Abstract
Purpose
Apalutamide plus androgen-deprivation therapy (ADT) has been approved for treatment of patients with metastatic castration-sensitive prostate cancer (mCSPC) based on data from phase 3 TITAN study. This analysis was conducted to describe pharmacokinetics of apalutamide and N-desmethyl-apalutamide and explore relationships between apalutamide exposure and selected clinical efficacy and safety observations.
Methods
1052 patients were randomized to apalutamide + ADT (n = 525) or placebo + ADT (n = 527). A previously developed population pharmacokinetic model was applied. Cox regression analysis investigated the relationships between apalutamide exposure and overall survival (OS; n = 1004) and radiographic progression-free survival (rPFS; n = 1003). Logistic regression analysis assessed the relationships between apalutamide exposure and selected clinically relevant adverse events (n = 1051).
Results
Apalutamide + ADT treatment was efficacious in extending rPFS and OS versus placebo + ADT. Within a relatively narrow apalutamide exposure range (coefficient of variation: 22%), no statistical association was detected between rPFS, OS and apalutamide exposure quartiles. Incidence of skin rash and pruritus increased significantly with increasing apalutamide exposure.
Conclusions
Differences in apalutamide exposure were not associated with clinically relevant differences in rPFS or OS in patients with mCSPC. Patients with increased apalutamide exposure are more likely to develop skin rash and pruritus. Dose reductions may improve these adverse events, based on an individual risk–benefit approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to GLOBOCAN estimates, prostate cancer is the second most frequent cancer in men worldwide, with 1.28 million new cases in 2018 and responsible for 3.8% of all deaths caused by cancer in men (approximately 359,000 deaths) [1]. Androgen-deprivation therapy (ADT) through medical or surgical castration has been a mainstay of treatment for metastatic castration-sensitive prostate cancer (mCSPC) until several large, randomized, phase 3 clinical trials showed improved survival in patients with mCSPC when ADT was combined with either abiraterone acetate plus prednisone or docetaxel [2,3,4,5,6]. However, patients’ specific comorbidities, such as neuropathy and high risk of myelosuppression for docetaxel or liver disease for abiraterone plus prednisone might limit the use of these treatments [3].
Apalutamide is a next-generation, orally bioavailable, potent, and selective androgen receptor (AR) antagonist that inhibits downstream transcription of AR-regulated genes [7, 8]. Apalutamide has been approved for the treatment of patients with non-metastatic castration-resistant prostate cancer (nmCRPC) and mCSPC by the United States Food and Drug administration, the European Medicines Agency and other global health authorities, at a recommended dose of apalutamide at 240 mg once-daily [9,10,11,12]. N-desmethyl-apalutamide is the main active metabolite of apalutamide, possessing about one-third of the pharmacological activity of apalutamide [10, 13]. Both apalutamide and N-desmethyl-apalutamide are mainly metabolically cleared, with the formation of N-desmethyl-apalutamide mediated predominantly by CYP2C8 and (to a lesser extent by) CYP3A4 [10]. Due to the auto-induction effect of apalutamide on its own metabolism the terminal half-life decreases from 6.4 to 4.2 days, arriving at steady state around 4 weeks after start of daily apalutamide administration [14]. Given the contribution of the active metabolite to the overall pharmacological effect of apalutamide, it is important to determine the exposure to apalutamide and N-desmethyl- apalutamide in subjects randomized in the TITAN study.
TITAN (NCT02489318) is a randomized, placebo-controlled, double-blind, phase 3 study assessing the addition of apalutamide to ADT for the treatment of mCSPC [15, 16]. Primary endpoints of overall survival (OS; [Hazard ratio] HR: 0.67; 95% confidence interval [CI) 0.51–0.89, p = 0.005) and radiographic progression–free survival (rPFS; HR: 0.48, 95% CI 0.39–0.60, p < 0.001) were significantly longer when apalutamide was combined with ADT compared to placebo plus ADT in a broad population of patients [15, 17]. Additionally, health-related quality of life was maintained for patients receiving apalutamide in the TITAN study [17] and adverse events (AEs) were manageable. These results are in line with those obtained with another AR antagonist used for the treatment of mCSPC, enzalutamide, which was evaluated in the ENZAMET trial [18].
The goals of this analysis were to quantify the exposure of apalutamide and N-desmethyl-apalutamide in patients with mCSPC and to understand the relationships between apalutamide exposure and selected clinical endpoints of efficacy (rPFS and OS) and safety (skin rash, pruritus, weight decrease, arthralgia, fall and fatigue) in this patient population.
Patients and methods
Study design and subject population
A total of 1052 eligible patients were enrolled in the TITAN study and randomized 1:1 to receive either apalutamide + ADT (n = 525) or placebo + ADT (n = 527). Patients were stratified by Gleason score at diagnosis, prior docetaxel use and geographic region. All patients received ADT (orchiectomy or medical castration) as standard-of-care therapy, and apalutamide 240 mg (4 × 60 mg tablets) or placebo (4 tablets) orally once-daily [15, 16].
The study was conducted in accordance with principles for human experimentation as defined in the Declaration of Helsinki and was approved by the Human Investigational Review Board of each study center and by the Competent Authority of each country. Informed consent was obtained from each subject before enrollment in the study, after being advised of the potential risks and benefits, as well as the investigational nature of the study.
Blood samples and bioanalytical method
Blood samples for pharmacokinetic (PK) analysis of apalutamide and N-desmethyl-apalutamide concentrations were collected prior to each next dose at trough, on day 1 of cycles 2, 3, 4, 5, and 6, each cycle consisting of 28 days. A validated liquid chromatography assay with tandem mass spectrometry was used to determine the plasma concentrations of apalutamide and N-desmethyl-apalutamide. The lower limit of quantification was 25.0 ng/mL for both apalutamide and N-desmethyl-apalutamide (internal data), the range was 25.0 to 25,000 ng/mL for both compounds. Accuracy was between 100.0 and 103.5% for apalutamide and between 98.7 and 104.0% for N-desmethyl-apalutamide. Precision was ≤ 10.3% for apalutamide and ≤ 7.0% for N-desmethyl-apalutamide. The method was selective with respect to Leuprolide and endogenous compounds.
