Abstract
Purpose
Ovarian cancer remains a most malignant female cancer nowadays. The acquisition of chemoresistance to common-used cisplatin-based chemotherapy results in a decreased overall patient survival. The present study is aimed to investigate the role and mechanism by which miR-139/ ATPases7A/B axis modulates the chemoresistance of ovarian cancer to cisplatin-based chemotherapy.
Methods
The expression of miR-139 in cisplatin-sensitive (n = 23) and cisplatin-resistant (n = 14) ovarian cancer tissues and cell lines (CAOV-3 and SNU119) was determined using real-time PCR assays; its effect on ovarian cancer cell chemoresistance to different concentrations of cisplatin was then assessed by measuring the cell viability using MTT assays. Next, miR-139 binding to the 3′UTR of ATP7A/B was confirmed using luciferase reporter gene assays. Finally, the combined effect of miR-139 and ATP7A/B on the chemoresistance of ovarian cancer cell was assessed.
Results
miR-139 expression was down-regulated in cisplatin-resistant ovarian cancer tissues (**P < 0.01) and reduced by cisplatin treatment in ovarian cell lines (*P < 0.05, **P < 0.01); miR-139 could enhance cisplatin-induced suppression on ovarian cancer cell viability, shown as reduced lC50 values; ATP7A and ATP7B protein levesincreased approximately 2 ~ fold-changein cisplatin-resistant cell lines. MiR-139 directly bound to the 3′UTR of ATP7A/B, respectively; miR-139 inhibition increased lC50 values whereas ATP7A/B knockdown reduced lC50 values of CAOV-3 and SNU119 cell lines under cisplatin treatment; the effect of miR-139 inhibition could be partially attenuated by ATP7A/B knockdown.
Conclusions
MiR-139/ATP7A/B axis can be a reliable biomarker for ovarian cancer diagnosis, and may affect the chemoresistance of ovarian cancer to cisplatin-based chemotherapy; rescuing miR-139 expression thus to inhibit ATP7A/B might contribute to dealing with the chemoresistance of ovarian cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer is the seventh most common cancer in women and eighth among causes of death in the female population with the highest mortality rate [1]. Due to nonspecific delayed symptoms and lack of suitable screening methods, most patients are diagnosed in advanced stages, when the curative effect of therapy is very limited [2, 3]. Ovarian cancer therapy usually consists of surgical removal of the tumor and subsequent chemotherapy using a combination of platinum derivatives and taxanes, mostly cisplatin or carboplatin and paclitaxel [4]. Despite a very good response to first-line chemotherapy, the common development of chemoresistance to conventional drugs results in a decreased overall patient survival [5, 6]. Searching for reliable and specific markers and fundamental solution of chemoresistance of ovarian cancer has become an urgent need during ovarian cancer treatment.
The acquisition of chemoresistance is complex and can be controlled by a variety of factors, including environmental factors and genetic factors. MicroRNAs (miRNAs) are a series of small non-coding RNAs with a length of 20–22 nt that can modulate a wide-range of biological processes, both normal developmental and disease-related processes. Dysregulation of miRNAs has been reported to be frequently observed in cancers, and is associated with the development of cancers from many perspectives, including cancer chemoresistance [7, 8]. Several let-7 members are differentially expressed in chemo-resistant ovarian cancer cells. Low levels of let-7i correlate with shorter progression-free survival in ovarian cancer. In addition, knock-down of let-7i decreased cisplatin-induced cell death with ∼40% in primary ovary and ovarian cancer cell lines [9]. Similarly, upregulation of miR-200c levels in an ovarian cancer cell line increased the sensitivity for microtubule-targeting drugs up to 85% [10]. The above findings inspired us to look for other miRNAs that are dysregulated in ovarian cancer, and further evaluate the role and mechanisms of miRNAs in regulating chemoresistance in ovarian cancer.
As we mentioned, miRNA dysregulation has been frequently observed in cancers, including ovarian cancer [7, 8, 11]. A total of 583 miRNAs that correlates with the tumor stage and grade from oncomir (http://oncomir.org/) was examined and the results suggest that miR-139-5p is significantly correlated with the ovarian cancer tumor stage and grade (Supplementary Table S1, P < 0.001, FDR < 0.01). Interestingly, miR-139 has also been reported to play an essential role in cancer development, including cancer chemoresistance. Through mediating Wnt/β-catenin signaling pathway, miR-139 is involved in the cell growth and metastasis of bladder cancer cells [12]. By direct targeting NOB1, miR-139 can induce the cell apoptosis and inhibit the metastasis of cervical cancer cells [13]. In glioblastoma multiforme, miR-139 can hinder the cell migration and invasion of cancer cells through targeting ZEB1/2 [14]. Regarding cancer chemoresistance, miR-139 also plays a potential role. By direct targeting Notch-1 signaling, miR-139 can mediate the chemo-sensitivity of colorectal cancer cell and breast cancer cell to chemo agents [15, 16], and reverse CD44+/CD133+-associated multidrug resistance of colorectal carcinoma cells [17]. By down-regulating Bcl2, miR-139 enhances the chemotherapeutic sensitivity of colorectal cancer cells [18]. Based on the above findings, we hypothesized that, in addition to tumor stage and grade of ovarian cancer, miR-139 might also affect the chemoresistance of ovarian cancer, most possibly through regulating downstream targets.
Copper-transporting ATPases are intracellular transporters that maintain cellular copper homeostasis [19, 20]. Reportedly, copper-transporting ATPases sequester platinum into vesicular structures, and thus prevent their cellular effect [21], thus to exert an indirect effect on chemoresistance. Dysregulated expression of ATP7A and ATP7B in ovarian cancer cells can alter the amount of cellular copper, which could then impact the activities of other transporters that import or export platinum derivatives [22, 23]. Increased expression of ATP7A and ATP7B in ovarian cancer cells predicts short survival for patients treated with platinum derivatives and suggests a role for these proteins in chemoresistance [24, 25]. Can ATP7A and/or ATP7B be regulated by miR-139 in ovarian cancer cells, thus to affect the chemoresistance? This remains to be studied. Using online tool Targetscan [26], it was found that ATP7A was predicted to possess a conserved miR-139 binding sites (position 3159–3166 of ATP7A 3′ UTR), suggesting that miR-139 might directly bind to the 3′ UTR of ATP7A to regulate its expression, therefore, affecting the chemoresistance of ovarian cancer to cisplatin-based chemo-therapy.
