Abstract
Purpose
The purpose of this analysis was to develop a population pharmacokinetic (PK) model for patritumab, a fully human monoclonal antibody that targets human epidermal growth factor receptor 3.
Methods
A total of 833 serum concentrations were included in this analysis; serum concentrations were obtained from 145 subjects (136 with non-small cell lung cancer, nine with solid tumors) treated with patritumab [9 or 18 mg/kg intravenously every 3 weeks (q3w)] in one phase 1 and one phase 1b/2 study. Data were analyzed by nonlinear mixed-effect modeling.
Results
Patritumab PKs were best described through a two-compartment model with first-order elimination and interindividual variability on clearance (CL), volume of the central compartment (V c), distributional clearance, and volume of the peripheral compartment. In the final model, CL and V c were estimated as 0.0238 L/h and 3.62 L, respectively. Body weight (BW) and baseline albumin were found to be covariates for CL and BW was a covariate for V c. Covariates associated with hepatic and renal impairment were not significant on CL. Simulations showed that BW-based dosing reduced interindividual variability in patritumab exposure compared with fixed dosing.
Conclusions
The PK of patritumab was linear at the doses studied and well described by the two-compartment model. Hepatic and renal impairment did not appear to affect PK. Our results support BW-based dosing of patritumab on a q3w schedule.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human epidermal growth factor receptor 3 (HER3) and its ligand heregulin form signaling complexes with other HER family receptors, leading to the activation of multiple signaling pathways that are associated with cancer cell growth and survival [1–3]. HER3 has been associated with poor prognosis and decreased survival in patients with various tumor types, including non-small cell lung cancer (NSCLC) and breast cancer [4–7]. HER3 contains six docking sites for phosphoinositide 3-kinase and is a key dimerization partner for other HER family members. Activation of HER3 has been implicated in the development of resistance to anticancer treatment, including resistance to treatment targeting HER2 or epidermal growth factor receptor (EGFR) [7–10].
Patritumab is a fully human anti-HER3 antibody that prevents the receptor from binding heregulin [11–13]. Patritumab has been assessed as monotherapy in subjects with solid tumors [14, 15] and in combination with the EGFR inhibitor erlotinib in subjects with advanced NSCLC [16].
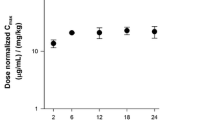
In a phase 1 study, patritumab pharmacokinetics were found to be similar to those observed for many other monoclonal antibodies, with a terminal elimination half-life of approximately 8 days [14] and a central volume of distribution of approximately 3 L [17]. Patritumab appears to follow dual pathways of elimination, including target-mediated nonlinear clearance and linear clearance through the reticuloendothelial system [18]. At patritumab doses of 9 and 18 mg/kg, linear pharmacokinetics were observed [8]. These doses were predicted to produce blood trough levels in mice approximately tenfold greater than those associated with maximal efficacy (3 µg/mL) [19].
To support the dose recommendations for patritumab, a pooled population pharmacokinetic model was developed on the basis of data from a phase 1 and a phase 1b/2 patritumab study. The objectives of the population pharmacokinetic model were to characterize the pharmacokinetics of patritumab, to evaluate the effect of potential covariates on patritumab pharmacokinetics, and to compare fixed dosing with body weight-adjusted dosing.
Materials and methods
Study design and methods
The data used in this analysis were collected from two studies that were approved by the institutional review board of participating institutions and conducted in accordance with the principles of the Declaration of Helsinki.
All subjects provided written informed consent.
An international phase 1b/2 study (HERALD; NCT01211483) examined patritumab in combination with erlotinib in EGFR treatment-naive subjects with advanced NSCLC after the failure of at least one prior chemotherapy [16]. In the phase 1b portion of the study, seven subjects received patritumab 18 mg/kg every 3 weeks (q3w) with erlotinib 150 mg/day. In the phase 2 portion of the study, 215 subjects were randomized to one of the following treatments: patritumab 18 mg/kg q3w with erlotinib 150 mg/day (n = 72); patritumab 18 mg/kg loading dose followed by 9 mg/kg q3w maintenance dose, with erlotinib 150 mg/day (n = 72); or placebo q3w with erlotinib 150 mg/day (n = 71).
An open-label, phase 1 study examined patritumab (9 or 18 mg/kg q3w) in Japanese subjects with advanced solid tumors (U31287-A-J101 [J101]) [15]. Nine subjects received one of the following: patritumab 9 mg/kg q3w (n = 3) or patritumab 18 mg/kg q3w (n = 6).