Pharmacokinetic analysis and exposure metrics
A previously developed population PK model was used to characterize apalutamide and N-desmethyl-apalutamide pharmacokinetics in the TITAN study [14]. In total, 2302 apalutamide and 2303 N-desmethyl-apalutamide plasma concentrations from 501 patients (randomized to the apalutamide arm) with evaluable data were used in the PK analysis. One additional subject, randomized but never treated with apalutamide, was assigned having no exposure. Apalutamide was dosed at 240 mg once-daily for patients entering the trial. This dose could be reduced due to the occurrence of AEs (and eventually re-escalated) as part of the management of toxicities, including rash in this study. The predictive performance of the previously developed population PK model was evaluated using visual predictive check (VPC), allowing visual comparison between distributions of observed and simulated data (n = 500 replicates) [14].
For each subject, the individual area under the concentration–time curve was computed during a steady-state dosing interval (AUC0-24 h). This was computed since the plasma concentrations of apalutamide attain steady state between 4 and 8 weeks, around the time of the first visit scheduled for efficacy assessment (scheduled first day of cycle 3). The AUC0-24 h was corrected by the individual average daily dose, incorporating any dose modifications for AE as well up to the last day of dosing prior to the event of interest. AUC0-24 h at steady state as exposure metric for exposure–response analysis was supported by previous exposure–response results of the SPARTAN study [19,20,21]. More specifically, the correlations between the individual exposure metrics at steady state (AUC0–24 h,ss, Cmin,ss, Cmax,ss) for both apalutamide and N-desmethyl apalutamide were high (r2 > 0.95), further suggesting similar results for ER analysis irrespective of the chosen exposure metric. Furthermore, since AUC0–24 h,ss represents the average drug concentration over a longer time period following multiple dosing, it is useful to correlate long-term drug effects with steady-state AUC0–24 h (as was done in this analysis). The PK analysis was based on the maximum a posteriori estimation and was conducted using the MAXEVAL = 0 option in NONMEM® software (Icon Development Solutions, Ellicott City, MD) [20, 22]. The data management, diagnostic graphics and post-processing of NONMEM® analysis results were carried out using R Project for Statistical Computing, Version 3.4.1 or higher [23].
Exposure–response analyses for efficacy endpoints
The primary endpoints of the exposure-efficacy analysis were rPFS and OS [15, 16]. Beside the effect of stratification factors on rPFS and OS (Gleason score at diagnosis [≤ 7 versus > 7], prior docetaxel use, and geographic region [North America and European Union versus other countries]), the effect of prognostic factors identified to have a statistically significant association at the p < 0.05 level in TITAN study were considered in the exposure–efficacy analysis [15, 16]. The statistically significant prognostic factors for rPFS were baseline prostate-specific antigen (PSA), baseline lactate dehydrogenase (LDH), baseline age, number of bone lesions at baseline and presence of visceral disease. The statistically significant prognostic factors for OS were baseline PSA, baseline LDH, baseline Eastern Cooperative Oncology Group performance status (ECOG PS; 1 versus 0), number of bone lesions at baseline and presence of visceral disease. In addition, the type of ADT (medical castration [942/1004] versus surgical castration [62/1004], or both in which case patients were classified having medical castration) was included in the exposure–response analysis for both rPFS and OS. Twenty-five out of 527 patients (4.7%) in the placebo + ADT arm and 23 out of 525 patients (4.4%) in the apalutamide + ADT arm had missing values in the prognostic factor LDH at baseline. These patients were excluded from the analysis dataset for rPFS and OS. One additional subject with ECOG = 2 was excluded from the analysis dataset for OS as the inclusion criteria only allowed patients of ECOG PS grade 0 or 1. The rationale of excluding patients with missing prognostic factors for the exposure–response analysis was to maintain similarity with the population used for primary efficacy analysis. As a result, 1004 (placebo + ADT and apalutamide + ADT, n = 502 each) and 1003 (501 placebo + ADT, n = 501 and apalutamide + ADT, n = 502) patients were included in the analysis dataset for rPFS and OS, respectively (Supplementary Table S1). Twenty-two out of 502 patients (4.4%) treated with apalutamide did not have apalutamide PK samples available (e.g., samples not collected or not analyzed) and their exposure metrics were predicted from the typical values of the reference population PK model parameters, given the subject-specific covariates. This approach allowed inclusion of data from these patients in the exposure–response analysis. There was one subject in the apalutamide + ADT arm that was randomized but never treated. This subject was included in the analysis dataset and the exposure metric for this subject was set to zero.
The influence of apalutamide exposure on rPFS and OS was analyzed separately with a 2-tiered approach. First, Kaplan–Meier (KM) curves were stratified by quartiles of apalutamide exposure (including placebo patients without exposure). Second, a multivariate Cox regression analysis was conducted per efficacy endpoint, including the apalutamide treatment effect, apalutamide exposure, stratification and significant prognostic factors. The HR and its 95% CI were used to assess the impact of apalutamide on rPFS and OS (after adjusting for the prognostic factors). The p-values as well as the change in − 2 log-likelihood (–2LL) were used for model comparison. Of note, the proportional hazard assumption was visually checked for rPFS and OS and was considered valid.