To validate the above hypothesis, miR-139 expression was first examined, and the role of miR-139 in ovarian cancer chemoresistance to cisplatin was assessed by measuring the effect of miR-139 on ovarian cancer cell proliferation under cisplatin treatment; further, whether miR-139 acts on ATP7A and ATP7B to affect their function, thus to regulate the chemoresistance of ovarian cancer cell to cisplatin was estimated. Taken together, we demonstrated a miR-139/ATP7A/ATP7B axis which plays an essential role in ovarian cancer chemoresistance, and presents a potential targets in ovarian cancer treatment.
Materials and methods
Clinical tissues samples
A total of 37 patients (23 cisplatin-sensitive and 14 cisplatin-resistant) who received surgical resection and cisplatin-based treatment at the Third Xiangya hospital of Central South university were recruited in the present study. Inform content was obtained from every patient before the surgery. Ovarian cancer tissue was collected immediately after resection and was stored in liquid nitrogen before further use.
Information on histologic subtype, cancer stage (using International Federation of Gynaecology and Obstetrics (FIGO) staging criteria and WHO guidelines [27,28,29]), grade, and residual disease after surgery was extracted from histopathology reports and medical records. Pathological examination was performed. The mean follow-up time was 62.6 months.
Adjuvant chemotherapy was administered in 37 cases and was cisplatin-based with paclitaxel (175 mg/m2, 3 h infusion) at an area under the concentration time curve of 5 or 6, every 3 weeks for six cycles as the standard of care except for patients who had allergic reactions or medical contraindications. Resistance to chemotherapy is defined as disease progression or recurrence within 6 months after end of therapy/within a 6-month therapy-free interval. Sensitive status was defined as a therapy-free interval of at least 6 months without the evidence of tumor progression or recurrence. In our cohort, 23 (62.16%) cases were classified as sensitive (relapse > 6 months) and 14 (37.84%) as resistant (relapse < 6 months).
Cell lines and cell transfection
Human ovarian cancer cell lines: CAOV3 was obtained from the American Type Culture Collection (ATCC, USA). SNU119 was obtained from Korean Cell Line Bank (Korea). CAOV3 and SNU119 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma, UK) with 10% FBS along with 1% MEM vitamins, MEM nonessential amino acids and Penicillin–Streptomycin. The cell line authentication was done using 20-STR analysis (report shown in Supplementary Materials). DNA was extracted using Axygen® AxyPrep™ Multisource Genomic Miniprep DNA (AP-MN-MS-GDNA-50) and amplified by 20-STR amplification. STR loci and gender-specific gene Amelogenin were detected using 96-capillary 3730xl DNA Analyzer (ABI, Waltham, MA, USA).
MiR-139 mimics or miR-139 inhibitor was transfected into CAOV3 and SNU119 cells to achieve ectopic miR-139 expression or miR-139 inhibition (GenePharma, China). Si-ATP7A or si-ATP7B was used to achieve knockdown of ATP7A or ATP7B (GenePharma, China).
Establishment of cisplatin-resistant subclones from CAOV3 and SNU119 cells
To establish cisplatin-resistant subclones, CAOV3 and SNU119 cells were cultured with various concentrations of cisplatin (0.25, 0.5, 1, 2, 4, 6, 8, 16, 32, 64 μM, courtesy of Nihon-Kayaku Co. Ltd., Tokyo, Japan) for 3–5 weeks, and the surviving cells were collected. This collection procedure after cisplatin exposure was repeated four times. Finally, CAOV3- or SNU119-derived cisplatin-resistant subclones, named CAOV3/cDDP and SNU119/cDDP were established by the limiting dilution method. The selected cells were also supplemented with cisplatin (1 μM, 2–3 passages) to maintain their resistant phenotype.
Real-time PCR
Trizol reagent (Invitrogen) was used for total RNA extraction following the manufacturer’s instructions. Using miRNA-specific primer, total RNA was reverse transcribed and the miScript Reverse Transcription kit (Qiagen, Germany) was used for miR-139 qRT-PCR. The SYBR green PCR Master Mix (Qiagen) was used following the manufacturer’s instructions. The Ct method was used to evaluate the relative expression and normalized to U6 and GAPDH mRNA expression, respectively. Primer sequences, amplification mix composition, cycling conditions as well as raw data on melting curves showing the reaction specificity and efficiency were provided as Supplementary material.
Immunoblotting assays
RIPA buffer (Cell-Signaling Tech., US) was used to homogenize the cells. The protein levels of ATP7A and ATP7B in cervical cancer cells was detected by performing immunoblotting. Cells were lysed cultured, or transfected in 1% PMSF supplemented RIPA buffer. Proteins were extracted, examined for protein concentration by the bicinchoninic acid (BCA) assays using Pierce™ BCA Protein Assay Kit (Cat. 23225, Pierce, Waltham, MA, USA) and then loaded onto SDS–PAGE minigel, and then transferred onto PVDF membrane. The blots were probed with the following antibodies: anti-ATP7A (1/1000, ab13995, Abcam, USA), anti-ATP7B (1/1000, Cat# EPR6794, ab124973, Abcam, USA) and anti-GAPDH (mouse monoclonal to GAPDH, 1/500, ab8245, Abcam, USA) at 4 °C overnight, and incubated with HRP-conjugated secondary antibody (1:5000). Signals were visualized using ECL Substrates (Millipore, USA). The protein expression was normalized to endogenous GAPDH.
Prediction of miRNA targets
The potential miR-139 binding site on the 3′ UTR of ATP7A, as well as other miRNAs that might regulate ATP7A/B were predicted using Targetscan [26] (http://www.targetscan.org/vert_71/).