Blood sampling and analysis
In the phase 1b/2 HERALD study, blood sampling for pharmacokinetic analysis occurred prior to patritumab dosing in cycles 1–5, 7, and 9. In cycles 1 and 3, additional sampling occurred at the end of infusion and 3 h post-infusion. A subgroup of subjects (n = 22) participated in a substudy for intense patritumab pharmacokinetic assessment: in cycle 3, sampling was also performed 6, 24, and 72 h post-infusion and on days 8 and 15.
In the phase 1 J101 study, blood sampling for pharmacokinetic assessment occurred prior to patritumab dosing, at the end of infusion, and on day 22 in cycles 1–3. In cycle 1, additional sampling occurred 4, 7, 24, and 72 h after the start of infusion and on days 8 and 15. In cycle 4, sampling was only performed prior to patritumab dosing.
Serum patritumab concentrations were measured using a sandwich immunoassay. Samples were bound by murine antihuman monoclonal antibody (Amgen, Inc., Thousand Oaks, CA) that was immobilized to an assay plate. Following washing, patritumab was detected through the addition of biotin-conjugated anti-patritumab polyclonal antibody (Amgen, Inc.) and streptavidin HRP (R&D Systems, Inc., Minneapolis, MN). A tetramethylbenzidine peroxide solution (KPL Inc., Gaithersburg, MD) was added to produce a colorimetric signal, and absorbance was correlated with serum patritumab concentrations. The range of quantitation for this method was 100–4500 ng/mL. The assay method was validated, and samples were analyzed by Pharmaceutical Product Development, LLC (Richmond, VA). The intra-assay variability ranged from 3 to 9 % and the interassay variability ranged from 3 to 9 %. The total error for the validation samples ranged from 6 to 12 %. The % coefficient of variation of individual replicates ranged from 0 to 14 %.
Structural model
Nonlinear mixed-effects modeling was performed using NONMEM® program version 7.1.2 (ICON Development Solutions, Ellicott City, MD). Data set preparation for NONMEM® and graphical and exploratory analysis were performed using TIBCO Spotfire® S+8.1J (TIBCO Software Inc., Palo Alto, CA).
Pharmacokinetic parameters were estimated using a first-order conditional estimation method with interaction. The population pharmacokinetic model was developed in a stepwise fashion with evaluation at each step. Model selection was based on examination of objective function and visual inspection of goodness-of-fit plots.
Covariate analyses
Covariate analyses were performed on clearance and volume of distribution of the central compartment (V c). The relationships between individual estimates and covariates were initially investigated graphically. The demographic values of sex and baseline body weight (kg), which were measured at screening or from cycle 1 day 1, were used as baseline covariates candidates for V c and clearance. To assess the effect of renal impairment on patritumab, baseline creatinine clearance (mL/min; derived using the Cockcroft–Gault formula [20]) and baseline estimated glomerular filtration rate (mL/min; derived using the Modification of Diet in Renal Disease study equation [21]) were assessed as covariates for clearance. To assess the effect of hepatic impairment, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total albumin (µmol/L), and baseline albumin (g/L) were used as baseline covariate candidates for clearance [22]. Missing covariate values at baseline were replaced with the population medians.
Continuous covariates were entered into the model using the following equation (Eq. 1):
Categorical covariates were entered into the model using the following equation (Eq. 2):
where P indiv is the individual parameter of each subject, P typical is the population mean of the parameter, COVindiv is the individual covariate values of each subject, and θ represents the effect of the covariate on the parameter.
Covariates were selected using a forward selection approach (P < 0.05) with backward elimination (P < 0.01). Covariates were modeled individually, and only those found to significantly influence pharmacokinetic parameters were included in the final model.
Model evaluation
The model was evaluated using a visual predictive check. Covariates were randomly drawn from the population data set, and individual pharmacokinetic parameters were simulated based on the parameter estimates from the final model to simulate the patritumab concentrations for 4000 individuals. The median, 2.5th, and 97.5th percentiles were then calculated, as were the 95 % confidence intervals.
Subsequently, pharmacokinetic profiles following a fixed dose of 1260 mg patritumab (18 mg/kg × 70 kg) or a body weight-based dose of 18 mg/kg patritumab × body weight (kg) q3w were simulated for the first and second 2000 subjects, respectively.