Exposure–response analyses of safety
The exposure–safety analysis focused on AEs of clinical relevance using the following selection criteria: (1) occurrence > 10%; (2) at least one Grade ≥ 3 event; (3) ≥ twofold higher occurrence in the apalutamide + ADT arm than in the placebo + ADT arm. Only skin rash and pruritus met the pre-specified criteria and were included in the analysis. Although weight decrease, arthralgia, fall and fatigue did not meet the criteria, they were still considered in this analysis based on results from a previous exposure–response analysis in patients with nmCRPC in SPARTAN study [20]. The selected safety observations were dichotomized into presence or absence of the AE at any grade. Patients with multiple occurrences of events were counted once, at the time of the first event.
The safety population consisted of 1051 patients, 527 patients in the placebo + ADT armand 524 patients in the apalutamide + ADT arm. One subject, randomized but never treated, was excluded. Twenty-three out of 524 patients (4.4%) treated with apalutamide did not have apalutamide PK samples available and their exposure metrics were predicted from the typical values of the reference population PK model parameters, given the subjects-specific covariates (Supplementary Table S1). Similar to the efficacy analysis, this approach allowed inclusion of data from these patients in the exposure–response analysis.
The possible association between the treatment effect (apalutamide + ADT versus placebo + ADT), the apalutamide exposure and the selected AEs was assessed using univariate and multivariate logistic regression analyses. The corresponding odds ratio (OR), 95% CI, Pearson's Chi-squared (χ2) and p-values were calculated. The p-values as well as the change in –2LL were used for model comparison.
Software and statistical considerations
The data management, diagnostic graphics and statistical analysis were carried out using the R Project for Statistical Computing, Version 3.4.1 or higher [23]. P < 0.05 were considered statistically significant and no correction for multiple statistical testing was implemented. The nature of the current analyses was purely exploratory. Thus, the estimates of the parameters of interest, 95% CI and p-values were determined only to assist in evaluating the exposure–response relationships and should be cautiously interpreted.
Results
PK analysis
The previously developed population PK model adequately described the central tendency of the apalutamide and N-desmethyl-apalutamide plasma concentrations at steady state as observed from the VPC (Fig. 1). Although the variability in plasma concentrations observed in the TITAN study was slightly lower than the model-predicted variability, these results indicate a similar apalutamide PK in patients regardless of clinical presentation of prostate cancer (nmCRPC and mCSPC).
Visual predictive check applying the previously developed population pharmacokinetic model to TITAN data for apalutamide (upper panel—a) and N-desmethyl-apalutamide (lower panel—b). The open circles represent the observed concentrations of apalutamide (panel a) and N-desmethyl-apalutamide (panel b). The median and 5th and 95th percentiles of the observations are indicated as dashed lines. The simulation-derived median and 90% PI accompanied by their 95% CI are indicated as blue lines and shaded blue area, respectively. CI confidence interval, PI prediction interval
The mean AUC0-24 h of apalutamide and N-desmethyl-apalutamide, dose-normalized to 240 mg, was 118 and 155 µg·h/mL, respectively (Table 1) and its variability (coefficient of variation, CV%) was considered low (22.0% for apalutamide and 15.3% for N-desmethyl-apalutamide). Individual AUC0-24 h, based on average daily dose up to the last day of dosing prior to rPFS or censoring date, (mean [CV%] of 114 µg·h/mL [23.5%] and 149 µg·h/mL [17.7%] for apalutamide and N-desmethyl-apalutamide, respectively] were similar to AUC0-24 h, dose-normalized to 240 mg, due to the relatively low number of dose modifications (7.3% vs 2.1% dose reductions and 23.5% vs 12.2% dose interruptions in the apalutamide treatment arm vs placebo arm) observed in the TITAN study. Because patients discontinued treatment upon progression of disease, average daily dose and AUC0-24 h corrected for average daily dose prior to OS were relatively unchanged as compared to those corrected prior to rPFS. In addition, after sorting the apalutamide exposure metrics by rank order, four approximately equal-sized groups were created, based on the quartiles of apalutamide exposure (Table 1). Although the table mentions dose-normalized exposure metrics for comparative purposes, the exposure–response analysis for efficacy and safety effectively considered the actual exposure metrics, after correcting by the average daily dose up to the event of interest.
rPFS analysis
The stratification factors and significant prognostic factors were balanced among the treatment and exposure groups evaluated, except for prior docetaxel use, geographic region and visceral disease, which showed an imbalance in the exposure quartiles (Supplementary Table S2). Potential imbalances should be accounted for in the multivariate analysis.
The KM curves of rPFS stratified by quartiles of apalutamide AUC0-24 h indicated a statistically significant increase in rPFS for apalutamide treated patients in each pairwise comparison of the exposure subgroup with placebo (Fig. 2). However, within apalutamide-treated patients, including those with short term (or short duration) dose reductions due to AEs, no statistical association could be detected between rPFS and quartiles of apalutamide exposure.
