Abstract
Several studies have investigated the effects of polymorphisms in the GSTP1, GSTT1, and GSTM1 genes on responsiveness to chemotherapy in breast cancer, but the results have been inconsistent. The aim of this study was to determine the association between polymorphisms of GSTP1, GSTT1, and GSTM1 genes and response to chemotherapy in patients with breast cancer. The relevant studies were retrieved from PubMed, Embase, ISI Web of Knowledge, China National Knowledge Infrastructure, and Wanfang databases. The articles evaluating the correlations between response to chemotherapy and GSTP1, GSTT1, and GSTM1 polymorphisms in breast cancer patients were comprehensively reviewed. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated to measure the strength of the associations. These associations were assessed with the χ 2 test in this meta-analysis. Subgroup analysis by chemotherapy protocol and ethnicity were conducted to explore the source of heterogeneity among studies. A total of 14 articles with 31 studies involving GSTP1, GSTT1, and GSTM1 polymorphisms with response to chemotherapy were identified in the final meta-analysis. In the overall analysis, a significant association of GSTM1-present/GSTM1-null polymorphism with responsiveness to chemotherapy was observed in breast cancer patients (OR 0.74, CI 0.60–0.92, P = 0.006), whereas the GSTT1-present/GSTT1-null and GSTP1rs1695 polymorphisms were not significantly associated with clinical response to chemotherapy. The subgroup analysis by chemotherapy protocol indicated that the patients who harboring GSTP1rs1695 AA or AG variant had a higher response rate to anthracycline-based chemotherapy than those carrying GSTP1rs1695 GG variant [AA vs. GG: OR 0.48, CI 0.29–0.80, P < 0.05; AA vs. AG: OR 0.60, CI 0.43–0.83, P < 0.05; A vs. G: OR 0.60, CI 0.47–0.77, P < 0.05; AA vs. (AG + GG): OR 0.56, CI 0.42–0.76, P < 0.05; (AA + AG) vs. GG: OR 0.57, CI 0.34–0.94, P < 0.05]. In addition, the heterogeneity existed among studies for GSTP1 polymorphism, while no obvious heterogeneity was detected for GSTT1 and GSTM1 polymorphisms. And the heterogeneity present in different studies, evaluating the association of GSTP1 polymorphism with response to anthracycline-based chemotherapy, disappeared in breast cancer patients after subgroup analysis by chemotherapy regimen was performed. In conclusion, this meta-analysis suggested that GSTP1rs1695 and GSTM1-present/GSTM1-null polymorphisms could be considered as reliable predictors of response to anthracycline-based chemotherapy in patients with breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common cancer in women worldwide, and 1.68 million new patients occured all over the world in 2012 [1, 2]. Over 14% of female death is due to breast cancer every year, which is the leading cause of cancer-related deaths in women [3, 4]. Radiotherapy, chemotherapy, targeted therapy, and hormone therapy are the common therapeutic methods of breast cancer. Chemotherapy is often used as a therapeutic strategy before or after surgical resection of breast cancer. And chemotherapy is usually the most useful treatment to suppress cancer cell growth and division when metastasis happens in breast cancer patients. Chemotherapy will be utilized as an adjuvant treatment (ATC) after primary surgery in early stage of cancer. When this treatment is used in patients with locally advanced breast cancer or large operable breast tumors before surgery, it is called neoadjuvant chemotherapy (NACT) [5]. In addition, the combined use of drug therapy will be more effective than a single drug in the process of breast cancer chemotherapy [6]. Anthracycline is one of the most effective cytotoxic agents, and therefore, the anthracycline-based chemotherapy regimens is usually considered as mainly therapeutic protocol for patients with breast cancer [7]. Anthracycline contains doxorubicin and epirubicin, which are often applied in the chemotherapy of breast cancer. Furthermore, cyclophosphamide, 5-fluorouracil, and paclitaxel are also used in the therapy of breast cancer [8, 9]. However, responsiveness to chemotherapy is variable in individual and cannot be predicted for breast cancer patients. Previous studies have found breast cancer patients had a higher susceptibility to drug resistance, and these chemotherapy drugs often had a greater risk of side effects [10]. Consequently, the predictors of response to chemotherapy would be critical to individualizing treatment.
