expressed at high level on bone marrow stromal cells [1, 22, 36], normal B lymphocytes [25], and malignant B lymphocytes of patients with chronic lymphocytic leukemia and other low-grade B cell non-Hodgkin’s lymphoma [3, 38]. In addition, CXCR4 plays a key role in homing and mobilization of hematopoietic stem cells [20]. CXCL12, the stromal cell-derived factor-1 (SDF-1), was originally isolated as a pre-B cell stimulatory factor [22]. CXCL12 plays a critical role in the regulation of trafficking, transendothelial migration, proliferation, and differentiation of hematopoietic cells [2, 23]. In addition, CXCL12 is a potent chemoattractant for microglial cells [34], crucial for neuronal guidance in development [16, 18] and expressed in vascular endothelia and neurons but not in glial cells of the mature human brain [33]. The function of CXCL12 in the adult CNS is still uncertain. The B cell-attracting chemokine CXCL13 is expressed on follicular dendritic cells and responsible for homing of CXCR5-positive B lymphocytes as well as directing T-helper cells to the lymphoid follicle [9, 19, 40, 42]. The CC chemokine receptor 5 (CCR5), the receptor of the pro-inflammatory chemokines CCL3, CCL3L1, CCL4, and CCL5, plays an important role in the adaptive immune response due to its expression on effector and memory T cells, Th1 T cells, macrophages, and dendritic cells [28, 39]. CCR6 is the unique receptor for the inflammatory chemokine CCL20, which is expressed in Peyer’s patches [5, 11, 13, 27]. CCR6 is highly expressed on immature dendritic cells [16, 27]. In addition, CCR6 was detected on tumor cells of the vast majority of low-grade B cell non-Hodgkin’s lymphoma as well as on normal B lymphocytes [38].
The present study addresses the expression pattern of a set of chemokines and their receptors in PCNSL with special emphasis on those chemokines which are known to play a role in B and T cell homing to the brain. We revealed a characteristic, cell type-associated chemokine and chemokine receptor expression pattern in PCNSL indicating a potential role of these molecules in the development and intracerebral dissemination of PCNSL.
Materials and methods
Ten PCNSL of immunocompetent patients (4 males and 6 females; mean age 63 years; range 52–75 years) were obtained by stereotactic biopsy. In each patient, systemic lymphoma was excluded by clinical staging of the disease. All studies were approved by local ethics committees. Informed consent was provided according to the Declaration of Helsinki. Diagnosis of PCNSL was based on morphological and immunohistochemical criteria as described previously [21]. All tumors corresponded to CD20 + BCL6 + DLBCL [10].
RNA Extraction and Reverse Transcriptase (RT)-PCR Analysis
RNA was extracted from frozen tumor tissue samples by using the trizol/chloroform method (Invitrogen, Karlsruhe, Germany). Histological investigation confirmed tumor cell content of at least 80% in each specimen. First strand cDNA was synthesized using the Super-Script II kit (Invitrogen). All cDNAs were tested for transcripts of beta-actin as housekeeping gene by RT-PCR. The primer sequences and cycling conditions are listed in Table 1.
Immunohistochemistry
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded 4 μm sections as described [21]. In brief, for detection of chemokines, monoclonal mouse anti human CXCL12, polyclonal goat anti human CCR5 and CXCL13, respectively, polyclonal rabbit anti human CXCR4 and CCR6, and monoclonal rat anti human CXCR5 were used (Table 2). Tumors were considered positive for expression of the respective chemokine, when more than 20% of the tumor cells were immunopositive.
Results
The results of chemokine expression at the mRNA and protein level are detailed in Table 3.
CXCR4 transcripts were detected in each of nine investigated tumors. The PCR data were confirmed at the protein level by demonstrating CXCR4 immunoreactivity in more than 20% of the tumor cells in 7 out of 7 cases investigated (Fig. 1a).