Kaplan–Meier plot for radiographic progression-free survival (a) and overall survival (b) as a function of placebo and apalutamide area under the concentration curve during 24 h at steady-state (AUC0-24 h) quartiles (Q1 to Q4). Q1 is the lowest exposure quartile and Q4 is the highest exposure quartile for apalutamide. P-value signifies the difference between exposure-quartiles of apalutamide and placebo. AUC area under the concentration curve, OS overall survival, rPFS radiographic progression-free survival
The multivariate Cox regression analysis evidenced a statistically significant association between apalutamide treatment, prior docetaxel use (HR = 0.66, 95% CI 0.43–1.00, p = 0.048), PSA (HR = 1.11, 95% CI 1.06–1.16, p < 0.0001), LDH (HR = 2.80, 95% CI 1.87–4.18, p < 0.0001), age (HR = 0.98, 95% CI 0.97–1.00, p = 0.0183), number of bone lesions (HR = 1.97, 95% CI 1.57–2.47, p < 0.0001), presence of visceral disease (HR = 1.58, 95% CI 1.19–2.09, p = 0.002) and rPFS (Table 2). The apalutamide treatment effect on rPFS was found to be similar in the multivariate analysis (HR = 0.45, 95% CI 0.36–0.56, p < 0.0001) after adjusting by all prognostic factors, compared to the univariate analysis previously described [15]. This suggests that (i) the prognostic factors can be considered independently associated with rPFS; and (ii) the imbalance reported in prior docetaxel use, geographic region and visceral disease does not have a major influence on the estimation of treatment effect. The addition of apalutamide AUC0-24 h as a covariate did not result in a statistically significant improvement of the model fit (χ2 = 0.202; df = 1, p = 0.653), but led to a substantial change of the treatment effect and its uncertainty (HR = 0.54, 95% CI 0.23–1.24; Supplementary Table S3), explained by the presence of collinearity. The effect of apalutamide exposure as a continuous covariate was also evaluated in absence of treatment effect, but did not improve the description of the data, indicating that the treatment effect of 240 mg daily apalutamide is not dependent on apalutamide AUC0-24 h (Supplementary Table S3). The lack of statistical association between rPFS and apalutamide exposure was confirmed in a multivariate Cox regression analysis after excluding placebo subjects (stratified HR of AUC0-24 h: 0.997 μg·h/mL; 95% CI 0.990–1.004). Both the univariate and multivariate KM analyses show a significant effect of 240 mg daily dosing of apalutamide on rPFS that is not dependent on apalutamide AUC0-24 h.
OS analysis
The prognostic factors’ distribution across treatment arms and apalutamide exposure quartiles was balanced, as observed for rPFS (Supplementary Table S2) and potential imbalances by prior docetaxel use, geographic region, and visceral disease should be controlled in the multivariate analysis. The KM curves of OS stratified by quartiles of apalutamide AUC0-24 h indicated no statistically significant difference between each of the quartile of exposure and placebo, except for the highest exposure quartile (p = 0.0482) (Fig. 2). This can probably be explained by the limited number of events in each exposure quartile. In addition, when placebo subjects were excluded, no statistical association could be detected between OS and the quartiles of apalutamide AUC0-24 h.
The multivariate Cox regression analysis evidenced a statistically significant association between apalutamide treatment, PSA (HR = 1.14, 95% CI 1.06–1.21, p = 0.0002), LDH (HR = 5.51, 95% CI 3.44–8.83, p < 0.0001), ECOG (HR = 1.54, 95% CI 1.14–2.08, p = 0.005), number of bone lesions (HR = 1.92, 95% CI 1.40–2.65, p < 0.0001), presence of visceral disease (HR = 1.90, 95% CI 1.34–2.69, p = 0.0003) and OS (Table 2). The apalutamide treatment effect on OS was found to be similar in the multivariate analysis after adjusting by all prognostic factors (HR = 0.64, 95% CI 0.48–0.86, p = 0.003) compared to the univariate analysis results [15]. This suggests that (i) the prognostic factors can be considered independently associated with OS; and (ii) the imbalance reported in prior docetaxel use, geographic region and visceral disease does not have a major influence on the estimation of treatment effect. The addition of apalutamide AUC0-24 h as a continuous covariate did not result in a statistically significant improvement of the model fit (χ2 = 1.862; df = 1, p = 0.172; Supplementary Table S4). On the contrary, it resulted in a substantial modification of apalutamide treatment effect and its associated uncertainty, thus suggesting the presence of collinearity issues. The effect of apalutamide exposure as a continuous covariate was evaluated in the absence of treatment effect (Supplementary Table S4). Although the -2LL was lower for this model, the proportion of treatment effect calculated using Li’s method was considered small and not relevant (22.5%) [24]. The lack of statistical association between OS and quartiles of apalutamide AUC0-24 h was confirmed in a multivariate Cox regression analysis after excluding placebo subjects and including prognostic factors as well as AUC0-24 h as a continuous covariate (stratified HR of AUC0-24 h: 0.994 μg·h/mL; 95% CI of 0.986–1.003). Similar to the observations in the rPFS analysis, both the univariate and multivariate KM analyses support that the significant effect of apalutamide on OS is not solely dependent on apalutamide AUC0-24 h in patients with mCSPC. Of note, specific variables (such as age) were treated as continuous variables in the multivariate analysis of both rPFS and OS, as opposed to their categorical use in the primary statistical analysis, previously published [15, 16]. Although categories may sometimes be more illustrative for clinical practice, using covariates continuously where possible, allows to leverage all the information in the data. Moreover, prior to the multivariate analysis the selected significant prognostic factors were also explored as categorical variables in a univariate KM analysis (Fig. 2, Supplementary Figures S1, S2).
Exposure–safety analysis
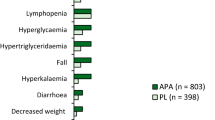
From the selected AEs, only skin rash and pruritus had a statistically significant higher incidence in the apalutamide + ADT arm compared to the placebo + ADT arm (p < 0.0001; Table 3). The univariate logistic regression analysis for the treatment effect showed that the probability of experiencing skin rash (OR = 3.98; 95% CI 2.80–5.77, p < 0.001) and pruritus (OR = 2.51; 95% CI 1.55–4.18, p < 0.001) was significantly higher in the apalutamide + ADT arm compared to placebo + ADT arm. Comparable incidence rates were observed in the two treatment arms for weight decrease (OR = 1.28; 95% CI 0.77–2.18, p = 0.345), fall (OR = 1.06; 95% CI 0.67–1.70, p = 0.792), fatigue (OR = 1.07; 95% CI 0.81–1.42, p = 0.631) and arthralgia (OR = 1.21; 95% CI 0.87–1.69, p = 0.258). Analogously, the univariate logistic regression showed that only skin rash (OR = 1.10 × 10 μg·h/mL; 95% CI 1.08–1.13, p < 0.001) and pruritus (OR: 1.06 × 10 μg·h/mL; 95% CI 1.03–1.10, p < 0.001) at any grade, had a statistically significantly increase in incidence with increasing apalutamide exposure (Fig. 3). The magnitude of this association was most pronounced for skin rash compared to pruritus.
