Abstract
Purpose
Ceritinib 750 mg/day was approved for the treatment of patients with untreated anaplastic lymphoma kinase (ALK)-rearranged non-small cell lung cancer (NSCLC) based on ASCEND-4 study. The objective of this article is to introduce the use of time-dependent modeling approach in the updated exposure–efficacy analysis of ceritinib for the first-line indication.
Methods
Exposure–efficacy analyses, including data from 156 patients, were first conducted using time-independent logistic regression model for response of complete or partial response and Cox regression model for progression-free survival (PFS). The exposure measure used was average Ctrough, which is defined as the geometric mean of all evaluable Ctrough for each patient. To further investigate the impact of exposure measure on exposure–efficacy analyses, a time-dependent modeling approach was used, where exposure at different time intervals was associated with the corresponding response endpoints in a longitudinal manner.
Results
With exposure measure being average Ctrough, it was observed that higher exposure was associated with reduced efficacy in terms of response (odds ratio = 0.77) and PFS [hazard ratio (HR) = 1.12]. These time-independent models do not account for the impact of time-varying concentration due to dose modifications. Subsequently, a new time-dependent modeling approach was used, where exposure and efficacy were associated longitudinally in the analyses. The results showed that the odds ratio of response became 1.07, and the HR of PFS became 1.04, indicating no apparent reverse relationship between exposure and efficacy across the exposure range studied.
Conclusion
The drug effect on efficacy in clinical trials could be better characterized using time-dependent exposure–response models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anaplastic lymphoma kinase (ALK) rearrangements serve as a key oncogenic driver and occur in 3–7% of patients with non-small cell lung cancer (NSCLC) [1, 2]. In the US, ceritinib (LDK378, Zykadia®; Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA), a selective oral ALK inhibitor, was initially approved as a second-line treatment at a dose of 750 mg once a day for treatment of patients with ALK-rearranged metastatic NSCLC who have progressed on or are intolerant to crizotinib, based on the results from ASCEND-1, a phase 1, dose escalation and expansion first-in-human study [3]. In April 2017, it was approved for the treatment of previously untreated patients with ALK-rearranged metastatic NSCLC [4]. To support this most recent indication, an open-label, randomized global phase 3 study, ASCEND-4 (NCT01828099), was conducted to assess the efficacy and safety of ceritinib at 750 mg/day against platinum-based chemotherapy as a first-line therapy in untreated advanced ALK-rearranged NSCLC patients. The median progression-free survival (PFS) and objective response rate with first-line ceritinib were 16.6 months [95% confidence interval (CI) 12.6, 27.2] and 72.5% (95% CI 65.5, 78.7), respectively, compared with 8.1 months (95% CI 5.8, 11.1) and 26.7% (95% CI 20.5, 33.7), respectively, with chemotherapy, indicating a statistically significant and clinically meaningful improvement in PFS versus chemotherapy in patients with advanced ALK-rearranged NSCLC [5].
To date, exposure–response (E–R) analyses have been well recognized and frequently utilized in support of regulatory approval [6]. The quantitative relationship between drug exposure and response with regard to efficacy and safety is critical in justifying or confirming a dosing regimen in the context of the overall risk/benefit balance. For such analyses, one of the commonly used drug exposure measures is the average trough plasma concentration (average Ctrough), which is defined as the geometric mean of all evaluable Ctrough values observed for each patient, assuming log-normal distribution of plasma concentrations. From a practical consideration, average Ctrough is the preferred exposure measure as it is more feasible to be obtained. Since trough concentrations are usually collected at multiple time points throughout the treatment duration in a clinical trial, each patient is likely to have more than one Ctrough values contributing to the calculation of average Ctrough.