Genetic variations in drug metabolizing enzymes have been conformed to be associated with the response to chemotherapy [11]. Glutathione S-transferases (GSTs) is a superfamily of multifunctional enzymes involving in the detoxification of exogenous and endogenous reactive components [12]. Furthermore, GSTs, consisting of six members: GSTT1 (theta), GSTP1 (pi), GSTM1 (mu), GSTA (alpha), GSTK1 (kappa), and GST (sigma) in human, can detoxify chemotherapy drugs or their metabolites by catalyzing the conjugation of mutagenic electrophilic substrates to glutathione [13]. Particularly, GSTT1 (theta), GSTP1 (pi), GSTM1 (mu), and GSTA (alpha) enzymes could protect numerous molecules from reactive oxidant damage [14]. It is reported that GSTP1 rs1695 (313A>G) which converts isoleucine 105 (Ile) to valine (Val) could lead to a low specific activity of GSTP1 enzyme [15]. Specifically, deletion polymorphisms of GSTM1 and GSTT1 genes were associated with reduced enzyme activity [16]. Recent studies have found that the genetic polymorphisms of GSTP1, GSTM1, and GSTT1 genes provided a stronger scientific basis for response to chemotherapy [17]. At the same time, several in vitro studies have also observed that the expression of GSTP1, GSTM1, and GSTT1 genes had a significant association with resistance to chemotherapy [18, 19]. However, the results of GSTP1, GSTM1, and GSTT1 polymorphisms with responsiveness to chemotherapy in breast cancer were still inconclusive, probably because the sample size of each study included was so small that it lacked sufficient evidence to demonstrate the comprehensive conclusion. Nevertheless, meta-analysis could synthesize information from various investigations on the same issue to provide a reliable result concerning the correlation between GSTP1, GSTM1, and GSTT1 polymorphisms and response to chemotherapy in breast cancer.
Therefore, we conducted a meta-analysis to determine the potential role of GSTP1, GSTM1, and GSTT1 polymorphisms in predicting response to chemotherapy in breast cancer patients. Moreover, a subgroup analysis based on chemotherapy regimens was also performed, to investigate whether SNPs in the GSTP1, GSTM1, and GSTT1 genes could work as biomarkers to predict the outcomes of the responsiveness to anthracycline-based chemotherapies for breast cancer. As far as we know, this is the first meta-analysis to investigate the associations of GSTP1, GSTM1, and GSTT1 polymorphisms with responsiveness to chemotherapy in breast cancer patients.
Materials and methods
Searching strategy
PubMed, Embase, ISI Web of Knowledge, China National Knowledge Infrastructure (CNKI), and Wanfang database were comprehensively searched. The latest retrieval was updated in May 2016 with the following items: “Breast Neoplasms, Breast cancer, glutathione S-transferase T1, glutathione S-transferase M1, Glutathione S-Transferase pi, GSTP1, GSTT1, GSTM1, polymorphism, chemotherapy.” The following retrieval schemes were used on the PubMed database: “(Breast Neoplasms [Mesh] OR Breast cancer) AND (glutathione S-transferase T1 [Mesh] OR glutathione S-transferase M1 [Mesh] OR Glutathione S-Transferase pi [Mesh] OR GSTP1 OR GSTT1OR GSTM1).” In addition, “(Breast Neoplasms [Mesh] OR Breast cancer) AND chemotherapy” was also searched to find relevant articles. After the title and abstract screening, all the retrieved articles were screened by reading full text to assess their eligibility for the meta-analysis. Only studies conducted in humans were used in this meta-analysis. Reviews and references of included studies were searched for additional relevant studies.