Logistic regression representing the probability of experiencing skin rash, weight decrease, fall, fatigue, arthralgia, and pruritus as function of apalutamide area under the concentration curve during 24 h at steady-state (AUC0-24 h) for patients in the treatment and placebo arms. The upper and lower open circles represent the presence (y = 1) or absence (y = 0) of a given treatment-emergent event across the range of apalutamide AUC0-24 h exposure. The red closed circles depict the observed incidence for the placebo arm, whereas the dark blue circles represent the observed incidence for the quartiles of apalutamide exposure in the treatment arm. The corresponding vertical bars represent the exact 95% CI calculated using Wilson's method. Finally, the middle line and its corresponding shaded area represent the model-based exposure-safety relationship and the associated 95% CI, respectively. AUC area under the concentration curve, CI confidence interval, OR odds ratio
A multivariate logistic regression model including treatment effect and apalutamide AUC0-24 h at steady state for each AE was assessed. However, collinearity issues impaired the interpretation of results. Based on the modeled exposure–safety relationships, the incidence of experiencing skin rash or pruritus was predicted for apalutamide exposures expected for doses of 240, 180, and 120 mg once-daily. The predicted incidence decreased with lower doses and was more pronounced for skin rash compared to pruritus (Supplementary Table S5). For skin rash, dose reductions will likely improve apalutamide-related skin rash and pruritus in patients who develop toxicity after apalutamide treatment starting at 240 mg once daily.
Discussion
The population PK analysis suggests that the steady-state PK of apalutamide and N-desmethyl-apalutamide is similar for patients suffering from various stages of prostate cancer (nmCRPC in SPARTAN and mCSPC in TITAN, respectively), when accounting for weight and albumin [14]. Although only trough concentrations were available in the TITAN study, the population PK modeling approach allowed characterization of the PK of apalutamide and N-desmethyl-apalutamide in patients randomized in the TITAN study. The variability in apalutamide (CV: 22%) and N-desmethyl-apalutamide (CV: 15.3%) exposure metrics (normalized to 240 mg once-daily dosing) was modest, with no known significant genetic polymorphisms in CYP2C8 or CYP3A4 affecting the PK in a clinically meaningful way.
In TITAN, rPFS and OS were significantly longer when apalutamide was combined with ADT compared to placebo plus ADT. In general, the safety profile was consistent between the two groups, with the frequency of grade 3 or 4 AEs being 42.2% in the apalutamide group and 40.8% in the placebo group [15, 16].
Apalutamide exposure–efficacy analysis showed that no statistical association could be detected between rPFS, OS and quartiles of apalutamide AUC0-24 h at steady state (corrected by the average daily dose up to the event of interest), either in the univariate or multivariate Cox regression analyses [20]. Furthermore, AUC is considered a good reflection of the daily exposure following multiple dosing and is frequently used to correlate long-term treatment effects to drug exposure [25]. Although N-desmethyl-apalutamide, a pharmacologically active metabolite, contributes to the overall pharmacological activity observed for apalutamide, the exposure to N-desmethyl-apalutamide did not appear to add value to the exposure–response analysis in nmCRPC subjects and was therefore not considered in this exposure–response analysis. This is because the steady-state exposure of N-desmethyl-apalutamide is similar to the one previously observed in patients with nmCRPC and it has been shown that addition of N-desmethyl-apalutamide exposure did not improve the model prediction of exposure–response relationships and resulted in collinearity issues [20]. The absence of a relationship between the range of observed apalutamide exposures and clinical benefit was previously reported in a different patient population (nmCRPC patients) and using a different efficacy endpoint (metastasis-free survival) [20]. Furthermore, the absence of a clear association between exposure and clinical efficacy may be linked to the narrow exposure range and favorable tolerance profile, as was also observed for enzalutamide, another androgen receptor inhibitor [26,27,28].
In mCSPC, skin rash and pruritus were the only identified AEs to have a statistical association with apalutamide exposure. Skin rash showed the most pronounced relationship with apalutamide exposure, both in mCSPC and nmCRPC patients [20]. Although the association between apalutamide exposure and incidence of skin rash and pruritus in the TITAN study is statistically significant (Fig. 3), the observed association with apalutamide AUC0-24 h quartiles seems less evident (Table 3). This could potentially be attributed to the relatively narrow apalutamide exposure range in TITAN study. Nonetheless, in nmCRPC patients from the SPARTAN study, a clear association between apalutamide exposure and rash was also observed [20]. Furthermore, there is no clear indication that the development of rash would be different between patients with nmCRPC (SPARTAN) and mCSPC (TITAN). An effect of apalutamide exposure on weight decrease was previously identified in nmCRPC patients but was not observed in mCSPC patients [15, 16, 19, 20]. Due to the nature of the disease (nonmetastatic in SPARTAN study [19] versus metastatic in TITAN study [15, 16]), the health status of patients may have been better in the SPARTAN study (77.4% versus 64.3% of patients had ECOG score 0 at baseline in SPARTAN study and TITAN study, respectively) [2, 16, 17, 19, 21]. A lower incidence of weight gain was observed in the apalutamide + ADT arm vs the placebo + ADT arm of mCSPC patients included in the TITAN study. This might be explained by the counterbalancing effects of ADT (weight gain often associated with initiation of ADT) and apalutamide (weight decrease previously observed in the nmCRPC population). This could potentially explain why no significant additional weight loss due to increased apalutamide exposure was observed for metastatic patients of the TITAN study.