In support of the initial regulatory submission of second-line indication of ceritinib [7, 8], the E–R analysis approach utilizing the average Ctrough as exposure measure was previously applied to assess the relationship between ceritinib’s exposure and response (efficacy and safety) using data from ASCEND-1, which was primarily driven by data from the 750 mg dose group collected in the expansion phase. Since average Ctrough was well correlated with AUCtau at steady state, it could serve as a surrogate for total exposure for the E–R analysis (data on file). The analyses showed no apparent relationship between average Ctrough and efficacy endpoints, including best overall response (BOR) and PFS. This finding is not uncommon in oncology given that the dose range studied in pivotal trials is generally limited, which does not permit an adequate characterization of the full exposure–efficacy curve. However, based on the efficacy data of ASCEND-1, the objective response rate of 54.6% (95% CI 47, 62) and the median duration of response of 7.4 months (95% CI 5.4, 10.1) suggested that the proposed dose of 750 mg daily did provide meaningful therapeutic benefit to ALK-rearranged NSCLC patients over existing treatments. In addition, treatment with ceritinib at 750 mg daily was generally well tolerated over a prolonged period of time with a low rate of discontinuation (10%) due to adverse events (AEs). Therefore, the proposed dose of 750 mg daily was acceptable as the recommended dose of ceritinib given the positive risk/benefit profile [9].
For the submission of ceritinib’s first-line indication in which ceritinib was administered at the recommended second-line dose of 750 mg daily, E–R analyses using average Ctrough were also performed. The exposure–efficacy analysis results using data from ASCEND-4 showed a decreasing trend, with lower systemic exposure having better efficacy performance. Further data exploration indicated that this could potentially be due to the limitation of using the average Ctrough as the drug exposure measure.
It should be acknowledged that in many cases, dose reduction and/or interruption can lead to fluctuations in PK concentration over time. It is not uncommon to observe the proportion of patients who stayed on the planned starting dose continuously declined over time due to dose adjustments as the trial progressed [10, 11]. Consequently, averaging all Ctrough during the treatment course as a single measure for the E–R analysis (i.e., time-independent E–R analysis) could mask the concentration changes over time and potentially lead to a bias in the estimation of the E–R relationship.
E–R analysis ignoring time dependency may lead to biased results. Such bias typically exists whenever an analysis that is timed from enrollment or randomization, such as PFS, is compared across groups defined by a classifying event occurring some time post enrollment/randomization [12,13,14]. For instance, it is well recognized that comparing survival by response category yields invalid inferences about treatment effect, since the length of survival will influence the chance of a patient being classified into one group or the other. Similarly, E–R analysis that ignores the impact of the length of survival or duration of follow-up on the exposure may also lead to false conclusions. In this article, we argue that a more appropriate E–R analysis approach is to take into consideration the entire concentration–time profile, i.e., to associate the exposure at different time intervals with the corresponding efficacy endpoints in a longitudinal fashion. In the subsequent sections, we refer to it as the time-dependent E–R analysis.
The objective of this article is to introduce the use of time-dependent modeling approach in the updated exposure–efficacy analysis of ceritinib for the first-line indication. A comparison with the conventional time-independent exposure–efficacy analysis was also made. The application of the time-dependent approach in E–R analysis could potentially address the aforementioned limitation of time-independent E–R analysis approach using average exposure measure.
Methods
Study design
Data from ASCEND-4 were used in the exposure–efficacy analyses for the submission of first-line indication of ceritinib. The study design and methodology of ASCEND-4 have been reported previously [5]. Briefly, adult untreated patients with stage IIIB/IV ALK-rearranged non-squamous NSCLC were randomized in a 1:1 ratio to receive 750-mg oral ceritinib daily or platinum-based chemotherapy every 3 weeks for 4 cycles followed by maintenance pemetrexed. The patient characteristics at baseline can be found in Soria JC et al. [5]. The treatment period began on day 1 of cycle 1. Each treatment cycle was 21 days. The study consisted of predose trough PK sampling in all patients who received ceritinib treatment on cycle 1 day 1, cycle 1 day 15, cycle 2 day 1, cycle 3 day 1, cycle 4 day 1, cycle 5 day 1, and cycle 6 day 1. Plasma concentrations of ceritinib were measured using a validated liquid chromatography/tandem mass spectrometry assay described previously, with a lower limit of quantification of 1.00 ng/mL [15].