Inclusion and exclusion criteria
Included studies in the meta-analysis had to fulfill the following criteria: (1) studies investigating the correlation between GSTP1, GSTM1, and GSTT1 polymorphisms and responsiveness to chemotherapy in breast cancer patients; (2) prospective or retrospective association studies evaluating the response to chemotherapy; (3) studies providing detailed data to response and non-response rate in different genotypes of GSTP1, GSTM1, and GSTT1. The exclusion criteria were as follows: (1) reviews and meta-analysis; (2) the article not involving the response rate of chemotherapy in breast cancer patients; (3) basic studies concerning animals and breast cancer cell lines; (4) studies lacking critical genotype information; (5) studies only investigating breast cancer susceptibility, progression, severity or survival.
Data extraction
Two investigators independently extracted the data from each included report. Discrepancies were settled by discussion with the research team. The following information was collected from each eligible study: first author’s name, publication year, country of studied population, ethnicity, chemotherapy protocol, genotype method, genotype frequency, number of patients and evaluation criterion of response to chemotherapy. To reduce heterogeneity, studies were classified, according to different population, into the following subgroups: Asians and Caucasians.
Statistical analysis
The odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated to evaluate the effects of GSTP1, GSTM1 and GSTT1 polymorphisms on response to chemotherapy in breast cancer, using Stata software (version 12.0, College Station, TX, USA). At the same time, the allele model, homozygous model, recessive model, dominant model, and codominant model were applied to assess the association between responsiveness of chemotherapy and GSTP1, GSTM1, and GSTT1 polymorphisms. Heterogeneity often occurs in a meta-analysis on account of many factors, for example, environmental factors, age, sample size, chemotherapy protocol, and ethnicity. In the meta-analysis, heterogeneity was assessed with Chi-square test-based Q test and I 2 statistic [20, 21]. The random effects model would be applied to compute a pooled estimate of the ORs from each study in case of heterogeneity existing (P > 0.05, I 2 < 50%), while the fixed effects model would be used if heterogeneity did not exist among studies (P < 0.05, I 2 > 50%) [22, 23]. The forest plots were drew to intuitively display whether the heterogeneity existed. Moreover, stratified analysis was carried out to investigate the influences of chemotherapy protocol and ethnicity in heterogeneity among studies. Funnel plots were carried out to estimate the potential publication bias with Begg’s test and Egger’s test [24]. Sensitivity analysis was performed to observe the stable of results and identify the effects of individual study on the pooled results by omitting each study. In this review, the Response Evaluation Criteria in Solid Tumors (RECIST) was applied to define the response to chemotherapy in breast cancer patients [25]. Complete responders (CR) and partial responders (PR) were considered as good response to chemotherapy, while stable disease (SD) and progressive disease (PD) were classified as non-response. The distribution difference for (CR + PR) versus (SD + PD) in various genotypes was evaluated with χ 2 test.
Results
Characteristics of eligible studies
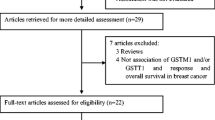
A total of 14 published articles with 31 studies were identified eligible for the systematic review according to the selection criteria. In the initial searching, 249 articles were retrieved from electronic databases. An additional 14 articles were excluded because they were duplicate, and 235 articles were remained. Forty articles concerning the correlation of the GSTP1, GSTM1, and GSTT1 polymorphisms with chemosensitivity to breast cancer were retained after reading titles and abstracts. Meanwhile, 21 articles were assessed for eligibility after scanning full text. Finally, 14 articles met the inclusion criteria were included in the meta-analysis after 7 articles which lacked detailed genotype information were removed [5, 9, 26–37]. Of these studies, 11 studies with 1727 breast cancer patients reported the association of response to chemotherapy with GSTP1 polymorphism, 9 studies with 1317 breast cancer patients were for GSTT1 polymorphism and 11 studies with 1468 breast cancer patients were for GSTM1 polymorphism. The process of literature searching and screening is shown in Fig. 1. And the characteristics of included studies are provided in Tables 1, 2 and 3. In addition, the detailed chemotherapy protocol or drugs used of breast cancer patients included in the meta-analysis were as follows: taxol and doxorubicin (TA), taxol, doxorubicin, and cyclophosphamide (TAC), cyclophosphamide, doxorubicin, and 5-fluorouracil (CAF), cyclophosphamide, epirubicin, and 5-fluorouracil (CEF), cyclophosphamide, intravenous methotrexate, and 5-fluorouracil (CMF), doxorubicin/docetaxel (A/D), doxorubicin/epirubicin (A/E), taxol and xeloda (TX), docetaxel and doxorubicin (DA), cyclophosphamide, taxol, and xeloda (CTX). Furthermore, no meta-analysis involving the association of GSTP1, GSTM1, and GSTT1 polymorphisms with the responsiveness to chemotherapy in breast cancer patients was found in literature searching (Tables 1, 2, 3; Fig. 1).
