Abstract
Purpose
The time-varying clearance (CL) of the PD-L1 inhibitor atezolizumab was assessed on a population of 1519 cancer patients (primarily with non-small-cell lung cancer or metastatic urothelial carcinoma) from three clinical studies.
Methods
The first step was to identify the baseline covariates affecting atezolizumab CL without including time-varying components (stationary covariate model). Two time-varying models were then investigated: (1) a model allowing baseline covariates to vary over time (time-varying covariate model), (2) a model with empirical time-varying Emax CL function.
Results
The final stationary covariate model included main effects of body weight, albumin levels, tumor size, anti-drug antibodies (ADA) and gender on atezolizumab CL. Both time-varying models resulted in a clear improvement of the data fit and visual predictive checks over the stationary model. The time-varying covariate model provided the best fit of the data. In this model, the main driver for change in CL over time was variations in albumin level with an increase in serum albumin (improvement in a patient’s status) mirroring a decrease in CL. Time-varying ADAs had a small impact (9% increase in CL). None of the covariates impacted atezolizumab CL by more than ± 30% from median. The estimated maximum decrease in CL with time was 22% with the Emax model.
Conclusion
The overall impact of covariates on atezolizumab CL did not warrant any change in atezolizumab dosing recommendations. The results support the hypothesis that variation in atezolizumab CL over time is associated with patients’ disease status, as shown with other checkpoint inhibitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tecentriq® (Atezolizumab) is a humanized immunoglobulin G1 monoclonal antibody that targets human PD-L1 on tumor-infiltrating immune cells and tumor cells, and inhibits PD-L1 interaction with programmed death 1 (PD-1) and B7.1 receptors, both of which can provide inhibitory signals to T cells [1,2,3]. Atezolizumab 1200 mg intravenous (IV) every three weeks (q3w) is approved to treat locally advanced or metastatic non-small-cell lung cancer (NSCLC), metastatic urothelial carcinoma (mUC), extensive-stage small-cell lung cancer (SCLC), and unresectable hepatocellular carcinoma (HCC) when atezolizumab 840 mg every two weeks (q2w) is approved to treat locally advanced or metastatic triple-negative breast cancer (TNBC) in the United States, Europe, and elsewhere [4, 5]. In addition, PK simulations, exposure–response, and safety analyses supported approval of the 840 mg q2w and 1680 mg every four weeks (q4w) IV dosing regimens through “exposure matching” with the clinically evaluated 1200 mg q3w IV dosing regimen for atezolizumab single agent [4,5,6].
The population pharmacokinetics (popPK) of atezolizumab has been previously assessed based on data from two Phase 1 clinical studies PCD4989g and JO28944 in patients with various solid tumors. The popPK model of atezolizumab developed with Phase 1 data (the “Phase 1 PopPK Model”) [7] was subjected to external validations using PK data collected in various phase 2 and phase 3 studies of atezolizumab 1200 mg IV q3w including IMvigor210 [7] in mUC as well as in other studies in approved indications (unpublished) or 840 mg IV q2w in study IMpassion130 in TNBC [6]. In an analysis during FDA review, atezolizumab clearance (CL) appeared to decrease over time, although this was not considered to be clinically relevant [4]. A similar trend was also observed for PD-1 inhibitors nivolumab [8, 9] and pembrolizumab [10], where the magnitude of decrease in CL (estimated using an empirical Emax model) was found to be associated with best overall response, resulting in a confounded relationship between steady-state exposure and response. A change in CL over time was also observed for the PD-L1 inhibitors avelumab [11] and durvalumab [12]. For the latter, time-dependent changes in longitudinal covariates better described the change in CL with time than the empirical Emax model used for nivolumab and pembrolizumab [8,9,10]. Li et al. recently proposed a model with time-dependent changes in covariates for pembrolizumab [13].
The objective of this analysis was to explore the impact of baseline and time-varying covariates on atezolizumab PK in a large population, including a majority of mUC and NSCLC patients, in three atezolizumab single-agent clinical studies: PCD4989g (Phase 1, various cancer types) [14], IMvigor211 (mUC) [15], and OAK (NSCLC) [16] in comparison with the empirical Emax model previously proposed for atezolizumab [4].