Tumor response was assessed locally and by a Blinded Independent Review Committee (BIRC) based on Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria [16]. Imaging procedures included CT/MRI of chest and abdomen, whole-body bone scan, and photography. The assessment was performed at baseline and post-baseline at cycle 3 day 1 and then every two cycles until month 33. After that, it was only conducted every three cycles if clinically indicated or positive at baseline.
Exposure–efficacy analyses of ceritinib were conducted based on data from patients in the ceritinib arm in ASCEND-4 to explore the relationship between systemic exposure and efficacy endpoints by BIRC, including tumor response and PFS, in ALK-rearranged metastatic NSCLC treatment-naïve (including ALK inhibitor) patients.
Statistical analysis
Time-independent E–R models
Logistic regression model for best overall response versus exposure
Modeling analysis was first conducted to describe the exposure–efficacy relationship using a time-independent approach. A logistic regression model was applied to model a patient’s probability of having BOR of confirmed complete response (CR) or partial response (PR) versus the observed average Ctrough (Eq. 1):
where xi is the average of individual Ctrough during the treatment course for patient i, \(\varvec{Z}_{\varvec{i}}\) is the covariate vector \(\{ Z_{i1} , Z_{i2} , \ldots , Z_{ik} \}^{\prime }\) with k corresponding to the number of covariates in the final model, π(xi, Zi) is the probability of having BOR of confirmed CR or PR, and \(\beta_{0}\), \(\beta_{1}\), and \(\varvec{\gamma}= \{ \gamma_{1} , \gamma_{2} , \ldots , \gamma_{k} \}^{\prime }\) are the intercept, slope for exposure, and slope vector for covariates, respectively.
Backward model selection was applied. Only covariates with a p value < 0.05 were retained in the final model. The initial covariates considered were baseline values of age, gender, race, weight, World Health Organization (WHO) status, brain metastases, and stage of disease at study entry.
Cox regression model for PFS versus exposure
A Cox regression model was used to estimate the relationship between PFS and ceritinib exposure in terms of the average Ctrough (Eq. 2):
where hi is the hazard of patient i, h0 is the baseline hazard, xi is the corresponding average Ctrough for patient i, and Zi is the covariate vector \(\{ Z_{i1} , Z_{i2} , \ldots , Z_{in} \}^{\prime }\) with n corresponding to the number of covariates in the final model.
Backward model selection was used for the Cox regression model with the same set of initial covariates as the logistic regression model.
Time-dependent E–R models
The idea of time-dependent E–R analysis is to associate exposure with the corresponding response over time in a longitudinal fashion. Before conducting such an analysis, the ceritinib plasma concentration–time profile along with dosing history was first examined in patients in ASCEND-4. This step was conducted to investigate whether there were any noticeable changes in ceritinib concentration during treatment that could potentially confound the exposure–efficacy analyses.
Model-based exposure–efficacy analyses were then conducted. A repeated-measures logistic regression model was used to explore the relationship between exposure and overall response, and a time-dependent Cox regression model was used to characterize the relationship between exposure and the primary endpoint, PFS by BIRC.
Repeated-measures logistic regression model for overall response versus exposure
For the repeated-measures logistic regression model, the purpose is to model the probability of having an overall response of CR or PR based on the corresponding PK exposure. The “corresponding PK exposure” was obtained by averaging the population PK (PopPK)-predicted PK concentrations two cycles prior to each tumor evaluation (i.e., Cavg,2-cycle). This PopPK-predicted Cavg,2-cycle is further described in the later section.
The choice of two cycles for the calculation of Cavg was based on the fact that tumor evaluation was performed every two cycles and the assumption that systemic exposure in the past two cycles is predictive of the anti-tumor response of ceritinib.