Results
Subjects
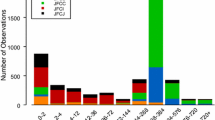
A total of 833 serum concentrations from 145 subjects (136 with NSCLC, nine with solid tumors) were included in the analysis. Subject demographics are given in Table 1. In the two studies, patritumab showed linear pharmacokinetics at doses of 9 and 18 mg/kg (Fig. 1).
Structural pharmacokinetic model
Combined data from the two studies were best described using a two-compartment model with first-order elimination. The model was parameterized in terms of clearance, V c, distributional clearance, and volume of the peripheral compartment (V p). Interindividual variability was added to clearance, V c, distributional clearance, and V p for the base model. Clearance was estimated to be 0.024 L/h (0.5 L/day), and V c was estimated to be 3.6 L. For the base model, shrinkage was low for clearance and V c (11.3 and 8.99 %, respectively) and high for distributional clearance and V p (69.5 and 66.0 %, respectively).
Covariate models
The effects of sex, baseline body weight, creatinine clearance, estimated glomerular filtration rate, and albumin were evaluated as covariates for clearance and/or V c. The effect of body surface area, lean body weight, and body mass index was not included in the model owing to their high correlation with body weight. Body weight has been reported to have a significant effect on clearance and volume of distribution of monoclonal antibodies [23]. In addition, the distributions of empirical Bayesian estimates of CL and V c based on the base model were similar among these body size descriptors. Therefore, body weight was added to the model as a covariate for both CL and V c as a first step. Further addition of sex as a covariate effect resulted in a statistically significant decrease in OFV. However, the estimate of the body weight effect decreased by 44 % after additionally including the effect of sex. This result revealed collinearity, as sex and body weight were highly correlated and, therefore, sex was not included in the model. Age, Eastern Cooperative Oncology Group performance status, ALT (U/L), AST (U/L), total bilirubin (µmol/L), creatinine clearance, and glomerular filtration rate at baseline were not included in the model since these were not significantly associated (P > 0.05) with patritumab pharmacokinetic parameter variability. Covariates on distributional clearance and V p were not considered because of the high shrinkage.
The pharmacokinetic parameter estimates of the final model were similar to those obtained in the base model and are given in Table 2. Clearance was estimated to be 0.0238 L/h (0.571 L/day). V c and V p were estimated to be 3.62 and 2.50 L, respectively, and distributional clearance was estimated to be 0.0178 L/h (0.426 L/day).
Body weight and baseline albumin were found to be significant covariates of clearance. For the range of body weights in the analysis population (42–121 kg), clearance ranged from 0.0178 to 0.0319 L/h (0.428–0.769 L/day), resulting in clearance values that were 25.1 % lower to 34.1 % higher compared with a subject with a typical body weight (71 kg). For the range of baseline albumin in the analysis population (21–59 g/L), clearance ranged from 0.0156 to 0.0421 L/h (0.374–1.01 L/day) or was 34.5 % lower or 77.0 % higher compared with a subject with typical baseline albumin (38 g/L).
Body weight was also found to be a significant covariate of V c. For the range of body weights in the analysis population (42–121 kg), V c ranged from 2.40 to 5.48 L, which was 33.6 % lower to 51.5 % higher compared with a subject with a typical body weight.
Model evaluation
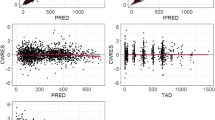
The visual predictive check for the final population pharmacokinetic model, stratified by study arm, indicated that the model provided adequate predictions of serum patritumab concentrations (Fig. 2).
Visual predictive check of the final population pharmacokinetic model by study arm. Dots are observations, full lines in blue are the median of the observations, and broken lines in blue are the 2.5th and 97.5th percentiles of the observations. Green bands are the 95 % prediction interval of median, and blue bands are the 95 % prediction interval of the 2.5th and 97.5th percentiles. LD loading dose, q3w every 3 weeks
Simulations of fixed versus body weight-based dosing
Pharmacokinetic simulations were performed to compare interindividual variability of patritumab exposure [i.e., area under the concentration time curve at steady state (AUCss) and maximum serum concentration at steady state (C max,ss)] following fixed or body weight-based dosing (Fig. 3). For fixed dosing, the simulated C max,ss values for the minimum to maximum body weights (42–121 kg) were 37.8 to +40.7 % relative to the median C max,ss. In contrast, for body weight-based dosing, the simulated C max,ss was −11.4 to +8.40 % relative to the median C max for the minimum and maximum body weights.
Similarly, for fixed dosing, the simulated AUCss values for the minimum to maximum body weights were −29.5 to +26.5 % relative to the median AUCss, compared with −16.5 to +19.5 % for body weight-based dosing.