Materials and methods
Studies and patients
Data from atezolizumab monotherapy studies, including the Phase 1 study PCD4989g in patients with locally advanced or metastatic solid or hematologic malignancies included in both dose escalation and expansion cohorts (ClinicalTrials.gov ID, NCT01375842) [3, 14, 17, 18] and the Phase 3 studies OAK in patients with locally advanced or metastatic NSCLC who have progressed during or following a platinum-containing regimen (GO28915; Clinical- Trials.gov ID, NCT02008227) [16] and IMvigor211 in patients with locally advanced or metastatic UC who have progressed during or following a platinum-containing regimen (GO29294; ClinicalTrials.gov ID, NCT02302807) [15], were used in the popPK analyses based on clinical data cut-off dates of December 2, 2014; July 7, 2016; and March 17, 2017, respectively. More details about the studies can be found in Supplementary Materials Table S1. Those 3 trials were selected as The Phase I study is the only study with full PK data while IMvigor211 and OAK are pivotal large Phase III studies. The study protocols were approved by institutional review boards and/or independent ethics committees at each site. All patients provided written informed consent.
Patients were defined as evaluable if they had at least one adequately documented atezolizumab administration and a corresponding PK sample collected after the dose (Supplementary Materials Figure S1).
Data analysis
All models were implemented in NONMEM 7.3 (ICON Development Solutions, Ellicott City, MD) [19]. Population model parameters were estimated using the FOCE method with η−ε interaction. Data exploration and visualization as well as descriptive statistics were performed using R® in addition to Comprehensive R Archive Network (CRAN) packages [20]. Perl-Speaks-NONMEM (PsN) (Uppsala University, Uppsala, Sweden) [21, 22] was used to perform the covariate selection and to evaluate/validate the popPK model using predictive checks [23]. For comparison of model performance, we used the objective function value (when applicable), relative standard errors of parameter estimates, plots of observed vs. predicted values, and visual predictive checks. Data handling consisted mainly of the last-observation-carry-forward (LOCF) technique that was employed to interpolate the missing time-varying covariate values in the PK dataset.
Population PK model development
The starting point of the PK analysis was the popPK model [7] which was developed with Phase 1 data and externally validated with data from over ten studies. The model without any covariates (the base model) estimated previously [7] was re-estimated with the pooled dataset (i.e., data from PCD4989g, OAK, and IMvigor211). Since the objective of this pooled analysis was to explore the impact of covariates on atezolizumab CL, the previously identified covariate effects on central volume of distribution (V1) and peripheral volume of distribution (V2) (i.e., sex, body weight (BWT) on V1 and sex on V2) were kept a priori in the base model and tested on the larger pooled database. The effect of albumin level on V1, which was included in the previous covariate model, was poorly estimated when using the pooled dataset and not kept in the model.
In a second step, baseline covariates were evaluated for their effect on atezolizumab CL. Univariate analysis of the impact of baseline covariates on CL was performed to rank the covariates by improvement (decrease) in the objective function value (OFV). All significant baseline covariates (ΔOFV > − 6.64 for one degree of freedom and a significance level of p < 0.01) were used to build a FULL model and then a backward elimination was performed. Each covariate in the FULL model was removed from the model in turn and the covariate removal that resulted in the smallest increase in OFV was eliminated. The process was repeated until the increase in OFV reached the threshold retained for statistical significance at p < 0.001 (ΔOFV > + 10.83 for one degree of freedom and 13.8 for two degrees of freedom). The baseline covariates time-stationary model was then obtained.