Equation 3 shows the repeated-measures logistic regression analysis for overall response of CR or PR versus the ceritinib average concentration:
where xij is the jth average 2-cycle PK concentration (Cavg,2-cycle) for patient i, Zi is the covariate vector, and \(\beta_{0}\), \(\beta_{1}\), and \(\varvec{\gamma}\) are the intercept, slope for exposure, and slope vector for covariates, respectively, in the model for the log of the odds ratio.
Equation 4 shows the probability of overall response being CR or PR:
A patient could have multiple tumor assessments over time according to the trial visiting schedule. As observed from the model, each tumor assessment is associated with a corresponding x, namely, the average concentration two cycles prior to the response, Cavg,2-cycle.
Time-dependent Cox regression model for PFS versus exposure
A time-dependent Cox regression model was used to describe PFS versus ceritinib average concentration (Eq. 5):
where hi(t) is the hazard of patient i at time t, h0(t) is the baseline hazard at time t, xi(t) is the average concentration of patient i at time t, and Zi is the covariate vector. Note that here t represents every two cycles. The calculation of Cavg,2-cycle is the same as that for the repeated-measures logistic regression model.
PFS is the time from randomization to disease progression or death. When applying the time-dependent Cox regression model, the time for PFS was segmented into 2-cycle intervals. For each interval, the censoring indicator was set to 0 if a patient died or progressed in that interval (event observed) and was set to 1 if otherwise (event not observed).
Population PK analysis
The PopPK-predicted ceritinib Cavg,2-cycle, calculated as accumulated AUC of the two cycles prior to the tumor assessment divided by the length of the two cycles in days, was used to drive the time-dependent E–R analysis as described above.
The PopPK model of ceritinib was previously described by Hong et al. [17]. Briefly, the analysis was performed using first-order conditional estimation with interaction, as implemented in NONMEM software (version VII, level 2.0; Icon Development Solutions, Ellicott City, Maryland) compiled with Intel Fortran Compiler (version 14.0.1.106) in a MODESIM high-performance computing environment. The final PopPK model was evaluated using a nonparametric bootstrap technique, and the prediction-corrected visual predictive check. Ceritinib concentration–time profile was described by a 1-compartment model with delayed first-order absorption and time-dependent elimination. The final model includes the effect of body weight, albumin and alanine aminotransferase on the apparent clearance at steady-state.
Results
Time-independent E–R analysis
In ASCEND-4, among the 189 patients randomly assigned to receive ceritinib, 156 patients were included in the PK analysis set (PAS). The exposure–efficacy analysis was based on patients in the PAS. The PAS included patients with an evaluable Ctrough, defined as patients with samples taken between 18 and 30 h after the last dose intake, and before the next dose intake. Although a decreasing number of Ctrough over time was observed, more than half of the patients in the PAS provided an evaluable Ctrough at each study visit (142 on cycle 1 day 1, 122 on cycle 1 day 15, 116 on cycle 2 day 1, 96 on cycle 3 day 1, 92 on cycle 4 day 1, 88 on cycle 5 day 1, and 82 on cycle 6 day 1).
Although high and clinically meaningful response rates were observed across all average Ctrough ranges (Fig. 1a) within the exposure achieved following a planned starting dose of 750 mg daily in ASCEND-4, there was a downward trend between PK and tumor response. With a 200 ng/mL (approximately the increase in average Ctrough based on an incremental change of 150 mg, the only dose strength of ceritinib) increase in ceritinib average Ctrough, there was a 23% decrease in the odds of having a BOR of confirmed CR/PR (odds ratio = 0.77; 95% CI 0.62, 0.94) (Table 1). Based on the backward model selection, baseline brain metastasis and gender were statistically significant covariates (p < 0.05) and were retained in the final model (Table 1). The estimated odds ratio for female versus male was 2.26 (95% CI 1.02, 5.01). However, efficacy subgroup analyses previously conducted for ASCEND-4 did not reveal any trend with regard to gender, suggesting that gender is unlikely to play a clinically meaningful role in the interpretation of efficacy results. The estimated odds ratio for brain metastasis absence versus presence was 3.39 (95% CI 1.50, 7.64), indicating that patients without brain metastasis at baseline had a higher chance of responding and vice versa.