Furthermore, as suggested by the logistic regression model linking exposure to occurrence of adverse events, dose reduction should improve apalutamide-related skin rash in patients experiencing this adverse event. Throughout the study, 92.7% of patients on active treatment with apalutamide in TITAN remained on 240 mg. While the number of dose reductions were few (one level to 180 mg: 4.6%; 2 levels to 120 mg: 2.7%), these patients were restarted on 240 mg apalutamide once the AEs were resolved. These dose reductions did not contribute to a loss of efficacy in the TITAN study. Although the proposed dose reductions may be performed for patients who experience a treatment-emergent AE, the risk–benefit of maintaining patients on a reduced dose needs to be assessed for each individual case based on medical judgment. Overall, apalutamide was administered to a broad population of mCSPC patients and had an acceptable safety profile.
The 240 mg once-daily dose was selected based on the safety and tolerability observed in the first-in-human study at doses up to 480 mg, evaluation of PSA responses across the dose range, observed clinical exposure relative to target exposure required for maximum tumor regression in mouse xenograft models, and inhibition of fluorodihydrotestosterone (FDHT, a biomarker for AR inhibition) [29]. AR inhibition, determined as standard uptake value (maximum–average, SUVmax–avg) by FDHT positron emission tomography/computed tomography after 4 weeks of apalutamide treatment, increased in a dose-dependent manner. The decline from baseline SUVmax–avg at 4 weeks was > 90% for apalutamide doses of ≥ 120 mg daily [29]. As confirmation, the exposure–response analysis based on safety and efficacy data obtained in subjects with nmCRPC in the SPARTAN phase 3 trial supported the adequacy of the 240 mg dose [20]. As this dose was selected to maximize the efficacy in the majority of patients, it is not surprising to only find an exposure–response for safety but not for efficacy endpoints within the relatively narrow exposure range observed in the TITAN trial.
In conclusion, differences in apalutamide exposure as defined by AUC0-24 h in this analysis were not associated with clinically relevant differences in rPFS or OS in patients with mCSPC. Of selected clinically relevant AEs, only skin rash and pruritus at any grade, had a statistically significant increase, albeit with a low effect size, in incidence with increasing apalutamide AUC0-24 h. In patients experiencing AEs dose reductions may be applied, based on a risk–benefit approach at an individual level, to alleviate apalutamide-related skin rash and pruritus.
Availability of data and material
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access [YODA] Project site at http://yoda.yale.edu.
References
Rawla P (2019) Epidemiology of prostate cancer. World J Oncol 10(2):63
Damodaran S, Lang JM, Jarrard DF (2019) Targeting metastatic hormone sensitive prostate cancer: chemohormonal therapy and new combinatorial approaches. J Urol 201(5):876–885. https://doi.org/10.1097/ju.0000000000000117
Hahn AW, Higano CS, Taplin ME, Ryan CJ, Agarwal N (2018) Metastatic castration-sensitive prostate cancer: optimizing patient selection and treatment. Am Soc Clin Oncol Educ Book Am Soc Clin Oncol Ann Meet 38:363–371. https://doi.org/10.1200/edbk_200967
James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, Ritchie AW, Parker CC, Russell JM, Attard G, de Bono J, Cross W, Jones RJ, Thalmann G, Amos C, Matheson D, Millman R, Alzouebi M, Beesley S, Birtle AJ, Brock S, Cathomas R, Chakraborti P, Chowdhury S, Cook A, Elliott T, Gale J, Gibbs S, Graham JD, Hetherington J, Hughes R, Laing R, McKinna F, McLaren DB, O’Sullivan JM, Parikh O, Peedell C, Protheroe A, Robinson AJ, Srihari N, Srinivasan R, Staffurth J, Sundar S, Tolan S, Tsang D, Wagstaff J, Parmar MK (2016) Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet (London, England) 387(10024):1163–1177. https://doi.org/10.1016/s0140-6736(15)01037-5
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S, Protheroe A, De Porre P, Kheoh T, Park YC, Todd MB, Chi KN (2017) Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 377(4):352–360. https://doi.org/10.1056/NEJMoa1704174
James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, Ritchie AWS, Amos CL, Gilson C, Jones RJ, Matheson D, Millman R, Attard G, Chowdhury S, Cross WR, Gillessen S, Parker CC, Russell JM, Berthold DR, Brawley C, Adab F, Aung S, Birtle AJ, Bowen J, Brock S, Chakraborti P, Ferguson C, Gale J, Gray E, Hingorani M, Hoskin PJ, Lester JF, Malik ZI, McKinna F, McPhail N, Money-Kyrle J, O’Sullivan J, Parikh O, Protheroe A, Robinson A, Srihari NN, Thomas C, Wagstaff J, Wylie J, Zarkar A, Parmar MKB, Sydes MR (2017) Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med 377(4):338–351. https://doi.org/10.1056/NEJMoa1702900