For this second step, the following baseline demographic and baseline pathophysiological covariates were considered: demographics: sex, age, BWT; cancer-related covariates: Eastern Cooperative Oncology Group (ECOG) performance status, sum of longest diameter of target lesions (SLD), presence of liver metastasis, and number of metastatic sites; liver function-related covariates: aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin (BIL), hemoglobin (HG), total protein (TPRO), alkaline phosphatase (ALP)]; inflammatory markers: albumin (ALBU), lymphocyte (LYM), neutrophil (NEU), platelet (PLA) counts, as well as the neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR), prognostic nutritional index (PNI) [24]; kidney function-related: estimated glomerular filtration rate (eGFR) [25]; smoking history; status of Anti-Therapeutic Antibodies (ADA) and PD-L1 status in tumor cells (TC) and tumor-infiltrating immune cells (IC) (PD-L1 scoring by immune-histochemistry is given in Supplementary Materials Table S3). Note that C-reactive protein (CRP), a well-known inflammatory marker was not explored in the main analysis because this information was not available in the Phase I study PCD4989g. However, a sensitivity analysis was performed on data from patients in the two Phase III studies with this information.
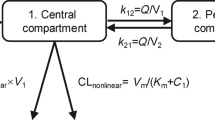
The effect of n covariates on CL, V1, and V2 was coded using a multiplicative model as follows for CL (Eq. 1):
where θ1 is the typical value of the parameter for patients having covariate values equal to the reference, and Effect1,i … Effectn,i are multiplicative factors of the effects for covariate 1 to n, respectively, for the set of covariates in patient i.
The multiplicative factor for continuous covariates was included in the base model using the following power function (Eq. 2):
where Effecti is the multiplicative factor of the covariate effect, Covi is the covariate value, Covref is the median of the covariate for all patients, and θeff is the exponent of the power function. Individual CL was expressed by (Eq. 3), inter-patient variability of the CL, and all other parameters (Vc, Vp, and Q) was included using a lognormal model:
where CLi is the individual clearance for patient i, TVCLcov is the typical value of clearance for a set of covariate as described above, ηCLi is the individual clearance random effect for patient i where ηCLi ~ N(0,ω2) is normally distributed, with mean 0 and variance ω2.
After the time-stationary baseline covariates model was developed, two time-varying models were investigated for CL; (1) A model allowing all covariates identified in the baseline covariates model, to vary with time (time-varying covariate model), and this model with time-varying covariates was subject to a new backward deletion at p < 0.001 to confirm all the covariate effects. (2) The previously proposed empirical time-dependent Emax model [9, 10, 12] added to the baseline covariates model according to equations 4 and 5:
where CLi is the individual clearance for patient i, TVCLcov is the typical value of clearance for a set of covariate as described below, ηCLi is the individual clearance random effect for patient i where ηCLi ~ N(0,ω2) is normally distributed, with mean 0 and variance ω2. t is time after the first dose, Tmax is the maximum fractional change in clearance over the trial time course (t), and T50 is the time at which the change is half of its maximum, while γ is an exponential shape parameter.
The three models: baseline covariates time-stationary model, empirical Emax time-dependent model, and time-varying covariates model were evaluated and compared based on OFV, precision, plausibility of parameter estimates, goodness-of-fit plots, and simulation-based visual predictive checks (VPC) [23, 26].
Results
Data
Only patients receiving doses of 1–20 mg/kg atezolizumab q3w, or the 1200 mg q3w fixed-dose in PCD4989g, 466 (out of 481, 96.9%) were included in this evaluation as already reported [7]. In Phase 3 studies, all patients received 1200 mg q3w fixed-dose. There were 598 (out of 613, 97.6%) and 455 (out of 467, 97.4%) evaluable patients in OAK and IMvigor211, respectively. A total of 9165 atezolizumab serum concentrations from 1519 patients [out of 1550 treated patients (98%)] were used for the popPK analysis (see Supplementary Materials Table S2 for more details). Descriptive statistics of continuous and categorical covariates in patients with evaluable PK are summarized in Table 1. Of the 1519 patients included in the popPK dataset, 685 had NSCLC (45.1%), 545 had mUC (35.9%) and 289 had other solid tumors (19%). The median patient age was 64 years and body weight ranged from 34 to 168 kg, 65% of patients were male, and approximately 74% of the patients were White. Patients had baseline ECOG PS of either 0 or 1 (42% and 58%, respectively). There were no obvious differences between studies in terms of covariates.