Luciferase activity
Wild-type ATP7A or ATP7B luciferase reporter gene vectors (named ATP7A-WT-3′UTR or ATP7B-WT-3′UTR) and mutant-type ATP7A or ATP7B luciferase reporter gene vectors (named ATP7A-MUT-3′UTR or ATP7B-MUT-3′UTR) were constructed. HEK293T cells were cultured overnight after being seeded into a 24-well plate, co-transfected with the indicated vectors and miR-139 mimics or miR-139 inhibitor. Forty-eight hours after transfection, Dual Luciferase Reporter Assay System (Promega, USA) was used to perform the luciferase assays.
MTT assay
Twenty-four hours after seeded into 96-well plates (5000 cells per well), cells were transfected with miR-139 mimics, or co-transfected with miR-139 inhibitor and si-ATP7A/B under a series of doses of cisplatin (0.25, 0.5, 1, 2, 4, 6, 8, 16, 32, 64 μM). Medium with cisplatin was applied at 24 h post-transfection. Forty-eight hours after transfection, 20 μl MTT (at a concentration of 5 mg/ml; Sigma–Aldrich) was added, and the cells were incubated for an additional 4 h in a humidified incubator. Then 200 μl DMSO was added after the supernatant discarded to dissolve the formazan. OD490 nm value was measured. The viability of the non-treatment cells (control) was defined as 100%, and the viability of cells from all other groups was calculated separately from that of the control group. Data were displayed as a percentage normalized to the viability of cells with no cDDP treatment. The abscissa was the logarithm of cDDP concentration (log-conc.). LC50 represented the concentration of cDDP when cell viability was reduced to 50%.
Statistics analysis
Data from three independent experiments were presented as mean ± SD, processed using SPSS 17.0 statistical software (SPSS, USA). A direct comparison between two groups was conducted using Student’s non-paired t test, and a one-way ANOVA with Dunnett’s post-test was used to compare the means of three or more groups. COX risk proportional regression model was used to identify the factors that have significant influence on survival. Age, cisplatin sensitivity, FIGO stage, grade of tumor, histologic subtype and miR-139 expression were used for univariate analysis; cisplatin sensitivity, FIGO stage and miR-139 expression were used for multivariate analysis. P values of < 0.05 were considered statistically significant.
Results
MiR-139 expression is down-regulated in cisplatin-resistant ovarian tissues and in response to cisplatin treatment
To investigate the role of miR-139 in ovarian cancer chemoresistance, miR-139 expression in cisplatin-resistant and cisplatin-sensitive ovarian tissues was first examined. As shown in Fig. 1a, miR-139 expression was significantly down-regulated in cisplatin-resistant ovarian cancer tissues, compared to cisplatin-sensitive tissues. Further, CAOV3 and SNU119 cells were treated with a series of doses of cisplatin (0, 0.5, 1, 2, 4, 8 μM), and then examined for miR-139 expression in response to cisplatin treatment. MiR-139 expression was down-regulated by cisplatin treatment in a dose-dependent manner, and was reduced to less than 0.5-fold by 4 and 8 μM cisplatin (Fig. 1b). These results suggested the potential role of miR-139 in ovarian cancer chemoresistance.
MiR-139 expression is down-regulated in cisplatin-resistant ovarian tissues and in response to cisplatin treatment. a miR-139 expression in cisplatin-sensitive (n = 23) and cisplatin-resistant (n = 14) ovarian cancer tissues was determined using real-time PCR assays. b and c miR-139 expression in CAOV3 and SNU119 cells under a series of dosed of cisplatin treatment was determined using real-time PCR assays. The data are presented as mean ± SD of three independent experiments. *P < 0.05, **P < 0.01
To further analyze the role of miR-139 in ovarian cancer, the above clinical samples were divided into two groups according to miR-139 expression: a high miR-139 expression group possessing miR-139 expression above the median value, and a low miR-139 expression group possessing miR-139 expression below the median value. The correlation between miR-139 expression and clinical parameters was analyzed and shown in Table 1. A high miR-139 expression was significantly correlated with cisplatin sensitivity and a low miR-139 expression was correlated with advanced FIGO stages. Furthermore, the results of univariate analysis showed that cisplatin sensitivity, FIGO stage and miR-139 expression caused significant differences to overall survival time; thus, these three factors were used for multivariate analysis. The results of multivariate analysis showed that a low miR-139 expression (HR = 0.29, 95% CI = 0.11–0.79) and advanced FIGO stage (HR = 0.17, 95% CI = 0.06–0.54) were of high risk (Table 2).
The role of miR-139 in ovarian cancer cell chemoresistance
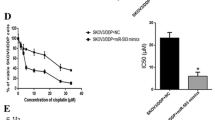
CAOV3 and SNU119 cells were transfected with miR-139 mimics to achieve ectopic miR-139 expression, as verified using real-time PCR assays (Fig. S1A and B). Twenty-four hours after transfection, CAOV3 and SNU119 cells were treated with a series of doses of cisplatin (0.125, 0.25, 0.5, 1, 2, 4, 8, 16, 32 μM for CAOV3 and 0.25, 0.5, 1, 2, 4, 8, 16, 32, 64 μM for SNU119) [30]; then the cell viability of CAOV3 and SNU119 was determined using MTT assays. As shown in Fig. 2a, c, the cell viability of CAOV3 and SNU119 was suppressed by cisplatin in a dose-dependent manner, and more strongly suppressed by miR-139 overexpression under a same cisplatin dose, compared to NC mimics group. Further, CAOV3- and SNU119-derived cisplatin-resistant subclones were established and verified for the evaluation of the function of miR-139 in ovarian cancer cell chemoresistance. The cell viability of cells with no treatment was defined as 100%. For CAOV3 cells, the cDDP concentration to reduce cell viability to 50% was about 1.36 μM (lC50 = 1.36); for CAOV3/cDDP cells this value was 7.94 μM (lC50 = 7.94) (Fig. 2b). For SNU119 and SNU119/cDDP cells, lC50 was promoted from 4.75 to 20.07 (Fig. 2d). CAOV3/cDDP and SNU119/cDDP cells were then transfected with miR-139 mimics. The suppressive effect of cisplatin on the indicated cells was amplified by miR-139 overexpression: the lC50 value for CAOV3/cDDP was reduced from 7.51 to 2.88, and for SNU119/cDDP was reduced from 21.13 to 9.85, compared to NC mimics group (Fig. 2e, f).