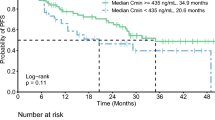
a Time-independent logistic regression model of tumor response (CR/PR) versus average Ctrough of ceritinib. The solid line represents the fitted curve and the shaded area represents the 95% CI. The predicted curve and 95% CI are generated as follows: numeric covariates at the median and categorical covariates at the highest frequency level. CI confidence interval, CR complete response, Ctrough average trough plasma concentration, PR partial response. b Kaplan–Meier plot of PFS by quartile of average Ctrough of ceritinib. PFS progression-free survival, Q quartile, NE not estimable
The Kaplan–Meier plot by average Ctrough quartiles revealed no strong trend for increase in PFS with increasing systemic exposure (Fig. 1b). The estimated median PFS was similar for the lower three quartiles, while it was shorter in the highest quartile. The results from Cox regression model showed a 12% increase in the risk of progression/death with a 200 ng/mL increase in average Ctrough; however, the 95% CI included 1, indicating that it was not statistically significant [Table 2, hazard ratio (HR) = 1.12; 95% CI 0.98, 1.28]. Body weight, gender, and brain metastasis at baseline were retained in the final Cox model based on backward model selection. Results also favored the absence of brain metastases at baseline, with a 60% reduction in the risk of progression/death (HR = 0.40; 95% CI 0.25, 0.66). Similarly, the estimated risk of progression/death also appeared to be lower in females (HR = 0.57; 95% CI 0.35, 0.93). Body weight produced no meaningful impact on HR, although the coefficient met the model selection criteria.
Results from both models indicated that a higher exposure was associated with reduced efficacy, namely lower overall response rate (ORR) of confirmed CR or PR and shorter PFS. To investigate the influence of any probable risk factors on the trend observed for the exposure–efficacy relationship, patient demographics and disease characteristics at baseline were reviewed. Patient demographics (such as age, gender, weight, and race), baseline WHO performance status, baseline brain metastasis status, and stage of disease at study entry were summarized for each average Ctrough quartile and appeared to be balanced across quartiles, except for body weight, gender, and baseline brain metastasis (Table 3). The median body weight in the first quartile was slightly higher than that in the other quartiles. Smaller proportions of females were included in the lower quartiles compared with that in the higher quartiles. These slight imbalances in demographics are unlikely to explain the exposure–efficacy trend observed, due to their lack of clinically meaningful impact on ceritinib PK [17]. It was first suspected that the lower response rate associated with higher exposure (Fig. 1a) was caused by more patients with brain metastasis at baseline, i.e., 35.9% at the highest exposure quartile (Table 3). However, similar number of patients with brain metastasis was also observed at the lower exposure range (38.5% at the lowest exposure quartile, Table 3). Hence, baseline brain metastasis could not explain the lower response seen at higher exposure. In addition, no other imbalance was observed for patient demographics or disease characteristics at baseline (Table 3).
Time-dependent E–R analysis
As depicted in Fig. 2, in a representative patient, ceritinib concentration was not constant over time due to dose modifications during the treatment period. This finding supports using a time-dependent modeling approach for exposure–efficacy analyses.
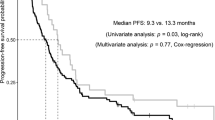
Figure 3 shows the predicted probability of having an overall response of CR/PR based on the repeated-measures logistic regression model, along with the 95% CI. With a 200 ng/mL increase in ceritinib concentration, there was an estimated 7% increase in the odds of having a confirmed CR/PR (odds ratio = 1.07; 95% CI 0.98, 1.17) (Table 1). Based on these results, there was no obvious trend between systemic exposure and overall tumor response. The downward trend observed using the time-independent modeling approach was no longer observed.