Covariate analysis
The results of the univariate covariate analysis on atezolizumab CL at a significance level of 0.01 as the entry criterion into the FULL model are provided in Supplementary Materials Table S4. The effects retained to enter the FULL model were ADA status and baseline ALBU, ALP, BIL, SLD, BWT, ECOG, HG, LDH, NEU, PNI, smoking, METSITES, SEX on CL; BWT, and SEX on V1 and on V2 (kept from the previous Phase 1 model, [7]). From the FULL model, a backward elimination was performed at p < 0.001, from which the following effects were retained in the final baseline model: BWT, ALBU, ADA, SLD, SEX, ALP, BIL, NEU on CL, BWT, SEX on V1 and SEX on V2.
Performance and parameter estimates for population PK models
The parameter estimates for the baseline covariate time-stationary population PK model are shown in Table 2 and pcVPC is illustrated in Fig. 1. The pcVPC shows a consistent under-prediction of observed median Cmin after long-term administration (from Cycle 6 onward). This trend is consistent with the previously reported decrease in atezolizumab CL over time (approximately 17.1%) reported in the US Package Insert [4]. To take into account the change of CL over time, two time-varying atezolizumab CL models were evaluated. (1) A more mechanistic time-varying CL model was implemented by incorporating longitudinal time-varying covariates to explain changes in atezolizumab CL over time. The covariates selected in the baseline covariates model (SLD, ALB, BWT, ALP, NEU, BIL, and ADA) were used in this approach and allowed to vary with time. (2) The previously proposed empirical [9, 10, 12] time-dependent CL model: the baseline covariates model was complemented with a time-dependent Emax CL function.
Prediction-corrected VPC of atezolizumab peaks and troughs with time-varying covariates model (semi-log scale). Orange areas: 95% PI of 5th and 95th percentiles across the 1000 simulated replicates; blue area: 95% PI of 50th percentile (median) across the 1000 simulated replicates; orange crosses observed 5th and 95th percentiles; blue cross: observed 50th percentile (median); gray open circles: prediction-corrected observed concentrations; n: number of observations in each bin
When covariates were allowed to vary with time to explain changes in atezolizumab CL over time, the fit of the covariates time-stationary model was markedly improved (difference in OFV: − 643 points for the same number of parameters). Backward elimination from this model indicated that all covariates remained in the model. The parameter estimates for the time-varying covariates population PK model are shown in Table 2 and the diagnostic plots are presented in Supplementary Materials Fig. S2. The pcVPC shows a clear improvement of the under-prediction of Cmin after long-term administration suggesting that time-varying covariates were able to explain the change in CL over time in the pooled dataset (Fig. 1).
When the empirical time-dependent Emax function [9] was added to the baseline covariates time-stationary model, the fit of the baseline covariates time-stationary model was also markedly improved but less than that of the model allowing covariates to vary with time (difference in OFV: − 308 points for 3 additional parameters). Backward elimination from this model indicated that all covariates remained in the model. Model parameter estimates for this empirical time-dependent CL model are presented in Table 2. The fixed effects of structural parameters are well estimated except for the parameters of the time-dependent CL function (RSE of T50 > 200%). Note that IIV estimate for CL was decreased compared to other models; in addition, one more random effect on CL in this model (Tmax) had to be fixed for improved model stability. The estimate of the maximum decrease in CL from baseline calculated as (1−exp(Tmax)) is similar to that in the previous post-hoc analysis [4]: 22% vs. 17%. The pcVPC in Supplementary Materials Fig. S3 shows a clear improvement of the under-prediction of Cmin after long-term administration compared to the baseline model, although a slight under-prediction of median (and 5th percentile) Cmin remains compared to the time-varying covariates model.
Model comparison
Model parameters comparison favored the time-varying covariates model with respect to the statistical fit, the model stability, and the predictive performance. The pcVPC of the empirical time-dependent CL model and the time-varying covariates model performed similarly (Fig. 1, Supplementary Materials Fig. S3). However, the time-varying covariates model performed better based on statistical criteria (delta in OFV) with − 643 points vs. − 308 points compared to baseline covariates model for time-varying covariates and empirical models, respectively. In addition, the time-varying covariate model provided better stability (successful minimization and covariance step vs. the need to fix a random effect and poor precision in gamma estimate) than the empirical model. This model also provides a broader mechanistic understanding of the relationship between the disease status (tumor burden, and cancer inflammation) and atezolizumab PK. The time-varying covariates model is more flexible as patients can improve and then worsen while on-treatment with successive CL decrease and increase (or vice versa). When using the empirical formula, individual patients have a continuous decrease (for most of them) or increase in CL all along the treatment as illustrated in Fig. 2.