The role of miR-139 in ovarian cancer cell chemoresistance. a and c miR-139 mimics-transfected CAOV3 and SNU119 cells were treated with a series of doses of cisplatin (0.125, 0.25, 0.5, 1, 2, 4, 8, 16, 32 μM for CAOV3 and 0.25, 0.5, 1, 2, 4, 8, 16, 32, 64 μM for SNU119); the cell viability was determined using MTT assays. The data are presented as mean ± SD of three independent experiments. *P < 0.05, **P < 0.01. b and d CAOV3 and SNU119 cells were exposed to a series of doses of cisplatin (as indicated) to establish cisplatin-resistant subclones; MTT assays were performed to verify the cell viability of cisplatin-resistant CAOV3/cDDP and SNU119/cDDP cells. Data were displayed as a percentage normalized to the viability of cells with no DDP treatment. The abscissa was the logarithm of DDP concentration (log-conc.). LC50 represented the concentration of DDP when cell viability was reduced to 50%. e and f CAOV3/cDDP and SNU119/cDDP cells were transfected with miR-139 mimics, and treated with a series of dosed of cisplatin (as indicated); the cell viability was then determined using MTT assays. Data were displayed as indicated
MiR-139 directly binds to the 3′UTR of ATP7A to inversely regulate its expression
As we mentioned, ATP7A/B plays an essential role in ovarian cancer chemoresistance. First, the expression of ATP7A and miR-139 in cisplatin-sensitive and resistant ovarian cancer cells was detected. The expression of ATP7A was higher in CAOV3/cDDP and SNU119/cDDP cells than cDDP sensitive cell lines (Fig. S1C). In contrast, miR-139 was lower expressed in CAOV3/cDDP and SNU119/cDDP cells than cDDP sensitive cells (Fig.S1D). Furthermore, whether miR-139 affects ovarian cancer chemoresistance through the regulation of ATP7A/B was verified. To achieve this goal, luciferase reporter gene vector was employed. Wild-type ATP7A luciferase reporter gene vectors (named ATP7A-WT-3′UTR) and mutant-type ATP7A luciferase reporter gene vectors (named ATP7A-MUT-3′UTR) containing an 8 or 5 bp mutation in the putative miR-139 binding sites were constructed (Fig. 3a). MiR-139inhibitor was transfected into CAOV3 and SNU119 cells to achieve miR-139 inhibition, as verified using real-time PCR assays (Fig. S1E). HEK293T cells were then co-transfected with the indicated vectors and miR-139 mimics or miR-139 inhibitor; the luciferase activity was determined using dual luciferase reporter assays. The luciferase activity of ATP7A-WT-3′UTR vectors was suppressed by miR-139 overexpression, whereas promoted by miR-139 inhibition; after mutation in either of the predicted miR-139 binding sites, the changes of the luciferase activity were abolished (Fig. 3b). These data indicated that miR-139 might regulate ATP7A expression through direct targeting.
MiR-139 directly binds to the 3′UTR of ATP7A to inversely regulate its expression. a Wild-type ATP7A luciferase reporter gene vector (named ATP7A-WT-3′UTR) and mutant-type ATP7A luciferase reporter gene vector (named ATP7A-MUT-3′UTR) containing an 8 or 5 bp mutation in the predicted miR-139 binding sites were constructed. b HEK293T cells were co-transfected with the indicated vectors and miR-139 mimics or miR-139 inhibitor; the luciferase activity was determined using dual luciferase assays. c and d CAOV3 and SNU119 cells were transfected with miR-139 inhibitor or miR-139 mimics; the protein levels of ATP7A were determined using Immunoblotting assays. The data are presented as mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, compared to CAOV3, SNU119, NC mimics or NC inhibitor group
Next, the protein levels of ATP7A in miR-139 mimics- or miR-139 inhibitor-transfected CAOV3 and SNU119 cells were examined to verify miR-139 regulation of ATP7A. In both CAOV3 and SNU119 cells, ATP7A protein levels were reduced by miR-139 overexpression, whereas increased by miR-139 inhibition (Fig. 3c, d). These data indicated that miR-139 inversely regulates ATP7A expression through direct targeting.
MiR-139 directly binds to the 3′UTR of ATP7B to inversely regulate its expression
Next, the indicated experiments were performed to verify miR-139 regulation of ATP7B. First, the expression of ATP7B was also higher in CAOV3/cDDP and SNU119/cDDP cells than cDDP sensitive cell lines (Fig. S1F). Wild-type ATP7B luciferase reporter gene vectors (named ATP7B-WT-3′UTR) and mutant-type ATP7B luciferase reporter gene vectors (named ATP7B-MUT-3′UTR) containing a 4 bp mutation in the putative miR-139 binding sites were constructed (Fig. 4a). HEK293T cells were then co-transfected with the indicated vectors and miR-139 mimics or miR-139 inhibitor; the luciferase activity was determined using dual luciferase reporter assays. Consistent with ATP7A, the luciferase activity of ATP7B-WT-3′UTR vectors was suppressed by miR-139 overexpression, whereas promoted by miR-139 inhibition; after mutation in either of the predicted miR-139 binding sites, the changes of the luciferase activity were abolished (Fig. 4b).
MiR-139 directly binds to the 3′UTR of ATP7B to inversely regulate its expression. a Wild-type ATP7B luciferase reporter gene vector (named ATP7B-WT-3′UTR) and mutant-type ATP7B luciferase reporter gene vector (named ATP7B-MUT-3′UTR) containing a 4 bp mutation in the predicted miR-139 binding site were constructed. b HEK293T cells were co-transfected with the indicated vectors and miR-139 mimics or miR-139 inhibitor; the luciferase activity was determined using dual luciferase assays. c and d CAOV3 and SNU119 cells were transfected with miR-139 inhibitor or miR-139 mimics; the protein levels of ATP7B were determined using Immunoblotting assays. The data are presented as mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, compared to CAOV3, SNU119, NC mimics or NC inhibitor group
Further, results from Immunoblotting assays indicated that in both CAOV3 and SNU119 cells, ATP7B protein levels were reduced by miR-139 overexpression, whereas increased by miR-139 inhibition (Fig. 4c, d), consistent with ATP7A. These data indicated that miR-139 inversely regulates ATP7B expression through direct targeting.