Repeated-measures logistic regression model of tumor response (CR/PR) versus time-varying concentration of ceritinib. The predicted curve and 95% CI are generated as follows: numeric covariates at the median and categorical covariates at the highest frequency level. CI confidence interval, CR complete response, PR partial response
According to the time-dependent Cox regression model, an estimated 4% increase in the risk of disease progression or death was obtained with a 200 ng/mL increase in ceritinib concentration (HR = 1.04; 95% CI 0.92, 1.17) (Table 2). The negligible estimated increase in the risk indicated that there was no clear relationship between systemic exposure and PFS.
Discussion
Frequently, E–R analyses performed in oncology were conducted using time-independent models such as logistic regression and Cox regression models. This type of models uses a summary-level exposure measure without accounting for the time-varying exposure due to dose modifications. However, dose reduction and interruption driven by AEs are quite common in oncology clinical trials. Dynamic adjustments in dose can lead to changes in systemic exposure throughout the treatment duration, causing bias in the results generated from time-independent exposure–efficacy analyses. Patients generally have a higher drug concentration in the earlier cycles compared to that in the later cycles. The longer the treatment duration, the higher chance there is for dose reduction and dose interruption. Potentially, patients who benefit from the treatment stay longer in the trial, and may, therefore, appear to have a lower PK concentration. On the other hand, patients who progress early would discontinue from the trial early with less opportunity for dose reduction or interruption. These patients would appear to have a higher PK, causing a spurious reverse relationship between PK and response.
To be consistent with the E–R modeling approach used in the initial second-line submission with ASCEND-1, the time-independent logistic regression model was first applied to the exposure–efficacy data from ASCEND-4, the pivotal phase 3 study supporting the first-line therapy of ceritinib given at a dose of 750 mg/day in untreated advanced ALK-rearranged NSCLC patients. The results showed that a lower response rate was observed at the higher average Ctrough concentration range compared to the lower concentration range (Fig. 1a). Similarly, the estimated median PFS was shorter in the highest quartile compared to the others (Fig. 1b), with a 12% increase in the risk of disease progression with a PK increment of 200 ng/mL, though not statistically significant (HR = 1.12; 95% CI 0.98, 1.28) (Table 2). It should be noted that the exposure measure of ceritinib utilized in the exposure–efficacy analyses was the average Ctrough during the entire course of ceritinib treatment, i.e., one exposure value per patient. In addition, this summary-level exposure measure implicitly assumes equal impact of trough concentration on the response regardless of how far it is from the time when efficacy was assessed. The time-varying ceritinib concentrations caused by dose modifications were not appropriately accounted for in the model. Therefore, the impact of time-varying ceritinib concentration as a result of dose modifications on the response was not estimated, and the underlying true E–R relationship was not appropriately described.
A more relevant modeling approach, namely, the repeated-measures logistic regression model, was subsequently fitted to the overall response against ceritinib exposure over time in to overcome the limitation of the time-independent model. The exposure measure used was the average PK concentration within a specific window prior to each tumor response assessment. In ASCEND-4, a time interval of two cycles was used, as tumor assessment was performed every two cycles. The repeated-measures logistic regression model estimates a patient’s probability of having an overall response of confirmed CR/PR using the PopPK-predicted average ceritinib concentration over the 2-cycle period prior to each tumor assessment as a time-dependent covariate (i.e., Cavg,2-cycle). Similarly, for PK versus PFS, a time-dependent Cox regression model was used. The PopPK-predicted ceritinib Cavg,2-cycle was also used as a time-dependent covariate in the Cox model. With the time-dependent modeling approach, the trend between PK as assessed by Cavg,2-cycle and tumor response showed a slightly positive trend (Fig. 3). The odds ratio of having a confirmed CR/PR changed from 0.77 (95% CI 0.62, 0.94) to 1.07 (95% CI 0.98, 1.17) with an increment of 200 ng/mL in ceritinib concentration (Table 1). As for the relationship between PK and PFS, an estimated 4% increase in the risk for disease progression (HR = 1.04; 95% CI 0.92, 1.17) was obtained with a PK increment of 200 ng/mL, indicating no apparent relationship between PK and PFS (Table 2).