Impact of covariate effects and clinical relevance
According to the final time-varying covariates population model (Table 2), the typical CL (TVCL) of atezolizumab for patient i is in Eq. 6:
The typical V1 (TVV1) and V2 (TVV2) for patient i are in Eqs. 7 and 8:
TVCLi typical value of clearance, TVV1i typical value of central volume, TVV2i typical value of peripheral volume for a patient i. Time-varying covariates: ALBU albumin (g/L), BWT body weight (kg), BIL bilirubin (µmol/mL), ALP alkaline phosphatase (U/L), NEU neutrophil count (109/L), SLD sum of longest diameters (mm), ADA post-baseline status of anti-therapeutic antibodies.
An exploration of the impact of covariates on atezolizumab PK using a tornado plot in Fig. 3 shows the isolated influence of each statistically significant covariate on CL. None of the covariates that were statistically significant (p < 0.001) predictors of atezolizumab CL were found to be clinically relevant as judged by the magnitude of covariate effects not exceeding 30% from the typical patient and the flat exposure–response relationship for atezolizumab [6, 7]. As in Phase 1 stationary model [7], the largest effect is seen with albumin with a 27% higher CL than the typical patient when evaluated at the 5th percentile (29 g/L). Females have a lower CL compared to males (− 20%). Patients with higher body weight have higher CL than patients with lower body weight (+ 20% vs. − 16% at 95th and 5th percentiles of body weight, respectively). Positive ADA has a small impact on CL (+ 9.3%). Other covariates (BSLD, ALP, NEU, and BIL) have minor impacts (< 10%). Of note, covariate effect estimates in the three models are consistent (Table 2). A sensitivity analysis based on Phase 3 data only to explore the impact of CRP on atezolizumab CL, showed that the effect of CRP was significant in addition to the other covariates in the model but small (< ± 10%).
Tornado plot of the impact of covariates on CL according to the time-varying covariates CL model. Black bold vertical line: predicted CL for a typical patient with median covariates (male, ADA negative, weighing 72 kg, albumin level 38 g/L, bilirubin level 5 µmol/L; alkaline phosphatase 93 U/L, neutrophil count 5.109/L, SLD 55 mm); Gray area: 30% change from the typical patient; Horizontal bars: percent change of CL from typical for each covariate; left label: evaluated covariate with 5th and 95th percentiles of the continuous covariate distributions
The limit of the tornado plot is that it does not assess the time variations. To investigate the impact of the covariates on time-varying CL, individual profiles of CL and covariates change over time were explored and some representative profiles are presented in Fig. 4. These plots focus on the most relevant covariates that were identified, i.e., ALBU and SLD as markers of disease severity as well as ADA status. The fractional changes from baseline for CL, ALBU, and SLD were derived and the locally-weighted smooth lines of these changes were plotted for each patient. Time-varying ADA was shown as a vertical pink area when a patient was positive. To visualize the magnitude of the change, a gray area displays a range of ± 30% fractional change of CL from baseline. For each panel, the patient identifier (patient ID, 1–3), the study, and the best overall response status are presented in the top band. Those plots indicate that changes in CL parallel changes in ALBU over time and to a lesser extent, changes in SLD over time. In addition, the plots suggest that the appearance of ADA has a minor impact on CL compared with the change in the patient’s disease status. Overall, the change in ALBU level over time seems to best predict the change in CL over time independent of ADA status. The strong reciprocal relationship between maximum change in CL and change in ALBU and to a lesser extent the corresponding change in SLD is illustrated in Supplementary Materials Fig. S4. Similar features were previously reported for durvalumab [12].