Smith MR, Antonarakis ES, Ryan CJ, Berry WR, Shore ND, Liu G, Alumkal JJ, Higano CS, Chow Maneval E, Bandekar R, de Boer CJ, Yu MK, Rathkopf DE (2016) Phase 2 study of the safety and antitumor activity of apalutamide (ARN-509), a potent androgen receptor antagonist, in the high-risk nonmetastatic castration-resistant prostate cancer cohort. Eur Urol 70(6):963–970. https://doi.org/10.1016/j.eururo.2016.04.023
Borno HT, Small EJ (2019) Apalutamide and its use in the treatment of prostate cancer. Fut Oncol (Lond, Engl) 15(6):591–599. https://doi.org/10.2217/fon-2018-0546
Erleada [Internet]. Eur. Med. Agency. 2018. . Available from: https://www.emaeuropaeu/en/medicines/human/EPAR/erleada. Accessed November 15, 2019
ERLEADATM (apalutamide) prescribing information. [Internet]. 2019. . Available from: https https://www.accessdatafdagov/drugsatfda_docs/label/2019/210951s001lblpdf. Accessed December 14, 2019
US Food and Drug administration. FDA approves apalutamide for metastatic castration-sensitive prostate cancer. FDA [Internet]. 2019. Available from: http: // wwwfdagov/drugs/resources-information-approved-drugs/fda-approves-apalutamide-metastatic-castration-sensitive-prostate-cancer Accessed November 15,2019
Lowrance WT, Breau RH, Chou R, Chapin BF, Crispino T, Dreicer R, Jarrard DF, Kibel AS, Morgan TM, Morgans AK, Oh WK, Resnick MJ, Zietman AL, Cookson MS (2021) Advanced prostate cancer: AUA/ASTRO/SUO guideline part I. J Urol 205(1):14–21. https://doi.org/10.1097/JU.0000000000001375
de Vries R, Jacobs F, Mannens G, Snoeys J, Cuyckens F, Chien C, Ward P (2019) Apalutamide absorption, metabolism, and excretion in healthy men, and enzyme reaction in human hepatocytes. Drug Metab Dispos 47(5):453–464. https://doi.org/10.1124/dmd.118.084517
Pérez-Ruixo C, Pérez-Blanco JS, Chien C, Yu M, Ouellet D, Pérez-Ruixo JJ, Ackaert O (2020) Population pharmacokinetics of apalutamide and its active metabolite N-desmethyl-apalutamide in healthy and castration-resistant prostate cancer subjects. Clin Pharmacokinet 59(2):229–244. https://doi.org/10.1007/s40262-019-00808-7
Chi KN, Agarwal N, Bjartell A, Chung BH, de Santana P, Gomes AJ, Given R, Juárez Soto Á, Merseburger AS, Özgüroğlu M, Uemura H, Ye D, Deprince K, Naini V, Li J, Cheng S, Yu MK, Zhang K, Larsen JS, McCarthy S, Chowdhury S (2019) Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med 381(1):13–24. https://doi.org/10.1056/NEJMoa1903307
Chi KN, Chowdhury S, Bjartell A, Chung BH, de Santana P, Gomes AJ, Given R, Juárez A, Merseburger AS, Özgüroğlu M, Uemura H, Ye D, Brookman-May S, Mundle SD, McCarthy SA, Larsen JS, Sun W, Bevans KB, Zhang K, Bandyopadhyay N, Agarwal N (2021) Apalutamide in patients with metastatic castration-sensitive prostate cancer: final survival analysis of the randomized, double-blind Phase III TITAN Study. J Clin Oncol. https://doi.org/10.1200/jco.20.03488
Agarwal N, McQuarrie K, Bjartell A, Chowdhury S, de Santana P, Gomes AJ, Chung BH, Özgüroğlu M, Juárez Soto Á, Merseburger AS, Uemura H, Ye D, Given R, Cella D, Basch E, Miladinovic B, Dearden L, Deprince K, Naini V, Lopez-Gitlitz A, Chi KN (2019) Health-related quality of life after apalutamide treatment in patients with metastatic castration-sensitive prostate cancer (TITAN): a randomised, placebo-controlled, phase 3 study. Lancet Oncol 20(11):1518–1530. https://doi.org/10.1016/s1470-2045(19)30620-5
Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, Coskinas X, Frydenberg M, Hague WE, Horvath LG, Joshua AM, Lawrence NJ, Marx G, McCaffrey J, McDermott R, McJannett M, North SA, Parnis F, Parulekar W, Pook DW, Reaume MN, Sandhu SK, Tan A, Tan TH, Thomson A, Tu E, Vera-Badillo F, Williams SG, Yip S, Zhang AY, Zielinski RR, Sweeney CJ (2019) Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med 381(2):121–131. https://doi.org/10.1056/NEJMoa1903835
Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, Olmos D, Mainwaring PN, Lee JY, Uemura H, Lopez-Gitlitz A, Trudel GC, Espina BM, Shu Y, Park YC, Rackoff WR, Yu MK, Small EJ (2018) Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med 378(15):1408–1418. https://doi.org/10.1056/NEJMoa1715546
Perez-Ruixo C, Ackaert O, Ouellet D, Chien C, Uemura H, Olmos D, Mainwaring P, Lee JY, Yu MK, Perez-Ruixo JJ, Smith MR, Small EJ (2020) Efficacy and safety exposure-response relationships of apalutamide in patients with nonmetastatic castration-resistant prostate cancer. Clin Cancer Res 26(17):4460–4467. https://doi.org/10.1158/1078-0432.Ccr-20-1041
Saad F, Cella D, Basch E, Hadaschik BA, Mainwaring PN, Oudard S, Graff JN, McQuarrie K, Li S, Hudgens S, Lawson J, Lopez-Gitlitz A, Yu MK, Smith MR, Small EJ (2018) Effect of apalutamide on health-related quality of life in patients with non-metastatic castration-resistant prostate cancer: an analysis of the SPARTAN randomised, placebo-controlled, phase 3 trial. Lancet Oncol 19(10):1404–1416. https://doi.org/10.1016/s1470-2045(18)30456-x
Beal S SL, Boeckmann A, Bauer R, editors. NONMEM 7.2.0 Users Guides. Ellicott City, MD: Icon Development Solutions; 1989–2011.