Immunohistochemical expression of chemokines and their receptors in PCNSL. While the vast majority of tumor cells show a cytoplasmic expression of CXCR4 (a), CXCL12 is expressed in tumor cells as well as in vascular endothelial cells (b). Expression pattern of CXCR5 (c) and CXCR13 (d) (PCNSL # 1). Note expression of CXCR5 in tumor cells (c) and expression of CXCL13 in both tumor cells (d) and vascular endothelial cells (insert d). Tumor cells exhibit a strong nuclear as well as cytoplasmic expression of CCR5 in PCNSL # 1 (e), while they are CCR5-negative in PCNSL # 6 (f). mRNA transcription in PCNSL # 6 (Table 3) is attributed to CCR5-expressing reactive astrocytes and small mature lymphocytes (insert f). Prominent nuclear and cytoplasmic expression of CCR6 by the tumor cells in PCNSL # 4 (g). In contrast, tumor cells in PCNSL # 8 are CCR6-negative (h). The detection of mRNA in PCNSL # 8 (Table 3) is explained by the CCR6-positive reactive astrocytes and small mature lymphocytes partially surrounding the reactive astrocytes and characterized by smaller, basophilic nuclei in the absence of mitoses as compared to the large nuclei of the pleomorphic blasts with prominent nucleoli (insert h). All sections are counterstained with hemalum. Original magnification, ×400 (a–h)
CXCL12 transcripts, which encoded the ligand of CXCR4, were also expressed in all tumors analyzed (8/8 cases). In addition, immunohistochemistry showed CXCL12 immunoreactive tumor cells and cerebral vascular endothelial cells in each of these tumors (Fig. 1b). Furthermore, activated microglial cells in the infiltration zone of the tumors were CXCL12-positive (data not shown).
CXCR5 protein was strongly expressed in the tumor cells in all cases analyzed (7/7; Fig. 1c) and, correspondingly, mRNA was detectable in all ten PCNSL investigated.
CXCL13 mRNA was transcribed in 7 of 7 cases available for PCR analysis. In all PCNSL investigated CXCL13 protein was detected by immunohistochemistry in both tumor cells and vascular endothelia (Fig. 1d).
CCR5 mRNA was detected in 6 out of 9 PCNSL samples (Fig. 2). In three PCNSL, tumor cells were immunohistochemically positive for CCR5 protein (cases no. 1, 4, 5; Fig. 1e). In two PCNSL (cases no. 6, 8), within the confines of light microscopy, CCR5-positive tumor cells were not detected, while reactive astrocytes, activated microglial cells, and small, mature lymphocytes within the associated reactive infiltrates were CCR5-positive (Fig. 1f), thereby most likely accounting for the presence of CCR5 mRNA. Two PCNSL lacked CCR5 mRNA and protein expression.
CCR5 mRNA transcription in PCNSL. CCR5 mRNA is transcribed in PCNSL #1 (lane 3) and PCNSL #4 (lane 6), while the 450 bp product is not detectable in PCNSL #2 (lane 4) and PCNSL #3 (lane 5). Lane 1 shows the 100 base pair (bp) marker. As negative controls, CCR5 mRNA-negative B cell-derived cDNA (lane 2) and H2O (lane 7) were used. Normal human tonsil-derived cDNA (lane 8) served as positive control
CCR6 mRNA was present in 5 out of 10 PCNSL. However, only one of these tumors demonstrated immunopositivity for CCR6 protein in the tumor cells (case no. 4, Fig. 1g), while tumor cells were negative in 6 of 7 cases investigated (<1%). Again, in three PCNSL (cases no. 2, 5, 8), CCR6 immunoreactivity was attributed to reactive astrocytes, activated microglial cells, and small, mature lymphocytes (Fig. 1h).
Discussion
This study demonstrates a cell type-associated expression pattern of a subset of chemokines and their receptors involved in B lymphocyte homing in PCNSL. The malignant B cells consistently expressed CXCR4 and its ligand CXCL12 as well as CXCR5 and its ligand CXCL13. Vascular endothelial cells, reactive astrocytes, microglial cells and T cells in PCNSL also showed distinct expression profiles of the investigated chemokines and their receptors. While cerebral vascular endothelial cells expressed CXCL12 and CXCL13, astrocytes, and microglial cells expressed CXCL12, CCR5, and CCR6. CCR5 and CCR6 were also produced by T lymphocytes. In contrast, CCR5 and CCR6 were only exceptionally expressed by malignant B cells.