Meta-analysis results
Association of GSTP1rs1695 polymorphism with response to chemotherapy in breast cancer patients
Eleven studies, investigating the association between GSTP1rs1695 polymorphism and response rate of chemotherapy in breast cancer patients, were included in the pooled analysis. In the total analysis, GSTP1rs1695 polymorphism did not have a significant effect on the response to chemotherapy in breast cancer patients. However, heterogeneity present in different studies was detected using Chi-square test-based on Q test (P < 0.05) and I 2 statistic (I 2 > 50%). Thus, subgroup analysis-based on ethnicity and chemotherapy protocol were conducted to eliminate the heterogeneity (Fig. 2). The results indicated GSTP1rs1695 polymorphism significantly was significantly associated with the responsiveness to anthracycline-based chemotherapy [A vs. G: OR 0.60, CI 0.47–077, P < 0.05; AA vs. GG: OR 0.48, CI 0.29–0.80, P < 0.05; AA vs. (AG + GG): OR 0.56, CI 0.42–0.76, P < 0.05; (AA + AG) vs. GG: OR 0.57, CI 0.34–0.94, P < 0.05; AA vs. AG: OR 0.60, CI 0.43–0.83, P < 0.05]. The breast cancer patients carried A allele of GSTP1rs1695 had a better response to anthracycline-based chemotherapy than patients carried G allele (A vs. G OR 0.60, CI 0.47–0.77, P < 0.05). And there was no significant correlation of GSTP1 polymorphism with responsiveness to chemotherapy in Asians and Caucasians (Table 4; Fig. 3).
Association of GSTM1 polymorphism with response to chemotherapy in breast cancer patients
The literature retrieval identified eleven studies which explored the association of response to chemotherapy with the GSTM1 polymorphism in breast cancer patients. In total, there was a significant association between GSTM1 polymorphism and the responsiveness to chemotherapy (OR 0.74, CI 0.60–0.92, P = 0006). The distribution of response rate to chemotherapy between GSTM1-present and GSTM1-null genotype really had a significant difference. No heterogeneity among studies was observed among studies for GSTM1 polymorphism. The results of subgroup analysis-based on chemotherapy protocol indicated that heterogeneity was decreased obviously. And the GSTM1 polymorphism had a significant effect on response to anthracycline-based chemotherapy in breast cancer patients (OR 0.61, CI 0.45–0.82, P = 0.001). In addition, the significant correlation was found in Caucasians (OR 0.75, CI 0.58–0.97, P = 0.03), but not in Asians. But two studies involving in Caucasians were too few to demonstrate the conclusion. In the cumulative analysis, the results indicated the breast cancer patients of GSTM1-null genotype had a poor effect on responsiveness to chemotherapy, especially in Caucasians (Table 5; Fig. 3).