R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2012. . Available from: https: // wwwr-projectorg/ Accessed November 15, 2019
Li Z, Meredith MP, Hoseyni MS (2001) A method to assess the proportion of treatment effect explained by a surrogate endpoint. Stat Med 20(21):3175–3188. https://doi.org/10.1002/sim.984
US Food and Drug administration. Guidance for industry: Exposure-Response Relationships — Study Design, Data Analysis, and Regulatory Applications [Internet]. 2003. Available from: https :// wwwfdagov/media/71277/download Accessed November 15,2019
van Nuland M, Bergman AM, Rosing H, de Vries N, Huitema ADR, Beijnen JH (2019) Exposure-response assessment of enzalutamide and its major metabolites in a real-world cohort of patients with metastatic castration-resistant prostate cancer. Pharmacotherapy 39(12):1137–1145. https://doi.org/10.1002/phar.2339
Joulia ML, Carton E, Jouinot A, Allard M, Huillard O, Khoudour N, Peyromaure M, Zerbib M, Schoemann AT, Vidal M, Goldwasser F, Alexandre J, Blanchet B (2020) Pharmacokinetic/pharmacodynamic relationship of enzalutamide and its active metabolite N-desmethyl enzalutamide in metastatic castration-resistant prostate cancer patients. Clin Genitourin Cancer 18(2):155–160. https://doi.org/10.1016/j.clgc.2019.05.020
Benoist GE, van Oort IM, Burger DM, Mehra N, van Erp NP (2020) The impact of patient characteristics on enzalutamide pharmacokinetics and how this relates to treatment toxicity and efficacy in metastatic prostate cancer patients. Cancer Chemother Pharmacol 85(4):753–760. https://doi.org/10.1007/s00280-020-04039-7
Rathkopf DE, Morris MJ, Fox JJ, Danila DC, Slovin SF, Hager JH, Rix PJ, Maneval EC, Chen I, Gönen M, Fleisher M, Larson SM, Sawyers CL, Scher HI (2013) Phase I study of ARN-509, a novel antiandrogen, in the treatment of castration-resistant prostate cancer. J Clin Oncol 31(28):3525–3530. https://doi.org/10.1200/jco.2013.50.1684
Acknowledgements
The authors would like to thank the patients, investigators, and their medical, nursing and laboratory staff who participated in the clinical studies included in the present work. Editorial assistance was provided by Akshada Deshpande, PhD (SIRO Clinpharm Pvt Ltd) and Namit Ghildyal, PhD (Janssen Global Services, LLC), funded by Janssen Global Services, LLC.
Funding
The clinical studies were supported by research funding from Janssen Research & Development, and the analyses presented here were supported by Janssen Research & Development.
Author information
Authors and Affiliations
Contributions
HT and OA were involved in development of methodology, analysis and interpretation of data and provided administrative, technical, or material support. CC contributed to the conception of the study and interpretation of data. ALG contributed to the conception of the study and supervised the study. SM contributed to the conception of the study. CPR contributed to the analysis and interpretation of data. LK, KC, SC, and NA were involved in acquisition of data. JJPR was involved in development of methodology and analysis and interpretation of data. All authors contributed to drafting, review and/or revisions of the manuscript, and all authors approved the final version of the manuscript for submission and publication.
Corresponding author
Ethics declarations
Conflict of interest
HT, OA, CC, ALG, SM, JJPR, and CPR: Employees of Janssen Research & Development at time of conduct of the study; CC, ALG, SM, and JJPR: Stock owners of Johnson & Johnson at time of conduct of the study. NA: Consultancy: Astellas, Astra Zeneca, Bayer, Bristol Myers Squibb, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Foundation Medicine, Genentech, Janssen, Merck, Nektar, Novartis, Pfizer, Pharmacyclics, and Seattle Genetics; Research funding: Astra Zeneca, Bavarian Nordic, Bayer, Bristol Myers Squibb, Calithera, Celldex, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Genentech, Glaxo Smith Kline, Immunomedics, Janssen, Medivation, Merck, Nektar, New Link Genetics, Novartis, Pfizer, Prometheus, Rexahn, Roche, Sanofi, Seattle Genetics, Takeda, and Tracon. KC: Honoraria: Novartis, Janssen, Astellas, Sanofi, Astra Zeneca, Roche, Daiichi Sankyo, Pfizer, and Point Biopharma; and Research funding: Novartis, Janssen, Astellas, Sanofi, Astra Zeneca, and Roche. SC: Consultancy: Clovis Oncology, Astellas Pharma, Bayer, Pfizer, and Janssen-Cilag; Honoraria: Clovis Oncology, and Novartis; Speakers’ Bureau: Pfizer; Research funding: Sanofi/Aventis. LK: Stock Owner: Swan Valley Medical; Honoraria: Astellas, Bayer, Janssen, Pfizer and Dendreon; Consultancy: 3D Biopsy, Astellas, Astra-Zeneca, Bayer, Dendreon, Ferring, Janssen, Pfizer, and Vaxiion; Speakers’ Bureau: Astellas, Bayer, Janssen, Pfizer, and Clovis; Travel, Accommodations, Expenses: Astellas, Bayer, Janssen, Pfizer, and Dendreon; Research funding: Astellas, Astra Zeneca, Bayer, BioXcel Therapeutics, Bristol Meyers Squibb, CU Optics, CUSP, Dendreon, Epizyme, Exact Sciences, Ferring, FKD, Genentech/Roche, GenomeDx, Genomic Health, Janssen, Merck, Myovant, Nucleix, OncoCell MDx, Pfizer, Pharmtech/Veru, Precision Med, QED Therapeutics, Siemens, Urogen, and Vaxiion.
Ethical approval
The study was conducted in accordance with principles for human experimentation as defined in the Declaration of Helsinki and was approved by the Human Investigational Review Board of each study center and by the Competent Authority of each country.
Consent to participate
Informed consent was obtained from each subject before enrollment in the study, after being advised of the potential risks and benefits, as well as the investigational nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
T’jollyn, H., Ackaert, O., Chien, C. et al. Efficacy and safety exposure–response relationships of apalutamide in patients with metastatic castration-sensitive prostate cancer: results from the phase 3 TITAN study. Cancer Chemother Pharmacol 89, 629–641 (2022). https://doi.org/10.1007/s00280-022-04427-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-022-04427-1