Individual profiles of fractional change in CL (black), albumin (red), and SLD (blue) according to the time-varying covariates model. Short vertical arrows: timing and result of ADA measurements; horizontal gray area: ± 30% change in CL; vertical pink area: period of time when the patient was ADA+ ; black line: locally-weighted smooth line of fractional change in CL from baseline; red line: locally-weighted smooth line of fractional change in albumin from baseline; blue line: locally-weighted smooth line of fractional change in sum of longest diameter from baseline. SD stable disease, PR partial response, PD progressive disease
Discussion
Time-varying CL driven by time-varying patient status is described, for atezolizumab. This analysis explored the impact of covariates on atezolizumab clearance (CL) over time during treatment using data pooled from one Phase 1 study (PCD4989g) and two Phase 3 studies where atezolizumab was administered as monotherapy (IMvigor211 study in mUC and OAK study in NSCLC). The results support the hypothesis that CL variation over time is associated with patients’ prognostic factors and disease status as shown with other checkpoint inhibitors [8,9,10,11,12, 27]. The popPK analysis dataset included a large population of 1519 patients with cancer, mainly mUC or NSCLC, receiving 1–20 mg/kg or 1200 mg of atezolizumab, q3w by IV infusion. As previously reported [7], atezolizumab concentrations were well described by a linear, two-compartment model with zero-order intravenous infusion and first-order elimination. The typical values of CL and V1 (0.235 L/day (9.79 mL/h) and 3.36 L, respectively) were comparable to those found in the previous analysis (0.200 L/day (8.33 mL/h) and 3.28 L, respectively), and the estimated half-life of 21 days was similar to that reported previously with Phase 1 patients [7].
The impact of baseline covariates on atezolizumab CL was re-assessed on this base model. The covariate analysis using baseline covariates found a positive relationship between weight and CL and V1, as well as a positive relationship between both SLD and ADA and CL, and a negative relationship for albumin with CL. In addition to the relationship with bodyweight, volumes of distribution (V1 and V2) and CL were lower in females compared to males. Compared to the Phase 1 Model [7], some additional covariates were found to impact CL in this larger population: neutrophil count, alkaline phosphatase, and bilirubin, all of which had small effects on CL (< ± 10%).
The change of CL over time was best described by a model with time-varying covariates [12] than with a previously proposed empirical time-dependent Emax function [8,9,10,11]. The maximum decrease in CL estimated with the Emax model (22%) is of similar magnitude to that estimated for other checkpoint inhibitors [28]. The main driver for change in CL over time was albumin level variations, with a decrease in CL mirroring an increase in albumin (improvement in patient’s status) and to a lesser extent the change in SLD over time (Supp Fig S4). This finding supports the hypothesis that atezolizumab PK is impacted by prognostic factors and disease status that could include cancer cachexia as suggested by others for PD-1 and PD-L1 inhibitors, i.e., nivolumab [8, 9], pembrolizumab [10, 27], avelumab [11] or durvalumab [12]. The time-varying covariate model provides a broader mechanistic understanding of the relationship between the disease status (tumor burden, and cancer inflammation) and atezolizumab PK. The current findings on the importance of time-varying covariates to drive change in CL during treatment for atezolizumab are in line with what is described by Baverel [12] for durvalumab and by Li [13] for pembrolizumab. The time-varying covariate model makes more mechanistic sense than the Emax model as it is more flexible with patients improving and worsening (or vice versa) while on-treatment with successive CL decreases and increases. When using the empirical time-dependent function, individual patients have to have a continuous decrease or an increase in CL but not both in sequence. This flexibility likely explains why the time-varying covariates model fits the data better, as previously observed [12]. Of note time-varying PK for checkpoint inhibitors has been mainly investigated for single-agent treatments. Similar investigations need be conducted with combination treatments. Since this phenomenon is related to treatment effect, some differences when combined with chemotherapies may be expected.
Evaluation of ADA impact on pharmacokinetics appears to be more sensitive when ADA is considered as time-varying compared to stationary in popPK models [29]. In both the baseline covariates stationary model [7] and the time-varying covariates model, treatment-emergent, ADA status had a small impact on CL (13.7% increase in the baseline model and 9.3% increase in the time-varying model). Of note a larger increase in clearance (22%; range 18–49%) was seen in a comparison of individual (posthoc) CL estimates in ADA-positive patients vs. ADA negative-patients [4]. Those estimates are unadjusted for covariate effects. Patients who later develop ADA tended to have poorer baseline prognostic factors, so the faster CL in ADA-positive patients is due to both the impact of ADA and that of associated baseline prognostic. The popPK model adjusts for baseline prognostic imbalances and estimates the independent effect of ADA, which is smaller.