MiR-139 affects ovarian cancer cell chemoresistance through the regulation of ATP7A/B
MiR-139 can inversely regulate ATP7A/B through direct binding to the 3′UTR of ATP7A/B; next, whether miR-139 affects ovarian cancer chemoresistance through the regulation of ATP7A/B was validated. CAOV3 and SNU119 cells were transfected with si-ATP7A/B to achieve ATP7A/B knockdown, as verified using Immunoblotting assays (Fig. 5a, b). Further, the cell viability of si-ATP7A/B and miR-139 inhibitor-co-transfected CAOV3 and SNU119 cell was determined using MTT assays, under the treatment of a series of doses of cisplatin. After miR-139 inhibition, the cell viability of CAOV3 and SNU119 cells was promoted, expressed as promoted lC50 values (Fig. 5c–f); after either ATP7A knockdown or ATP7B knockdown, the cell viability was suppressed, expressed as reduced lC50 values (Fig. 5c–f); moreover, the promotive effect of miR-139 inhibition on cell viability could be partially reversed by ATP7A/B knockdown (Fig. 5c–f). These data indicated that ATP7A/B can reverse the effect of miR-139 on cisplatin-induced changes of cell viability; miR-139 affects ovarian cancer cell chemoresistance through the regulation of ATP7A/B.
MiR-139 affects ovarian cancer cell chemoresistance through the regulation of ATP7A/B. a and b CAOV3 and SNU119 cells were transfected with si-ATP7A or si-ATP7B, as verified using Immunoblotting assays. The data are presented as mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, compared to NC mimics or NC inhibitor group. c and d CAOV3 cells were co-transfected with si-ATP7A or si-ATP7B and miR-139 inhibitor; the cell viability was determined using MTT assays. Data were displayed as indicated. e and f SNU119 cells were co-transfected with si-ATP7A or si-ATP7B and miR-139 inhibitor; the cell viability was determined using MTT assays. Data were displayed as indicated
The mRNA expression and protein levels of ATP7A/B in tissue samples and the correlation between miR-139 and ATP7A/B
To further confirm the above findings, the mRNA expression and protein levels of ATP7A/B in cisplatin-resistant and cisplatin-sensitive ovarian cancer tissue samples were examined using real-time PCR and Immunoblotting assays. ATP7A/B mRNA expression was remarkably up-regulated in cisplatin-resistant ovarian cancer tissues, compared with that in cisplatin-sensitive ovarian cancer tissue samples (Fig. 6a, b). Moreover, miR-139 expression was negatively correlated with ATP7A/B expression, respectively (Fig. 6c, d). In six randomly selected tissue samples (three resistant and three sensitive), ATP7A/B protein levels were higher in cisplatin-resistant tissues (Fig. 6e).
The mRNA expression and protein levels of ATP7A/B in tissue samples and the correlation between miR-139 and ATP7A/B. a–b The mRNA expression of ATP7A/B in cisplatin-resistant and –sensitive tumor tissue samples was examined using real-time PCR assays. The data are presented as mean ± SD of three independent experiments. **P < 0.01. c–d The correlation between miR-139 and ATP7A/B was analyzed using Spearman’s rank correlation analysis. e The protein levels of ATP7A/B in six randomly selected tumor tissue (three cisplatin-sensitive cases and three cisplatin-resistant cases) samples were examined using Immunoblotting assays
Discussion
In the present study, we demonstrated the detailed role and mechanism of miR-139 regulation of ovarian cancer chemoresistance. MiR-139 expression is down-regulated in cisplatin-resistant ovarian cancer tissues and cell lines; miR-139 overexpression amplifies the suppressive effect of cisplatin on cisplatin-resistant CAOV3/cDDP and SNU119/cDDP cells. Further, the expression of ATP7A/B was up-regulated in cisplatin-resistant ovarian cancer cell lines; miR-139 inversely regulates ATP7A/B expression through direct targeting, and affects ovarian cancer chemoresistance through regulation of ATP7A/B.
Nowadays, chemoresistance of cancer still remains a huge challenge. Chemoresistance mediated by miRNAs in ovarian cancer have been already validated by previous studies [31]. Park et al. showed overexpression of miR-23b, miR-27a, miR-27b, miR-346, miR-424, and miR-503 in chemo-resistant ovarian cancer cells [32]. In human ovarian cancer cells, the inhibition of CD44 by miR-199a reduced the expression of the multidrug resistance gene ABCG2, and thereby increased the chemo-sensitivity of ovarian cancer stem cells [33]. In addition, miR-199a was also implicated in cisplatin resistance by which its inhibition increased mTOR expression and decreased cisplatin-induced apoptosis in vitro [34]. Due to the common use of cisplatin-based chemotherapy, the present study aimed to find novel miRNA which is related to ovarian cancer chemoresistance to cisplatin-based therapy.
According to data from Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/), a large amount of miRNAs are dysregulated in cisplatin-treated ovarian cancer cell lines (GSE93794), including miR-139, which was significantly related to the tumor stage and grade of ovarian cancer. During the recent years, miR-139 dysregulation has been reported to be related with the invasion, metastasis and chemo-resistance of cancers, including colorectal cancer, breast cancer [15,16,17,18, 35]. In the present study, a higher expression level of miR-139 in cisplatin-resistant ovarian cancer tissues and cell lines was observed, indicating that miR-139 expression could be affected by cDDP treatment. Further, by achieving miR-139 overexpression in cisplatin-resistant CAOV3/cDDP and SNU119/cDDP cells, it was confirmed that miR-139 overexpression amplified the suppressive effect of cisplatin on the indicated cisplatin-resistant cells. The underlying mechanism of how miR-139 enhances the cellular effect of cisplatin on resistant ovarian cancer cell lines was hence further investigated.