Association of GSTT1 polymorphism with response to chemotherapy in breast cancer patients
Nine studies were included for GSTT1 polymorphism in this meta-analysis. In the overall analysis, we have found that no significant association between GSTT1 polymorphism and response to chemotherapy or anthracycline-based chemotherapy existed (total: OR 0.88, CI 0.69–1.10, P = 0.26; for anthracycline: OR 0.94, CI 0.67–1.32, P = 0.72). No evidence of heterogeneity was detected among studies for GSTT1 polymorphism. And the distribution of response rate to chemotherapy between GSTT1-present genotype and GSTT1-null genotype did not have a significant discrepancy (Table 5; Fig. 2).
Publication bias and sensitivity analysis
The funnel plots were drawn and symmetrical, while Egger’s test did not indicate significant publication bias for GSTT1 polymorphism (Begg’s test, z = 0.63. P = 0.53; Egger’s test, t = −0.34, P = 0.75). The data displayed that there might be small publication bias for the GSTP1 and GSTM1 polymorphisms (for GSTM1, Begg’s test, z = −1.17. P = 0.24; Egger’s test, t = −2.49, P = 0.03; for GSTP1, AA vs. GG, Begg’s test, z = −0.99. P = 0.32; Egger’s test, t = −3.63, P = 0.01). In subgroup analysis of anthracycline-based chemotherapy, the publication bias disappeared (for GSTM1, Begg’s test, z = −0.74. P = 0.46; Egger’s test, t = −1.27, P = 0.25; for GSTP1, AA vs. GG, Begg’s test, z = −0.94. P = 0.35; Egger’s test, t = −2.77, P = 0.05). The sensitivity analysis showed that the results were statistically robust since the corresponding combined ORs were relatively stable by removing each study (Figs. 4, 5).
Discussion
In the present study, we searched the relevant researches and performed the statistical analysis to test the hypothesis that the responsiveness to chemotherapy is dependent on genetic polymorphism of GSTP1, GSTM1, and GSTT1 genes. Overall, we have demonstrated that carriers of GG genotype of GSTP1rs1695 had a poor response to anthracycline-based chemotherapy than AA and AG genotypes in breast cancer patients. In other genetic models, similar results were also detected for GSTP1 polymorphism. The GSTM1-present genotype could increase the strength of response to chemotherapy compared with GSTM1-null genotype. However, significant association for GSTM1 polymorphism was only observed in Asians, but not in Caucasians. It is well known that the allelic distribution of metabolic genes was inconsistent throughout human populations and the allele frequencies of GSTM1-null/GSTM1-present variation might follow the diverse ethnic or geographic patterns [38]. No underlying association between GSTT1 polymorphism and responsiveness to chemotherapy was detected in the overall analysis and stratified analysis. The subgroup analysis-based on chemotherapy regimens presented that chemotherapy drug had a great influence in the association of GSTP1rs1695 and GSTM1-null/GSTM1-present polymorphisms with responsiveness to anthracycline-based chemotherapy. These data in the meta-analysis displayed that A allele of GSTP1rs1695 and GSTM1-present genotype might be considered as biomarkers of good response to anthracycline-based chemotherapy in breast cancer patients.
Anthracyclines had severe side effects like cardiovascular toxicity and bone marrow dysfunction [39]. Hence, the identification of biomarkers predicting the response to anthracycline-based chemotherapy in breast cancer patients was quite necessary. Anthracyclines could result in DNA damage, mitochondrial membrane disruption and occurring of apoptotic cascade, which displayed its antineoplastic effects [40]. Further, manganese catalase (CAT), superoxide dismutase (MnSOD), and glutathione-Stransferases (GSTs) involved in ROS neutralizing pathways might have a great effect on these processes [41]. GSTs could participate in the metabolism of DNA synthesis and regulate the cellular response to oxidative stress [28]. When DNA was damaged by reactive oxidant, GSTs would be activated by numerous products. For instance, GSTP1 gene was reported to be involved in anthracycline detoxification and had a high expression in breast cancer tissues [42, 43]. Although GSTs contained several genes which cantain a lot of polymorphic locus, the studies of GSTP1, GSTM1, and GSTT1 genes have found that functional polymorphisms existed in the three genes. From these results, GSTP1rs1695 and GSTM1-present/GSTM1-null polymorphisms might be specific molecular markers for clinical response to anthracycline-based chemotherapy in patients with breast cancer.