None of the covariates impact atezolizumab CL by more than a ± 30% threshold. This is similar to the estimates from the previous stationary Phase 1 model for atezolizumab and with data from other checkpoint inhibitors [8,9,10,11,12,13] and need be interpreted in the context of flat exposure response [6, 7] for atezolizumab. These results provided greater insights into the effects of covariates on atezolizumab PK over time but do not warrant any change in the current atezolizumab dosing recommendations.
Conclusion
Time-dependent changes in covariates, notably albumin level and tumor burden, best described the change in CL with time. This is consistent with the hypothesis that the main driver for change in CL over time is the improvement in the patient’s health status. The time-varying covariates PK model includes the effects of body weight, albumin levels, ADA, gender, neutrophil count, alkaline phosphatase, and bilirubin levels on atezolizumab CL. When adjusted for the effects of baseline covariates, the impact of time-varying ADA on atezolizumab CL is inconsequential (9%). The overall impact of covariates on atezolizumab CL is similar in magnitude to that found with the Phase 1 time-stationary popPK model with no change to atezolizumab dosing recommendations.
References
Chen DS, Mellman I (2013) Oncology meets immunology: the cancer-immunity cycle. Immunity 39:1–10. https://doi.org/10.1016/j.immuni.2013.07.012
Chen DS, Irving BA, Hodi FS (2012) Molecular pathways: next-generation immunotherapy–inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res 18:6580–6587. https://doi.org/10.1158/1078-0432.CCR-12-1362
Herbst RS, Soria J-C, Kowanetz M et al (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515:563–567. https://doi.org/10.1038/nature14011
TECENTRIQ (atezolizumab) [package insert] (2020) Genentech, Inc, South San Francisco
TECENTRIQ (atezolizumab) [summary of product characteristics] (2020) Roche Registration Limited, Welwyn Garden City
Morrissey KM, Marchand M, Patel H et al (2019) Alternative dosing regimens for atezolizumab: an example of model-informed drug development in the postmarketing setting. Cancer Chemother Pharmacol 84:1257–1267. https://doi.org/10.1007/s00280-019-03954-8
Stroh M, Winter H, Marchand M et al (2017) Clinical pharmacokinetics and pharmacodynamics of atezolizumab in metastatic urothelial carcinoma. Clin Pharmacol Ther 102:305–312. https://doi.org/10.1002/cpt.587
Liu C, Yu J, Li H et al (2017) Association of time-varying clearance of nivolumab with disease dynamics and its implications on exposure response analysis. Clin Pharmacol Ther 101:657–666. https://doi.org/10.1002/cpt.656
Bajaj G, Wang X, Agrawal S et al (2017) Model-based population pharmacokinetic analysis of nivolumab in patients with solid tumors. CPT Pharmacomet Syst Pharmacol 6:58–66. https://doi.org/10.1002/psp4.12143
Li H, Yu J, Liu C et al (2017) Time dependent pharmacokinetics of pembrolizumab in patients with solid tumor and its correlation with best overall response. J Pharmacokinet Pharmacodyn 44:403–414. https://doi.org/10.1007/s10928-017-9528-y
Wilkins JJ, Brockhaus B, Dai H et al (2019) Time-varying clearance and impact of disease state on the pharmacokinetics of avelumab in merkel cell carcinoma and urothelial carcinoma. CPT Pharmacomet Syst Pharmacol 8:415–427. https://doi.org/10.1002/psp4.12406
Baverel PG, Dubois VFS, Jin CY et al (2018) Population pharmacokinetics of durvalumab in cancer patients and association with longitudinal biomarkers of disease status. Clin Pharmacol Ther 103:631–642. https://doi.org/10.1002/cpt.982