MiRNAs can regulate post-transcriptional gene expressions and silence a broad set of target genes [36]. Through the regulation of downstream target genes, miRNAs play an important role in modulating gene expressions, thereby regulating downstream signaling pathways and affecting cancer formation and progression [36, 37]. In previous studies, miR-139 overexpression reduced the chemo-resistance and/or enhanced the chemo-sensitivity of cancer cells to docetaxel- or 5-fluorouracil-based chemo-therapy through regulating diverse downstream targets. As we mentioned, miR-139 targets Notch1 or Bcl2 to modulate the chemo-resistance of breast cancer or colorectal cancer [15,16,17,18, 35]. Since we demonstrate that miR-139 overexpression could enhance the suppressive effect of cisplatin on cisplatin-resistant ovarian cancer cells; herein, whether miR-139 exerts the above molecular effect through modulating downstream targets was further investigated.
Reportedly, dysfunction of Copper-transporting ATPases ATP7A and ATP7B can make contribution to dysregulated import or export of platinum derivatives [23, 24]; expression of the copper transporter ATP7B was inhibited by miR-15a and miR-16, resulting in enhanced sensitization of ovarian cancer cells to cisplatin [38]. In cisplatin-resistant ovarian cancer cell CAOV3/cDDP and SNU119/cDDP, ATP7A/B was up-regulated. Since miR-139 overexpression significantly enhances the suppressive effect of cisplatin on cisplatin-resistant ovarian cancer cell lines; herein, whether miR-139 was associated with ATP7A/B was validated. By prediction of Targetscan, the 3′UTR of ATP7A and ATP7B might contain miR-139 binding sites; next, luciferase reporter gene and Immunoblotting assays were employed to confirmed that miR-139 directly binds to the 3′UTR of ATP7A and ATP7B, respectively, to inversely regulate ATP7A and ATP7B expression.
After confirming miR-139 regulation of ATP7A/B, we further investigated whether miR-139 affects the cellular effect of cisplatin on ovarian cancer cell lines through regulating ATP7A/B. Cisplatin-resistant CAOV3/cDDP and SNU119/cDDP cells were transfected with miR-139 inhibitor and si-ATP7A/B under a series of doses of cisplatin treatment; then employed MTT assay to evaluate the detailed functions of miR-139/ATP7A/B. After miR-139 inhibition, the suppressive effect of cisplatin on CAOV3/cDDP and SNU119/cDDP cells was attenuated; on the contrary, either ATP7A or ATP7B knockdown amplified the suppressive effect of cisplatin on cell viability. In addition, the effect of miR-139 inhibition on cisplatin-induced cell viability suppression could be partially reversed by ATP7A and/or ATP7B knockdown. In other word, miR-139 might affect the cellular effect of cisplatin on cisplatin-resistant ovarian cancer cell lines through regulation of ATP7A/B.
As a further confirmation of the above findings, ATP7A/B mRNA expression and protein levels were all up-regulated in cisplatin-resistant tissue samples, compared those in cisplatin-sensitive tissue samples. Moreover, miR-139 expression was negatively correlated with ATP7A/B expression.
Taken together, we demonstrated that miR-139 overexpression could enhance the suppressive effect of cisplatin on ovarian cancer cell lines, and miR-139 may at least partially exerts its biological effect through targeting ATP7A/B. Since miR-139 expression is down-regulated whereas ATP7A/B expression is up-regulated in cisplatin-resistant ovarian cancer tissues, rescuing miR-139 expression thus to inhibit ATP7A/B might contribute to enhancing the cellular effect of cisplatin on ovarian cancer cell lines. However, one limitation of the present study is the lack of mouse model and validation in larger clinical samples, which should be addressed in our future studies.
Moreover, we also searched for other candidate upstream miRNAs that might regulate ATP7A/B expression using Targetscan (shown in Supplementary Table S2). Among the predicted miRNAs, miR-148a, miR-148b and miR-152 have been reported to play a key role in ovarian cancer via modulating cancer cell proliferation [39,40,41], migration [42], invasion [42], as well as chemoresistance to cisplatin [43] via targeting different downstream transcripts. These findings further indicate the potential of miRNA-mRNA network in dealing with cancer cell disorders and chemoresistance, which provides more possibilities for future studies.
References
Webb PM, Jordan SJ (2017) Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol 41:3–14. https://doi.org/10.1016/j.bpobgyn.2016.08.006
Jayde V, White K, Blomfield P (2009) Symptoms and diagnostic delay in ovarian cancer: a summary of the literature. Contemp Nurse 34(1):55–65
Jayde V, Boughton M (2012) The diagnostic journey of ovarian cancer: a review of the literature and suggestions for practice. Contemp Nurse 41(1):5–17. https://doi.org/10.5172/conu.2012.41.1.5
Winner KR, Steinkamp MP, Lee RJ, Swat M, Muller CY, Moses ME, Jiang Y, Wilson BS (2016) Spatial modeling of drug delivery routes for treatment of disseminated ovarian cancer. Cancer Res 76(6):1320–1334. https://doi.org/10.1158/0008-5472.CAN-15-1620