Our data on this chemokine pattern confirm and extend recent studies [14, 30, 31]. The expression of CXCL13 on vascular endothelial cells suggests a chemokine-mediated regulation of B cell homing to the CNS and their spread within the brain. However, it is still unknown whether B cells in PCNSL enter the brain already as malignant B cells, which are eliminated by the immune system in the periphery, or as non-transformed cells that possibly invade during a (viral) infection and subsequently undergo malignant transformation in the CNS, a scenario reminiscent of gastric MALT lymphoma. In addition, CXCR5/CXCL13 mediated reactions play an important role in infiltration, resistance to apoptosis, and proliferation of B cells in acute and chronic leukemia [4], effects which may also be operative in PCNSL, as PCNSL diffusely infiltrate the brain parenchyma and express genes of the NF-κB family indicating an activation of the NF-κB pathway, which may contribute to the high proliferative activity and the low level of apoptosis [6]. In PCNSL, CXCL12 was prominently upregulated on cerebral vascular endothelial cells as compared to the normal brain [17], which suggests that an increased expression facilitates lymphocyte crossing of the blood–brain barrier. Furthermore, in addition to their regulation of leukocyte homing, chemokines exert prominent effects on immune responses. CXCL12 stimulates the production of tumor necrosis factor, interleukin-1, and CCL5/RANTES in astrocytes and microglial cells [12], thus enabling these resident brain cell populations to interact with CCR5-expressing T cells, astrocytes, and microglial cells, respectively. These interactions may influence the composition of the inflammatory infiltrate as well as the magnitude and nature of the intracerebral immune response. In fact, reactive astrocytes have been described to influence the interaction with microglial cells and T cells via CXCL10 in secondary progressive multiple sclerosis [35] and murine Toxoplasma encephalitis [32]. In addition, the tumor cells may also have an impact on the phenotypic composition of the inflammatory infiltrates by their chemokine pattern with expression of CXCR4, CXCL12, CXCR5, CXCL13, and, rarely, of CCR5, and CCR6. A similar scenario has been reported for Hodgkin’s disease, in which tumor cell-derived eotaxin accounts for the conspicuous recruitment of eosinophils [15]. However, it is still unknown whether the immune reaction in PCNSL is directed against an antigen expressed by the malignant B cells, a resident CNS antigen or an infectious agent, possibly a virus, which may stimulate and perpetuate proliferation of the tumor cells. In fact, the observation of ongoing somatic hypermutation in PCNSL [21, 37] raises the question how a germinal center reaction, which requires the presence of B cells, antigen, antigen presenting cells as well as T cells, may occur in the microenvironment of the CNS. Thus, it is tempting to speculate that interactions between T cells, resident brain cell populations, and tumor cells may contribute to such an extranodal somatic hypermutation.
In conclusion, the distinct expression pattern of chemokines and their cellular sources as identified in our study may indicate a complex interaction between tumor cells, resident brain cells, and T cells in PCNSL, which likely contributes to the pathogenesis of these tumors and their typical histological appearance.
References
Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA (1996) A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J Exp Med 184:1101–1109
Broxmeyer HE, Kim CH, Cooper SH, Hangoc G, Hromas R, Pelus LM (1999) Effects of CC, CXC, C, and CX3C chemokines on proliferation of myeloid progenitor cells, and insights into SDF-1-induced chemotaxis of progenitors. Ann N Y Acad Sci 872:142–162, discussion 163
Burger JA, Burger M, Kipps TJ (1999) Chronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells. Blood 94:3658–3667
Chunsong H, Yuling H, Li W, Jie X, Gang Z, Qiuping Z, Qingping G, Kejian Z, Li Q, Chang AE, Youxin J, Jinquan T (2006) CXC chemokine ligand 13 and CC chemokine ligand 19 cooperatively render resistance to apoptosis in B cell lineage acute and chronic lymphocytic leukemia CD23 + CD5 + B cells. J Immunol 177:6713–6722