Based on these results, body weight-based dosing was found to reduce interindividual variability in exposure compared with fixed dosing.
Simulations comparing patritumab dosing regimens
Pharmacokinetic simulations were performed to compare the regimen of patritumab 18 mg/kg q3w, the regimen of patritumab 18 mg/kg loading dose (LD) followed by 9 mg/kg as the maintenance dose (MD), every 2 weeks (q2w), every 3 weeks (q3w), and every 4 weeks (q4w) (Fig. 4). For the regimens of 18 mg/kg q3w and 18 mg/kg LD followed by 9 mg/kg q2w as MD, 90 % prediction intervals exceeded 3 µg/mL, which was associated with maximal efficacy in preclinical in vivo studies [19], and the exposures were comparable between these two regimens. For the regimen of 18 mg/kg LD followed by 9 mg/kg q3w as MD, the majority of the trough serum patritumab concentrations exceeded 3 µg/mL after maintenance dose administration. Only approximately 8–9 % of trough serum patritumab concentration does not achieve or maintain the target concentration continuously. In contrast, the regimen of 18 mg/kg LD followed by 9 mg/kg q4w as MD resulted in approximately 20 % of subjects who did not achieve a trough concentration >3 µg/mL after maintenance dose administrations, a considerable percentage which may impact clinical benefit.
Simulated serum patritumab concentration versus time for the regimen of patritumab a 18 mg/kg every 3 weeks, 18 mg/kg loading dose followed by 9 mg/kg as the maintenance dose, b every 2 weeks, c every 3 weeks, and d every 4 weeks (N = 1000 subjects/regimen). Full lines in blue are the median of the predicted concentrations. Green bands are the 90 % prediction interval of the 5th and 95th percentiles across the simulated patient population. Broken lines in gray are the target serum concentration (3 µg/mL) which associated with maximal efficacy in vivo. LD loading dose, q2w every 2 weeks, q3w every 3 weeks, q4w every 4 weeks
Based on these results, the regimen of patritumab 18 mg/kg loading dose followed by 9 mg/kg q3w as the maintenance dose was recommended for use in continued clinical investigations.
Discussion
A population pharmacokinetic model for patritumab was developed from data obtained from 145 subjects with NSCLC or advanced solid tumors who were enrolled in one of two studies. The pharmacokinetics of patritumab have been observed as nonlinear up to a dose of 6 mg/kg owing to the pathways of dual elimination inherent to target-mediated disposition of a monoclonal antibody [14, 24]. Consistent with previous pharmacokinetic results [14], patritumab demonstrated linear pharmacokinetics at doses of 9 mg/kg and 18 mg/kg. In these studies, serum patritumab concentrations were well described with a two-compartment model with first-order elimination. Results showed that V c was approximately serum volume and clearance for patritumab (clearance = 0.57 L/day) was relatively consistent with those reported for immunoglobulin G antibodies (0.2–0.5 L/day), after saturation of the target-mediated elimination pathway [17]. Body weight and baseline albumin were significant covariates of clearance, and body weight was also a significant covariate of V c. Sex was a statistically significant covariate on clearance after accounting for body weight, but the estimate of body weight effect decreased about 44 % due to the collinearity. Therefore, sex was not included as a covariate for clearance in the model.
Although only subjects with mild hepatic impairment were included in this analysis set because of inclusion criteria for the phase 1b/2 study, mild hepatic impairment appears to have no effect on the pharmacokinetics of patritumab based on the lack of significant effect between ALT, AST, and/or bilirubin on clearance in this study. The lack of effect of hepatic impairment on the clearance of patritumab is consistent with known clearance mechanisms of monoclonal antibodies that are dependent on the reticuloendothelial system rather than liver metabolism [22].
Similarly, based on the lack of effect of creatinine clearance and estimated glomerular filtration rate on clearance, mild renal impairment has no apparent effect on patritumab pharmacokinetics. This is consistent with what would be predicted from clearance pathways for immunoglobulin G1 antibodies, which are not cleared by the kidneys [18].
The interindividual variability of patritumab clearance and V c was significantly related to body weight. These effects may be addressed with the use of body weight-based dosing, which, based on the simulations performed, provided lower variability in exposure compared with fixed dosing across subjects with low to high body weight. The exponential functions for body weight as a covariate of V c and clearance were >0.5. Therefore, these simulation results are consistent with previously reported results, suggesting that body weight-adjusted dosing is the preferred regimen over fixed dosing when body weight has a significant effect on V c and clearance [25].