Ween MP, Armstrong MA, Oehler MK, Ricciardelli C (2015) The role of ABC transporters in ovarian cancer progression and chemoresistance. Crit Rev Oncol Hematol 96(2):220–256. https://doi.org/10.1016/j.critrevonc.2015.05.012
Ali AY, Farrand L, Kim JY, Byun S, Suh JY, Lee HJ, Tsang BK (2012) Molecular determinants of ovarian cancer chemoresistance: new insights into an old conundrum. Ann N Y Acad Sci 1271:58–67. https://doi.org/10.1111/j.1749-6632.2012.06734.x
Mihanfar A, Fattahi A, Nejabati HR (2017) MicroRNA-mediated drug resistance in ovarian cancer. J Cell Physiol. https://doi.org/10.1002/jcp.26060
van Jaarsveld MT, Helleman J, Berns EM, Wiemer EA (2010) MicroRNAs in ovarian cancer biology and therapy resistance. Int J Biochem Cell Biol 42(8):1282–1290. https://doi.org/10.1016/j.biocel.2010.01.014
Yang N, Kaur S, Volinia S, Greshock J, Lassus H, Hasegawa K, Liang S, Leminen A, Deng S, Smith L, Johnstone CN, Chen XM, Liu CG, Huang Q, Katsaros D, Calin GA, Weber BL, Butzow R, Croce CM, Coukos G, Zhang L (2008) MicroRNA microarray identifies Let-7i as a novel biomarker and therapeutic target in human epithelial ovarian cancer. Cancer Res 68(24):10307–10314. https://doi.org/10.1158/0008-5472.CAN-08-1954
Cochrane DR, Spoelstra NS, Howe EN, Nordeen SK, Richer JK (2009) MicroRNA-200c mitigates invasiveness and restores sensitivity to microtubule-targeting chemotherapeutic agents. Mol Cancer Ther 8(5):1055–1066. https://doi.org/10.1158/1535-7163.MCT-08-1046
Pal MK, Jaiswar SP, Dwivedi VN, Tripathi AK, Dwivedi A, Sankhwar P (2015) MicroRNA: a new and promising potential biomarker for diagnosis and prognosis of ovarian cancer. Cancer Biol Med 12(4):328–341. https://doi.org/10.7497/j.issn.2095-3941.2015.0024
Hu Y, Deng C, Zhang H, Zhang J, Peng B, Hu C (2017) Long non-coding RNA XIST promotes cell growth and metastasis through regulating miR-139-5p mediated Wnt/beta-catenin signaling pathway in bladder cancer. Oncotarget 8(55):94554–94568. https://doi.org/10.18632/oncotarget.21791
Huang P, Xi J, Liu S (2016) MiR-139-3p induces cell apoptosis and inhibits metastasis of cervical cancer by targeting NOB1. Biomed Pharmacother 83:850–856. https://doi.org/10.1016/j.biopha.2016.07.050
Yue S, Wang L, Zhang H, Min Y, Lou Y, Sun H, Jiang Y, Zhang W, Liang A, Guo Y, Chen P, Lv G, Wang L, Zong Q, Li Y (2015) miR-139-5p suppresses cancer cell migration and invasion through targeting ZEB1 and ZEB2 in GBM. Tumour Biol 36(9):6741–6749. https://doi.org/10.1007/s13277-015-3372-8
Zhang HD, Sun DW, Mao L, Zhang J, Jiang LH, Li J, Wu Y, Ji H, Chen W, Wang J (2015) MiR-139-5p inhibits the biological function of breast cancer cells by targeting Notch1 and mediates chemosensitivity to docetaxel. Biochem Biophys Res Commun 465(4):702–713
Liu H, Yin Y, Hu Y, Feng Y, Bian Z, Yao S, Li M, You Q, Huang Z (2016) miR-139-5p sensitizes colorectal cancer cells to 5-fluorouracil by targeting NOTCH-1. Pathol Res Pract 212(7):643–649
Ke X, Ke S, Xin L, Li Y, Nagao N, Li J, Liu J, Yin P (2016) MiR-139-5p reverses CD44+/CD133+-associated multidrug resistance by downregulating NOTCH1 in colorectal carcinoma cells. Oncotarget 7(46):75118
Li Q, Liang X, Wang Y, Meng X, Xu Y, Cai S, Wang Z, Liu J, Cai G (2016) miR-139-5p inhibits the epithelial-mesenchymal transition and enhances the chemotherapeutic sensitivity of colorectal cancer cells by downregulating BCL2. Sci Rep 6:27157
Sun S, Cai J, Yang Q, Zhao S, Wang Z (2017) The association between copper transporters and the prognosis of cancer patients undergoing chemotherapy: a meta-analysis of literatures and datasets. Oncotarget 8(9):16036–16051. https://doi.org/10.18632/oncotarget.13917
Mangala LS, Zuzel V, Schmandt R, Leshane ES, Halder JB, Armaiz-Pena GN, Spannuth WA, Tanaka T, Shahzad MM, Lin YG, Nick AM, Danes CG, Lee JW, Jennings NB, Vivas-Mejia PE, Wolf JK, Coleman RL, Siddik ZH, Lopez-Berestein G, Lutsenko S, Sood AK (2009) Therapeutic targeting of ATP7B in ovarian carcinoma. Clin Cancer Res 15(11):3770–3780. https://doi.org/10.1158/1078-0432.CCR-08-2306
Kalayda GV, Wagner CH, Buss I, Reedijk J, Jaehde U (2008) Altered localisation of the copper efflux transporters ATP7A and ATP7B associated with cisplatin resistance in human ovarian carcinoma cells. BMC Cancer 8(1):175
Sabbatini G, Baldoni G (2004) Modulation of the cellular pharmacology of cisplatin and its analogs by the copper exporters ATP7A and ATP7B. Mol Pharmacol 66(1):25–32
Safaei R, Holzer AK, Katano K, Samimi G, Howell SB (2004) The role of copper transporters in the development of resistance to Pt drugs. J Inorg Biochem 98(10):1607–1613
Samimi G, Varki NM, Wilczynski S, Safaei R, Alberts DS, Howell SB (2003) Increase in expression of the copper transporter ATP7A during platinum drug-based treatment is associated with poor survival in ovarian cancer patients. Clin Cancer Res 9(16 Pt 1):5853
Nakayama K, Kanzaki A, Terada K, Mutoh M, Ogawa K, Sugiyama T, Takenoshita S, Itoh K, Yaegashi N, Miyazaki K (2004) Prognostic value of the Cu-transporting ATPase in ovarian carcinoma patients receiving cisplatin-based chemotherapy. Clin Cancer Res 10(8):2804–2811
Agarwal V, Bell GW, Nam JW, Bartel DP (2015) Predicting effective microRNA target sites in mammalian mRNAs. Elife. https://doi.org/10.7554/eLife.05005
Current FIGO. staging for cancer of the vagina, fallopian tube, ovary, and gestational trophoblastic neoplasia (2009). Int J Gynaecol Obstet 105 (1):3–4