In the included studies for GSTP1 polymorphism, Tang et al. have investigated the association of GSTP1rs1695 polymorphism with the response to chemotherapy and the chemotherapy regimens adopted in breast cancer patients were TA, TAC or CAF. The study of Tang et al. [26] observed that anthracycline-based chemotherapy had a better treatment effect on the A allele carriers of GSTP1 gene than C allele carriers. Other three studies also displayed the significant association of GSTP1rs1695 polymorphism with responsiveness to anthracycline-based chemotherapy in Chinese populations [28, 32, 34]. Two studies for GSTP1 polymorphism were selected in Caucasians, and no significant effects were found in breast cancer patients [5, 30]. There were two researches that studied population were not clearly reported and we considered the studied population as mixed population [27, 33]. Moreover, the results of five studies indicated that GSTP1rs1695 polymorphism was not related to response to chemotherapy [5, 27, 29–31]. For GSTT1-present/GSTT1-null polymorphism, one study described that GSTP1 polymorphism was significantly associated with responsiveness to chemotherapy [35]. For GSTM1 polymorphism, Tang et al. and Zhong et al. have detected a significant correlation between response rate to chemotherapy and GSTM1-present/GSTM1-null polymorphism [26, 28]. In addition, eight studies that adopted the anthracycline-based chemotherapy regimens for GSTM1 polymorphism were included in this meta-analysis [9, 26–29, 31, 36, 37]. From these studies, anthracycline-based chemotherapy was a common chemotherapy strategy both in Asians and Caucasians. But these studies were limited by the sample size, chemotherapy regimens and ethnicity. So the meta-analysis was a powerful tool by extracting and analyzing these data to obtain a more convincing and accurate conclusion.
Heterogeneity should be emphasized in this meta-analysis. No apparent between-study heterogeneity among studies involving GSTM1 and GSTT1 polymorphisms was detected. Although heterogeneity present in different studies concerning GSTP1 polymorphism was found, subgroup analysis showed chemotherapy strategy was the mainly cause of heterogeneity. And heterogeneity disappeared in the subgroup analysis-based on chemotherapy regimens of GSTP1 polymorphism. Ethnicity was not the leading cause of heterogeneity for GSTP1 polymorphism because heterogeneity still existed in the subgroup analysis-based on ethnicity. The sample size of included studies did not have a big difference, so we did not carry out the subgroup analysis-based on sample size. Moreover, the sample was all breast cancer patients and therefore the Hardy–Weinberg equilibrium (HWE) was not tested in the studied population. And sensitivity analysis displayed that the results were stable.
On the other hand, limitations of this meta-analysis should be noted. Firstly, the number of studies which involved single chemotherapy regimens was too few to precisely assess the role of GSTP1, GSTT1, and GSTM1 polymorphisms in responsiveness to chemotherapy. Secondly, the number of large-sized studies was limited. As a consequence, the sample size might lead to false negative results in some included studies. Third, the studies of Caucasians were so few that only two studies in Caucasians were included in this review. Therefore, large-scale studies with sufficient clinical information in Caucasians were still needed to demonstrate the conclusion. Finally, this meta-analysis did not extract the clinical information, environmental factors, and other genes information. These factors might affect the response to chemotherapy.
Conclusion
In summary, GSTP1rs1695 and GSTM1-present/GSTM1-null polymorphisms might be considered as reliable predictors of clinical response to anthracycline-based chemotherapy in breast cancer patients. However, studies that focused on gene–gene and gene–environment interactions were expected to be conducted to accurately establish the predictive values of GSTP1rs1695 and GSTM1-present/GSTM1-null polymorphisms in anthracycline-treated patients of breast cancer.