The regimen of patritumab 18 mg/kg q3w is considered to be comparable to the regimen of patritumab 18 mg/kg loading dose followed by 9 mg/kg q2w as the maintenance dose in exposure, as the total administration volume is same in first 6 weeks and total exposure is higher than the regimen of patritumab 18 mg/kg loading dose followed by 9 mg/kg q2w after the first 6 weeks. The regimen of patritumab 18 mg/kg loading dose followed by 9 mg/kg q3w is considered to be better than the regimen of patritumab 18 mg/kg loading dose followed by 9 mg/kg q4w; therefore, both regimens of patritumab 18 mg/kg q3w and 18 mg/kg as a loading dose, followed by 9 mg/kg q3w, are appropriate as related to pharmacokinetics.
The population pharmacokinetics analysis presented here supports the patritumab doses investigated in the phase 1b/2 study of NSCLC. The regimen of patritumab 18 mg/kg as a loading dose followed by 9 mg/kg q3w as the maintenance dose in combination with erlotinib is being further investigated in a phase 3 study in EGFR wild-type subjects with locally advanced or metastatic NSCLC who have progressed on at least one prior systemic therapy (NCT02134015) [26].
References
Hsieh AC, Moasser MM (2007) Targeting HER proteins in cancer therapy and the role of the non-target HER3. Br J Cancer 97:453–457
Liu J, Kern JA (2002) Neuregulin-1 activates the JAK–STAT pathway and regulates lung epithelial cell proliferation. Am J Respir Cell Mol Biol 27:306–313
Hegde GV, de la Cruz CC, Chiu C, Alag N, Schaefer G, Crocker L, Ross S, Goldenberg D, Merchant M, Tien J, Shao L, Roth L, Tsai SP, Stawicki S, Jin Z, Wyatt SK, Carano RA, Zheng Y, Sweet-Cordero EA, Wu Y, Jackson EL (2013) Blocking NRG1 and other ligand-mediated Her4 signaling enhances the magnitude and duration of the chemotherapeutic response of non-small cell lung cancer. Sci Transl Med 5:171ra18
Bièche I, Onody P, Tozlu S, Driouch K, Vidaud M, Lidereau R (2003) Prognostic value of ERBB family mRNA expression in breast carcinomas. Int J Cancer 106:758–765
Yi ES, Harclerode D, Gondo M, Stephenson M, Brown RW, Younes M, Cagle PT (1997) High c-erbB-3 protein expression is associated with shorter survival in advanced non-small cell lung carcinomas. Mod Pathol 10:142–148
Shames DS, Carbon J, Walter K, Jubb AM, Kozlowski C, Januario T, AnDo FuL, Xiao Y, Raja R, Jiang B, Malekafzali A, Stern H, Settleman J, Wilson TR, Hampton GM, Yauch RL, Pirzkall A, Amler LC (2013) High heregulin expression is associated with activated HER3 and may define an actionable biomarker in patients with squamous cell carcinomas of the head and neck. PLoS ONE 8:e56765
Xia W, Gerard CM, Liu L, Baudson NM, Ory TL, Spector NL (2005) Combining lapatinib (GW572016), a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases, with therapeutic anti-ErbB2 antibodies enhances apoptosis of ErbB2-overexpressing breast cancer cells. Oncogene 24:6213–6221
Baselga J, Swain SM (2009) Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer 9:463–475
Garrett JT, Olivares MG, Rinehart C, Granja-Ingram ND, Sánchez V, Chakrabarty A, Dave B, Cook RS, Pao W, McKinely E, Manning HC, Chang J, Arteaga CL (2011) Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci USA 108:5021–5026
Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, Moasser MM (2007) Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature 445:437–441
Freeman D, Ogbagabriel S, Rothe M, Radinsky R, Treder M (2008) Fully human anti-HER3 monoclonal antibodies (mAbs) have unique in vitro and in vivo functional and antitumor activities versus other HER family inhibitors. In: Abstract presented at 99th AACR annual meeting, San Diego, CA, 12–16 April 2008 (Abstract LB-21)
Treder M, Hartmann S, Ogbagabriel S, Borges E, Green L, Kang J, Radinsky R, Rothe M, Freeman D (2008) Fully human Anti-HER3 monoclonal antibodies (mAbs) inhibit oncogenic signaling and tumor cell growth in vitro and in vivo. In: Abstract presented at 99th AACR annual meeting, San Diego, CA, 12–16 April 2008 (Abstract LB-20)