Li H, Sun Y, Yu J et al (2019) Semimechanistically based modeling of pembrolizumab time-varying clearance using 4 longitudinal covariates in patients with non-small cell lung cancer. J Pharm Sci 108:692–700. https://doi.org/10.1016/j.xphs.2018.10.064
Horn L, Gettinger SN, Gordon MS et al (2018) Safety and clinical activity of atezolizumab monotherapy in metastatic non-small-cell lung cancer: final results from a phase I study. Eur J Cancer 101:201–209. https://doi.org/10.1016/j.ejca.2018.06.031
Powles T, Durán I, van der Heijden MS et al (2018) Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 391:748–757. https://doi.org/10.1016/S0140-6736(17)33297-X
Rittmeyer A, Barlesi F, Waterkamp D et al (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389:255–265. https://doi.org/10.1016/S0140-6736(16)32517-X
Powles T, Eder JP, Fine GD et al (2014) MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515:558–562. https://doi.org/10.1038/nature13904
Petrylak DP, Powles T, Bellmunt J et al (2018) Atezolizumab (MPDL3280A) monotherapy for patients with metastatic urothelial cancer: long-term outcomes from a phase 1 study. JAMA Oncol 4:537–544. https://doi.org/10.1001/jamaoncol.2017.5440
SL Beal, LB Sheiner, AJ Boeckmann, RJ Bauer (1989) NONMEM 7.4 users guides
R Core Team (2016) R: A Language and Environment for Statistical Computing. https://www.R-project.org/. Accessed 07 Sep 2020
Lindbom L, Ribbing J, Jonsson EN (2004) Perl-speaks-NONMEM (PsN)—a Perl module for NONMEM related programming. Comput Methods Programs Biomed 75:85–94. https://doi.org/10.1016/j.cmpb.2003.11.003
Lindbom L, Pihlgren P, Jonsson EN, Jonsson N (2005) PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed 79:241–257. https://doi.org/10.1016/j.cmpb.2005.04.005
Karlsson MO, Holford N (2008) Model evaluation. A tutorial on visual predictive checks. PAGE 17 (2008) Abstr 1434 www.page-meeting.org/?abstract=1434
Tomita M, Ayabe T, Maeda R, Nakamura K (2018) Comparison of inflammation-based prognostic scores in patients undergoing curative resection for non-small cell lung cancer. World J Oncol 9:85–90. https://doi.org/10.1440/wjon1097w
Levey AS, Bosch JP, Lewis JB et al (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130:461–470. https://doi.org/10.7326/0003-4819-130-6-199903160-00002
Karlsson MO, Savic RM (2007) Diagnosing model diagnostics. Clin Pharmacol Ther 82:17–20. https://doi.org/10.1038/sj.clpt.6100241
Turner DC, Kondic AG, Anderson KM et al (2018) Pembrolizumab exposure-response assessments challenged by association of cancer cachexia and catabolic clearance. Clin Cancer Res 24:5841–5849. https://doi.org/10.1158/1078-0432.CCR-18-0415
Centanni M, Moes DJAR, Trocóniz IF et al (2019) Clinical pharmacokinetics and pharmacodynamics of immune checkpoint inhibitors. Clin Pharmacokinet 58:835–857. https://doi.org/10.1007/s40262-019-00748-2
Wang Y-MC, Wang J, Hon YY et al (2016) Evaluating and reporting the immunogenicity impacts for biological products—a clinical pharmacology perspective. AAPS J 18:395–403. https://doi.org/10.1208/s12248-015-9857-y
Author information
Authors and Affiliations
Contributions
MM, PC, VQ, MB, NS, JYJ and RB wrote the article and designed the research. RZ prepared the datasets. MM performed the analyses.
Corresponding author
Ethics declarations
Conflict of interest
M. Marchand is employed by Certara Strategic Consulting. R. Zhang, B. Wu, P. Chan, V. Quarmby, M. Ballinger, N. Sternheim, J.Y. Jin, and R. Bruno are employed by Genentech, Inc.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Marchand, M., Zhang, R., Chan, P. et al. Time-dependent population PK models of single-agent atezolizumab in patients with cancer. Cancer Chemother Pharmacol 88, 211–221 (2021). https://doi.org/10.1007/s00280-021-04276-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-021-04276-4