Cook DN, Prosser DM, Forster R, Zhang J, Kuklin NA, Abbondanzo SJ, Niu XD, Chen SC, Manfra DJ, Wiekowski MT, Sullivan LM, Smith SR, Greenberg HB, Narula SK, Lipp M, Lira SA (2000) CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity 12:495–503
Courts C, Montesinos-Rongen M, Martin-Subero JI, Brunn A, Siemer D, Zuhlke-Jenisch R, Pels H, Jurgens A, Schlegel U, Schmidt-Wolf IG, Schaller C, Reifenberger G, Sabel M, Warnecke-Eberz U, Wiestler OD, Kuppers R, Siebert R, Deckert M (2007) Transcriptional profiling of the nuclear factor-kappaB pathway identifies a subgroup of primary lymphoma of the central nervous system with low BCL10 expression. J Neuropathol Exp Neurol 66:230–237
Cyster JG (1999) Chemokines and the homing of dendritic cells to the T cell areas of lymphoid organs. J Exp Med 189:447–450
Flugel A, Berkowicz T, Ritter T, Labeur M, Jenne DE, Li Z, Ellwart JW, Willem M, Lassmann H, Wekerle H (2001) Migratory activity and functional changes of green fluorescent effector cells before and during experimental autoimmune encephalomyelitis. Immunity 14:547–560
Forster R, Emrich T, Kremmer E, Lipp M (1994) Expression of the G-protein–coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood 84:830–840
Gatter K, Warnke R (2001) Diffuse large cell lymphomas. Lyon, France, pp 171–174
Greaves DR, Wang W, Dairaghi DJ, Dieu MC, Saint-Vis B, Franz-Bacon K, Rossi D, Caux C, McClanahan T, Gordon S, Zlotnik A, Schall TJ (1997) CCR6, a CC chemokine receptor that interacts with macrophage inflammatory protein 3alpha and is highly expressed in human dendritic cells. J Exp Med 186:837–844
Han Y, He T, Huang DR, Pardo CA, Ransohoff RM (2001) TNF-alpha mediates SDF-1 alpha-induced NF-kappa B activation and cytotoxic effects in primary astrocytes. J Clin Invest 108:425–435
Hieshima K, Imai T, Opdenakker G, Van Damme J, Kusuda J, Tei H, Sakaki Y, Takatsuki K, Miura R, Yoshie O, Nomiyama H (1997) Molecular cloning of a novel human CC chemokine liver and activation-regulated chemokine (LARC) expressed in liver. Chemotactic activity for lymphocytes and gene localization on chromosome 2. J Biol Chem 272:5846–5853
Jahnke K, Coupland SE, Na IK, Loddenkemper C, Keilholz U, Korfel A, Stein H, Thiel E, Scheibenbogen C (2005) Expression of the chemokine receptors CXCR4, CXCR5, and CCR7 in primary central nervous system lymphoma. Blood 106:384–385
Jundt F, Anagnostopoulos I, Bommert K, Emmerich F, Muller G, Foss HD, Royer HD, Stein H, Dorken B (1999) Hodgkin/Reed-Sternberg cells induce fibroblasts to secrete eotaxin, a potent chemoattractant for T cells and eosinophils. Blood 94:2065–2071
Klein RS, Rubin JB (2004) Immune and nervous system CXCL12 and CXCR4: parallel roles in patterning and plasticity. Trends Immunol 25:306–314
Krumbholz M, Theil D, Cepok S, Hemmer B, Kivisakk P, Ransohoff RM, Hofbauer M, Farina C, Derfuss T, Hartle C, Newcombe J, Hohlfeld R, Meinl E (2006) Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain 129:200–211
Lazarini F, Tham TN, Casanova P, Arenzana-Seisdedos F, Dubois-Dalcq M (2003) Role of the alpha-chemokine stromal cell-derived factor (SDF-1) in the developing and mature central nervous system. Glia 42:139–148
Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG (2000) BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity 12:471–481
Mohle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L (1998) The chemokine receptor CXCR-4 is expressed on CD34 + hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood 91:4523–4530
Montesinos-Rongen M, Kuppers R, Schluter D, Spieker T, Van Roost D, Schaller C, Reifenberger G, Wiestler OD, Deckert-Schluter M (1999) Primary central nervous system lymphomas are derived from germinal-center B cells and show a preferential usage of the V4–34 gene segment. Am J Pathol 155:2077–2086
Nagasawa T, Kikutani H, Kishimoto T (1994) Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci USA 91:2305–2309
Nagasawa T, Tachibana K, Kishimoto T (1998) A novel CXC chemokine PBSF/SDF-1 and its receptor CXCR4: their functions in development, hematopoiesis and HIV infection. Semin Immunol 10:179–185
Ohl L, Bernhardt G, Pabst O, Forster R (2003) Chemokines as organizers of primary and secondary lymphoid organs. Semin Immunol 15:249–255