Treder M, Ogbagabriel S, Moor R, Schulze-Horsel U, Hettmann T, Rothe M, Radinsky R, Freeman D (2008) Fully human anti-HER3 mAb U3-1287 (AMG 888) demonstrates unique in vitro and in vivo activities versus other HER family inhibitors in NSCLC models. Eur J Cancer 6(suppl):99 (Abstract 309)
LoRusso P, Jänne PA, Oliveira M, Rizvi N, Malburg L, Keedy V, Yee L, Copigneaux C, Hettmann T, Wu CY, Ang A, Halim AB, Beckman RA, Beaupre D, Berlin J (2013) Phase I study of U3-1287, a fully human anti-HER3 monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res 19:3078–3087
Wakui H, Yamamoto N, Nakamichi S, Tamura Y, Nokihara H, Yamada Y, Tamura T (2014) Phase 1 and dose-finding study of patritumab (U3-1287), a human monoclonal antibody targeting HER3, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol 73:511–516
Von Pawel J, Tseng J, Dediu M, Schumann C, Moritz B, Mendell-Harary J, Jin X, Feng W, Copigneaux C, Beckman RA (2014) Phase 2 HERALD study of patritumab (P) with erlotinib (E) in advanced NSCLC subjects (SBJs). J Clin Oncol 32(15 suppl):8045
Dirks NL, Meibohm B (2010) Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet 49:633–659
Wang W, Wang EQ, Balthasar JP (2008) Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 84:548–558
Lum P, Perez Ruixo JJ, Ogbagabriel S, DoshI S, Chen A, Yang BB, Hettman T, Radinsky R, Freeman D (2009) Identifying first in human (FIH) doses and schedule of U3-1287 (AMG 888), a fully human anti-HER3 mAb, based on preclinical pharmacokinetic (PK), pharmacodynamic (PD) and efficacy data. Mol Cancer Ther 8(12 suppl):B167. doi:10.1158/1535-7163.TARG-09-B167
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Stevens LA, Coresh J, Greene T, Levey AS (2006) Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med 354:2473–2483
Yang J, Shord S, Zhao H, Men Y, Rahman A (2013) Are hepatic impairment studies necessary for therapeutic proteins? Clin Ther 35:1444–1450
Xu Z, Vu T, Lee H, Hu C, Ling J, Yan H, Baker D, Beutler A, Pendley C, Wagner C, Davis HM, Zhou H (2009) Population pharmacokinetics of golimumab, an antitumor necrosis factor-alpha human monoclonal antibody, in patients with psoriatic arthritis. J Clin Pharmacol 49:1056–1070
Mager DE, Jusko WJ (2001) General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn 28:507–532
Bai S, Jorga K, Xin Y, Jin D, Zheng Y, Damico-Beyer LA, Gupta M, Tang M, Allison DE, Lu D, Zhang Y, Joshi A, Dresser MJ (2012) A guide to rational dosing of monoclonal antibodies. Clin Pharmacokinet 51:119–135
Paz-Ares L, Pawel Jv, Moritz B, Mendell J, Jin X, Copigneaux C, Beckman R (2014) Phase (Ph) 3 study of patritumab (P) plus erlotinib (E) in EGFR wild-type subjects with advanced non–small cell lung cancer (NSCLC). In: Poster presented at annual meeting of the European Society of Medical Oncology, Madrid, Spain, 26–30 September 2014 (Abstract 1336TiP)
Acknowledgments
Third-party writing assistance was provided by BlueMomentum, an Ashfield Company, part of UDG Healthcare plc, and supported by Daiichi Sankyo, Inc. and Daiichi Sankyo Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Satoshi Yoshiba, Mendel Jansen, Shuquan Chen, and Jeanne Mendell are employees of Daiichi Sankyo. Nobuko Matsushima is a former employee of Daiichi Sankyo.
Ethical standard
The data used in this analysis were collected from two studies that were approved by the institutional review board of participating institutions and conducted in accordance with the principles of the Declaration of Helsinki.
Rights and permissions
About this article
Cite this article
Yoshiba, S., Jansen, M., Matsushima, N. et al. Population pharmacokinetic analysis of patritumab, a HER3 inhibitor, in subjects with advanced non-small cell lung cancer (NSCLC) or solid tumors. Cancer Chemother Pharmacol 77, 987–996 (2016). https://doi.org/10.1007/s00280-016-3011-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3011-0