There are other solutions to mitigate the confounding factor in the context of E–R analysis. One good example is the tumor growth inhibition (TGI) model in which longitudinal tumor size is used as a pharmacodynamics (PD) biomarker [18, 19]. Another example is landmark analysis as proposed by Anderson et al. [12, 13].
The TGI model explicitly incorporates drug exposure over time to drive the drug effect on tumor size. However, one limitation of using tumor size as response measure is that new lesions and non-target lesions are not taken into account for evaluating anti-tumor response. Our proposed time-dependent E–R modeling approach adopts the same concept as the TGI model with respect to utilizing longitudinal PK and response data to estimate drug effect. The only difference is that our model directly analyzes the clinical RECIST endpoints which are the primary endpoints in oncology clinical studies and are important for regulatory submission and approval. It is acknowledged that using the RECIST-defined odds ratio of CR/PR or stable disease (SD), quantitative anti-tumor activity information is dichotomized (1 or 0); therefore, some information may be lost. For future work, TGI modeling approach may also be applied to ceritinib E–R analysis, so that results from both approaches can be compared to help us better understand the relationship between systemic exposure, PD biomarker, and efficacy.
For landmark analysis, in the context of exposure–PFS characterization, it can be performed by selecting a fixed timepoint, so that the analysis would not be impacted by patients who progressed or died earlier than this timepoint, and therefore likely to have higher exposure. Conditional on the patient still at risk for progression at the landmark timepoint, the analysis excluded patients who progressed or died prior to the landmark timepoint. The average Ctrough was re-derived using trough concentrations up to the landmark timepoint. Based on the conditional HR estimate from an exploratory landmark analysis at week 6 post randomization (data on file), a timepoint close to the median time to response of 6.1 weeks [5], there is no strong relationship between exposure and efficacy in terms of PFS. Even though these results do not contradict with that from the time-dependent Cox analysis, the conditional nature of the landmark analysis limits the generalization of the results to the overall effect of ceritinib exposure on PFS as established by the time-dependent modeling approach. Another recognized limitation of the landmark analysis is the arbitrary selection of the landmark timepoint and the exclusion of events prior to the landmark timepoint, leading to inclusion of only a subset of population for the analysis.
In conclusion, the time-dependent exposure–efficacy analysis of ceritinib illustrate that the dynamic E–R relationship in clinical trials with long duration could be better analyzed using time-dependent models. Using time-varying PK and the paired response outcome at each assessment interval, richer information at each time interval can be fully utilized for better characterization of drug effect on efficacy outcome.