References
Jemal A, Siegel R, Ward E et al (2009) Cancer statistics. CA Cancer J Clin 59(4):225–249
Ferlay J, Soerjomataram I, Dikshit R et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386
Parkin DM, Bray F, Ferlay J et al (2005) Global cancer statistics, 2002. CA Cancer J Clin 55(2):74–108
Ibrahim NI, Dahlui M, Aina EN et al (2012) Who are the breast cancer survivors in Malaysia? Asian Pac J Cancer Prev 13(5):2213–2218
Yao S, Barlow WE, Albain KS et al (2010) Gene polymorphisms in cyclophosphamide metabolism pathway, treatment-related toxicity, and disease-free survival in SWOG 8897 clinical trial for breast cancer. Clin Cancer Res 16(24):6169–6176
Zurrida S, Veronesi U (2015) Milestones in breast cancer treatment. Breast J 21(1):3–12
Roche H, Fumoleau P, Spielmann M et al (2006) Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 Trial. J Clin Oncol 24(36):5664–5671
Afsar NA, Haenisch S, Mateen A et al (2010) Genotype frequencies of selected drug metabolizing enzymes and ABC drug transporters among breast cancer patients on FAC chemotherapy. Basic Clin Pharmacol Toxicol 107(1):570–576
Ji M, Tang J, Zhao J et al (2012) Polymorphisms in genes involved in drug detoxification and clinical outcomes of anthracycline-based neoadjuvant chemotherapy in Chinese Han breast cancer patients. Cancer Biol Ther 13(5):264–271
Davidson A, Gelmon K (2011) Do anthracyclines still have a role in adjuvant chemotherapy of breast cancer? Future Oncol 7(1):37–55
Huang MY, Wang YH, Chen FM et al (2008) Multiple genetic polymorphisms of GSTP1 313AG, MDR1 3435CC, and MTHFR 677CC highly correlated with early relapse of breast cancer patients in Taiwan. Ann Surg Oncol 15(3):872–880
Armstrong RN (1997) Structure, catalytic mechanism, and evolution of the glutathione transferases. Chem Res Toxicol 10(1):2–18
Zhong S, Huang M, Yang X et al (2006) Relationship of glutathione S-transferase genotypes with side-effects of pulsed cyclophosphamide therapy in patients with systemic lupus erythematosus. Br J Clin Pharmacol 62(4):457–472
Hayes JD, McLellan LI (1999) Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res 31(4):273–300
Srivastava SK, Singhal SS, Hu X et al (1999) Differential catalytic efficiency of allelic variants of human glutathione S-transferase Pi in catalyzing the glutathione conjugation of thiotepa. Arch Biochem Biophys 366(1):89–94
Townsend DM, Tew KD (2003) The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene 22(47):7369–7375
Hirshfield KM, Rebbeck TR, Levine AJ (2010) Germline mutations and polymorphisms in the origins of cancers in women. J Oncol 2010:297671
Lourenco GJ, Lorand-Metze I, Delamain MT et al (2010) Polymorphisms of glutathione S-transferase mu 1, theta 1, and pi 1 genes and prognosis in Hodgkin lymphoma. Leuk Lymphoma 51(12):2215–2221
Satta T, Isobe K, Yamauchi M et al (1992) Expression of MDR1 and glutathione S transferase-pi genes and chemosensitivities in human gastrointestinal cancer. Cancer Am Cancer Soc 69(4):941–946
Higgins JP, Thompson SG, Deeks JJ et al (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22(4):719–748
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Egger M, Davey SG, Schneider M et al (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Jinhai T, Jianhua Z, Jianzhong W et al (2009) Establishment of a multiplex ligation-dependent SNP genotyping method and its application in the detection of genes related to chemotherapeutic drugs in breast cancer. Chin J Oncol 31(2):0253–3766
Oliveira AL, Rodrigues FF, Santos RE et al (2010) GSTT1, GSTM1, and GSTP1 polymorphisms and chemotherapy response in locally advanced breast cancer. Genet Mol Res 9(2):1045–1053