Palmesino E, Moepps B, Gierschik P, Thelen M (2006) Differences in CXCR4-mediated signaling in B cells. Immunobiology 211:377–389
Paulus W, Jellinger K, Morgello S, Deckert-Schlüter M (2000) Malignant lymphomas. Lyon, France, pp 198–203
Power CA, Church DJ, Meyer A, Alouani S, Proudfoot AE, Clark-Lewis I, Sozzani S, Mantovani A, Wells TN (1997) Cloning and characterization of a specific receptor for the novel CC chemokine MIP-3alpha from lung dendritic cells. J Exp Med 186:825–835
Sallusto F, Mackay CR, Lanzavecchia A (2000) The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol 18:593–620
Schlegel U, Schmidt-Wolf IG, Deckert M (2000) Primary CNS lymphoma: clinical presentation, pathological classification, molecular pathogenesis and treatment. J Neurol Sci 181:1–12
Smith JR, Braziel RM, Paoletti S, Lipp M, Uguccioni M, Rosenbaum JT (2003) Expression of B-cell-attracting chemokine 1 (CXCL13) by malignant lymphocytes and vascular endothelium in primary central nervous system lymphoma. Blood 101:815–821
Smith JR, Falkenhagen KM, Coupland SE, Chipps TJ, Rosenbaum JT, Braziel RM (2007) Malignant B cells from patients with primary central nervous system lymphoma express stromal cell-derived factor-1. Am J Clin Pathol 127:633–641
Strack A, Asensio VC, Campbell IL, Schluter D, Deckert M (2002) Chemokines are differentially expressed by astrocytes, microglia and inflammatory leukocytes in Toxoplasma encephalitis and critically regulated by interferon-gamma. Acta Neuropathol (Berl) 103:458–468
Stumm RK, Rummel J, Junker V, Culmsee C, Pfeiffer M, Krieglstein J, Hollt V, Schulz S (2002) A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J Neurosci 22:5865–5878
Tanabe S, Heesen M, Yoshizawa I, Berman MA, Luo Y, Bleul CC, Springer TA, Okuda K, Gerard N, Dorf ME (1997) Functional expression of the CXC-chemokine receptor-4/fusin on mouse microglial cells and astrocytes. J Immunol 159:905–911
Tanuma N, Sakuma H, Sasaki A, Matsumoto Y (2006) Chemokine expression by astrocytes plays a role in microglia/macrophage activation and subsequent neurodegeneration in secondary progressive multiple sclerosis. Acta Neuropathol (Berl) 112:195–204
Tashiro K, Tada H, Heilker R, Shirozu M, Nakano T, Honjo T (1993) Signal sequence trap: a cloning strategy for secreted proteins and type I membrane proteins. Science 261:600–603
Thompsett AR, Ellison DW, Stevenson FK, Zhu D (1999) V(H) gene sequences from primary central nervous system lymphomas indicate derivation from highly mutated germinal center B cells with ongoing mutational activity. Blood 94:1738–1746
Trentin L, Cabrelle A, Facco M, Carollo D, Miorin M, Tosoni A, Pizzo P, Binotto G, Nicolardi L, Zambello R, Adami F, Agostini C, Semenzato G (2004) Homeostatic chemokines drive migration of malignant B cells in patients with non-Hodgkin lymphomas. Blood 104:502–508
Wu L, Paxton WA, Kassam N, Ruffing N, Rottman JB, Sullivan N, Choe H, Sodroski J, Newman W, Koup RA, Mackay CR (1997) CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med 185:1681–1691
Wu MT, Hwang ST (2002) CXCR5-transduced bone marrow-derived dendritic cells traffic to B cell zones of lymph nodes and modify antigen-specific immune responses. J Immunol 168:5096–5102
Yoshie O, Imai T, Nomiyama H (1997) Novel lymphocyte-specific CC chemokines and their receptors. J Leukoc Biol 62:634–644
Yu P, Wang Y, Chin RK, Martinez-Pomares L, Gordon S, Kosco-Vibois MH, Cyster J, Fu YX (2002) B cells control the migration of a subset of dendritic cells into B cell follicles via CXC chemokine ligand 13 in a lymphotoxin-dependent fashion. J Immunol 168:5117–5123
Acknowledgments
The expert technical assistance of Irmgard Henke and Elena Fischer is gratefully acknowledged. This study was supported by the Deutsche Krebshilfe/Dr. Mildred Scheel Stiftung für Krebsforschung (grant no: 10-2153-De1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brunn, A., Montesinos-Rongen, M., Strack, A. et al. Expression pattern and cellular sources of chemokines in primary central nervous system lymphoma. Acta Neuropathol 114, 271–276 (2007). https://doi.org/10.1007/s00401-007-0258-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-007-0258-x