References
Soda M, Choi YL, Enomoto M et al (2007) Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 448:561–566. https://doi.org/10.1038/nature05945
Shaw AT, Yeap BY, Mino-Kenudson M et al (2009) Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 27:4247–4253. https://doi.org/10.1200/JCO.2009.22.6993
Shaw AT, Kim D-W, Mehra R et al (2014) Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 370:1189–1197. https://doi.org/10.1056/NEJMoa1311107
US Food and Drug Administration. Zykadia prescribing information. https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/zykadia.pdf (2017). Accessed 13 Dec 2017
Soria JC, Tan DSW, Chiari R et al (2017) First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 389:917–929. https://doi.org/10.1016/S0140-6736(17)30123-X
US Food and Drug Administration. Guidance for industry: exposure-response relationships-study design, data analysis, and regulatory applications. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM072109.pdf (2003). Accessed 29 Jan 2019
US Food and Drug Administration (2014) Clinical pharmacology and biopharmaceutics review(s) of ceritinib. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205755Orig1s000ClinPharmR.pdf. Accessed 13 Dec 2017
Khozin S, Blumenthal GM, Zhang L et al (2015) FDA approval: ceritinib for the treatment of metastatic anaplastic lymphoma kinase-positive non-small cell lung cancer. Clin Cancer Res 21:2436–2439. https://doi.org/10.1158/1078-0432.CCR-14-3157
US Food and Drug Administration (2014) Medical review(s) of ceritinib. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205755Orig1s000MedR.pdf. Accessed 13 Dec 2017
US Food and Drug Administration (2014) Clinical pharmacology and biopharmaceutics review(s) of panobinostat. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/205353Orig1s000ClinPharmR.pdf. Accessed 13 Dec 2017
Liu C (2016) Dose adjustment integrated exposure-response analysis (DAIER) for dose optimization lenvatinib in renal cell carcinoma FDA-AACR Oncology Dose Finding Workshop. http://www.aacr.org/AdvocacyPolicy/GovernmentAffairs/Documents/6.13.16%20FDA-AACR%20Dose%20Finding%20for%20Online.pdf. Accessed 11 May 2018
Anderson JR, Cain KC, Gelber RD (1983) Analysis of survival by tumor response. J Clin Oncol 11:710–719. https://doi.org/10.1200/JCO.1983.1.11.710
Anderson JR, Cain KC, Gelber RD (2008) Analysis of survival by tumor response and other comparisons of time-to-event by outcome variables. J Clin Oncol 26:3913–3915. https://doi.org/10.1200/JCO.2008.16.1000
Giobbie-Hurder A, Gelber RD, Regan MM (2013) Challenges of guarantee-time bias. J Clin Oncol 31:2963–2969. https://doi.org/10.1200/JCO.2013.49.5283
Heudi O, Vogel D, Lau YY, Picard F, Kretz O (2014) Liquid chromatography tandem mass spectrometry method for the quantitative analysis of ceritinib in human plasma and its application to pharmacokinetic studies. Anal Bioanal Chem 406:7389–7396. https://doi.org/10.1007/s00216-014-8125-9
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Hong Y, Passos VQ, Huang PH, Lau YY (2017) Population pharmacokinetics of ceritinib in adult patients with tumors characterized by genetic abnormalities in anaplastic lymphoma kinase. J Clin Pharmacol 57:652–662. https://doi.org/10.1002/jcph.849
Bruno R, Claret L (2009) On the use of change in tumor size to predict survival in clinical oncology studies: toward a new paradigm to design and evaluate phase II studies. Clin Pharmacol Ther 86:136–138. https://doi.org/10.1038/clpt.2009.97
Claret L, Bruno R (2014) Assessment of tumor growth inhibition metrices to predict overall survival. Clin Pharmacol Ther 96:135–137. https://doi.org/10.1038/clpt.2014.112
Acknowledgements
The authors thank Zhe Chen for his contribution to the exploratory data analysis. Medical editorial assistance for this manuscript was provided by Shiva Krishna Rachamadugu, Novartis Healthcare Pvt. Ltd.
Funding
This study was funded by Novartis Pharmaceutical Corporation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
YY Lau, W Gu, YY Ho, X Zhang and P Urban are employees of Novartis Pharmaceutical Corporation. YY Lau, YY Ho, X Zhang and W Gu also own Novartis stocks. Y Hong declares no conflicts of interest.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the studies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ying Hong was an employee of Novartis at the time of analysis and initiation of the manuscript.
Rights and permissions
About this article
Cite this article
Lau, Y.Y., Gu, W., Ho, YY. et al. Application of time-dependent modeling for the exposure–efficacy analysis of ceritinib in untreated ALK-rearranged advanced NSCLC patients. Cancer Chemother Pharmacol 84, 501–511 (2019). https://doi.org/10.1007/s00280-019-03830-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03830-5