Shanliang Zhong, Shenggao Jiang, Jinhai Tang et al (2010) Association of GSTs gene polymorphism with response to chemotherapy in breast cancer. Chin J Clin Lab Sci 28(6):438–440
Bai YL, Zhou B, Jing XY et al (2012) Predictive role of GSTs on the prognosis of breast cancer patients with neoadjuvant chemotherapy. Asian Pac J Cancer Prev 13(10):5019–5022
Romero A, Martin M, Oliva B et al (2012) Glutathione S-transferase P1 c.313A>G polymorphism could be useful in the prediction of doxorubicin response in breast cancer patients. Ann Oncol 23(7):1750–1756
Tulsyan S, Chaturvedi P, Agarwal G et al (2013) Pharmacogenetic influence of GST polymorphisms on anthracycline-based chemotherapy responses and toxicity in breast cancer patients: a multi-analytical approach. Mol Diagn Ther 17(6):371–379
Xinlan L, Yanjiao Z, Min J et al (2013) Association of polymorphisms of GSTP1 (rs1695) with the efficacy of paclitaxel/anthracycline—based chemotherapy in stage breast cancer. Acad J Second Mil Med Univ 34(8):0879–0884
Islam MS, Islam MS, Parvin S et al (2015) Effect of GSTP1 and ABCC4 gene polymorphisms on response and toxicity of cyclophosphamide-epirubicin-5-fluorouracil-based chemotherapy in Bangladeshi breast cancer patients. Tumour Biol 36(7):5451–5457
Zhou L, Huang A, Zhang D et al (2015) Genetic variability of glutathione S-transferases influences treatment outcome of breast cancer. Tumour Biol 36(8):5925–5929
Khedhaier A, Remadi S, Corbex M et al (2003) Glutathione S-transferases (GSTT1 and GSTM1) gene deletions in Tunisians: susceptibility and prognostic implications in breast carcinoma. Br J Cancer 89(8):1502–1507
Mishra A, Chandra R, Mehrotra PK et al (2011) Glutathione S-transferase M1 and T1 polymorphism and response to neoadjuvant chemotherapy (CAF) in breast cancer patients. Surg Today 41(4):471–476
Saadat M, Khalili M, Nasiri M et al (2012) Clinical response to chemotherapy in locally advanced breast cancer was not associated with several polymorphisms in detoxification enzymes and DNA repair genes. Biochem Biophys Res Commun 419(1):117–119
Sivonova M, Waczulikova I, Dobrota D et al (2009) Polymorphisms of glutathione-S-transferase M1, T1, P1 and the risk of prostate cancer: a case-control study. J Exp Clin Cancer Res 28:32
Hershman D, Neugut AI, Jacobson JS et al (2007) Acute myeloid leukemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J Natl Cancer Inst 99(3):196–205
Ambrosone CB, Ahn J, Singh KK et al (2005) Polymorphisms in genes related to oxidative stress (MPO, MnSOD, CAT) and survival after treatment for breast cancer. Cancer Res 65(3):1105–1111
Bewick MA, Conlon MS, Lafrenie RM (2008) Polymorphisms in manganese superoxide dismutase, myeloperoxidase and glutathione-S-transferase and survival after treatment for metastatic breast cancer. Breast Cancer Res Treat 111(1):93–101
Sau A, Pellizzari TF, Valentino F et al (2010) Glutathione transferases and development of new principles to overcome drug resistance. Arch Biochem Biophys 500(2):116–122
Arun BK, Granville LA, Yin G et al (2010) Glutathione-s-transferase-pi expression in early breast cancer: association with outcome and response to chemotherapy. Cancer Invest 28(5):554–559
Acknowledgements
We would like to acknowledge all the participants in the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Kong, X., Li, Z. & Li, X. GSTP1, GSTM1, and GSTT1 polymorphisms as predictors of response to chemotherapy in patients with breast cancer: a meta-analysis. Cancer Chemother Pharmacol 78, 1163–1173 (2016). https://doi.org/10.1007/s00280-016-3173-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3173-9