Scully RE, Sobin LH (1987) Histologic typing of ovarian tumors. Arch Pathol Lab Med 111(9):794–795
Shepherd JH (1989) Revised FIGO staging for gynaecological cancer. Br J Obstet Gynaecol 96(8):889–892
Zhu X, Shen H, Yin X, Long L, Chen X, Feng F, Liu Y, Zhao P, Xu Y, Li M, Xu W, Li Y (2017) IL-6R/STAT3/miR-204 feedback loop contributes to cisplatin resistance of epithelial ovarian cancer cells. Oncotarget 8(24):39154–39166. https://doi.org/10.18632/oncotarget.16610
Wang Y, Kim S, Kim IM (2014) Regulation of metastasis by microRNAs in ovarian cancer. Front Oncol 4:143. https://doi.org/10.3389/fonc.2014.00143
Park YT, Jeong JY, Lee MJ, Kim KI, Kim TH, Kwon YD, Lee C, Kim OJ, An HJ (2013) MicroRNAs overexpressed in ovarian ALDH1-positive cells are associated with chemoresistance. J Ovarian Res 6(1):18. https://doi.org/10.1186/1757-2215-6-18
Cheng W, Liu T, Wan X, Gao Y, Wang H (2012) MicroRNA-199a targets CD44 to suppress the tumorigenicity and multidrug resistance of ovarian cancer-initiating cells. FEBS J 279(11):2047–2059. https://doi.org/10.1111/j.1742-4658.2012.08589.x
Wang Z, Ting Z, Li Y, Chen G, Lu Y, Hao X (2013) microRNA-199a is able to reverse cisplatin resistance in human ovarian cancer cells through the inhibition of mammalian target of rapamycin. Oncol Lett 6(3):789–794. https://doi.org/10.3892/ol.2013.1448
Chen X, Lu P, Wang DD, Yang SJ, Wu Y, Shen HY, Zhong SL, Zhao JH, Tang JH (2016) The role of miRNAs in drug resistance and prognosis of breast cancer formalin-fixed paraffin-embedded tissues. Gene 595(2):221–226. https://doi.org/10.1016/j.gene.2016.10.015
Chen Y, Gao DY, Huang L (2015) In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv Drug Deliv Rev 81:128–141. https://doi.org/10.1016/j.addr.2014.05.009
Aigner A (2011) MicroRNAs (miRNAs) in cancer invasion and metastasis: therapeutic approaches based on metastasis-related miRNAs. J Mol Med (Berl) 89(5):445–457. https://doi.org/10.1007/s00109-010-0716-0
Dwivedi SK, Mustafi SB, Mangala LS, Jiang D, Pradeep S, Rodriguez-Aguayo C, Ling H, Ivan C, Mukherjee P, Calin GA, Lopez-Berestein G, Sood AK, Bhattacharya R (2016) Therapeutic evaluation of microRNA-15a and microRNA-16 in ovarian cancer. Oncotarget 7(12):15093–15104. https://doi.org/10.18632/oncotarget.7618
Zhao M, Su Z, Zhang S, Zhuang L, Xie Y, Li X (2016) Suppressive role of MicroRNA-148a in cell proliferation and invasion in ovarian cancer through targeting transforming growth factor-beta-induced 2. Oncol Res 24(5):353–360. https://doi.org/10.3727/096504016X14685034103275
Zhao S, Wen Z, Liu S, Liu Y, Li X, Ge Y, Li S (2015) MicroRNA-148a inhibits the proliferation and promotes the paclitaxel-induced apoptosis of ovarian cancer cells by targeting PDIA3. Mol Med Rep 12(3):3923–3929. https://doi.org/10.3892/mmr.2015.3826
Zhou X, Zhao F, Wang ZN, Song YX, Chang H, Chiang Y, Xu HM (2012) Altered expression of miR-152 and miR-148a in ovarian cancer is related to cell proliferation. Oncol Rep 27(2):447–454. https://doi.org/10.3892/or.2011.1482
Wen Z, Zhao S, Liu S, Liu Y, Li X, Li S (2015) MicroRNA-148a inhibits migration and invasion of ovarian cancer cells via targeting sphingosine-1-phosphate receptor 1. Mol Med Rep 12(3):3775–3780. https://doi.org/10.3892/mmr.2015.3827
Benson EA, Skaar TC, Liu Y, Nephew KP, Matei D (2015) Carboplatin with decitabine therapy, in recurrent platinum resistant ovarian cancer, alters circulating miRNAs concentrations: A Pilot Study. PLoS One 10(10):e0141279. https://doi.org/10.1371/journal.pone.0141279
Author information
Authors and Affiliations
Contributions
Fang Xiao designed and performed the experiments, wrote the manuscript. Fang Xiao and Yueran Li have contributed to experimental work and data analysis. Yajun Wan and Min Xue conducted the experiments and revised the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Author Fang Xiao declares that she has no conflict of interest. Author Yueran Li declares that she has no conflict of interest. Author Yajun Wan declares that she has no conflict of interest. Author Min Xue declares that she has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
280_2018_3548_MOESM1_ESM.tif
Fig. S1 Transfection efficiency and expression level confirmation (A-B) miR-139 mimics was transfected into CAOV3 and SNU119 cells to achieve ectopic miR-139 expression, as verified using real-time PCR assays. (C) The expression of ATP7A in CAOV3, SNU119, CAOV3/cDDP and SNU119/cDDP cells was determined by Immunoblotting. (D) The expression of miR-139 in CAOV3, SNU119, CAOV3/cDDP and SNU119/cDDP cells was determined by RT-PCR. (E) CAOV3 and SNU119 cells were transfected with miR-139 inhibitor to achieve miR-139 inhibition, as verified using real-time PCR assays. (F) The expression of ATP7B in CAOV3, SNU119, CAOV3/cDDP and SNU119/cDDP cells was determined by Immunoblotting. (TIF 773 KB)
Rights and permissions
About this article
Cite this article
Xiao, F., Li, Y., Wan, Y. et al. MircroRNA-139 sensitizes ovarian cancer cell to cisplatin-based chemotherapy through regulation of ATP7A/B. Cancer Chemother Pharmacol 81, 935–947 (2018). https://doi.org/10.1007/s00280-018-3548-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3548-1