Abstract
Purpose
The objective of this study was to investigate the predictive value of F-18-fluorodeoxyglucose positron emission tomography (FDG-PET) for early assessment of tumor response to chemotherapy and for patient survival in gynecologic malignancies.
Methods
We performed CT and FDG-PET scans before initiation of chemotherapy to determine baseline conditions. PET scan was repeated after the first cycle of chemotherapy. The tumor response was later evaluated by CT scans after three cycles of chemotherapy, using RECIST. The PET response was analyzed in terms of the difference in SUVmax for FDG of the patient’s largest lesion between the baseline scan and after the first cycle of chemotherapy. The metabolic response for the tumor was defined as a 30 % reduction in its SUVmax.
Results
Eleven patients received platinum-based regimens, and 20 patients received non-platinum-based regimens. The mean progression-free survival (PFS) for the patients with a metabolic response was 13 months (range 5–29). In contrast, the mean PFS of the patients with no metabolic response was only 4.3 months (range 1–18). There was a statistically significant difference between the metabolic response and PFS (p = 0.002, Mann–Whitney U test). There was a strong correlation between the metabolic response and RECIST, regardless of the chemotherapy regimens used (platinum-based group, p = 0.006; non-platinum group, p = 0.046, Fisher’s exact test). The metabolic change in SUVmax was clearly predictive of tumor response in 93.5 % of patients.
Conclusion
Early FDG-PET assessment by measuring the single largest lesion is a very promising tool for rapidly predicting tumor responses and patient survival, regardless of the chemotherapy regimen.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The most widely accepted criteria for assessing a solid tumor response to treatment are the Response Evaluation Criteria in Solid Tumors (RECIST) [1]. For chemotherapy, RECIST requires at least two or three cycles of treatment before response assessment can begin, which has the potential risk of providing chemotherapy with less than optimal effects to patients. Avoiding providing inappropriate chemotherapy and minimizing individual-specific toxicity to the patients are two important goals for cancer clinicians. It is essential to keep a balance between maintaining quality of life (QOL) and continuing life-saving but invasive anti-cancer therapy, especially for recurrent cases. However, an effective method for predicting a chemotherapeutic response in individual cases has yet to be established.
F-18-fluorodeoxyglucose positron emission tomography (FDG-PET) has widely been used in oncology for evaluation of tumor status, treatment planning and detection of recurrent disease. However, the role of FDG-PET as a predictor of chemotherapeutic response is in its infancy and the ideal number of target lesions to be measured is still a major issue [2]. Measuring the sum of the standardized uptake values (SUVs) of all target lesions, in the same way as RECIST, is time-consuming, and the selection of the target lesions depends on a clinician’s preferences.
In the current study, we investigated the predictive value of a metabolic change, measured by FDG-PET, in a single largest lesion in a very early response to chemotherapy.
Materials and methods
Study design
The PEACH study (FDG-PET for Early Assessment of CHemotherapeutic response) was conducted from February 2011 to May 2015. A written informed consent was obtained from all patients. The institutional review board of Osaka University Hospital approved the present study. Thirty-one patients with measurable lesions were enrolled, with two primary and 29 recurrent/refractory cases. The one exclusion criterion used was for patients who had diabetes mellitus.
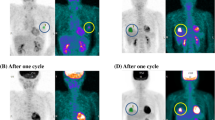
We performed CT and FDG-PET scans before initiation of chemotherapy to determine baseline conditions, and the PET scan was repeated after the completion of the first cycle of chemotherapy (within 7 days before the second cycle). The tumor response to treatment was evaluated by CT scan images after three chemotherapy cycles, according to RECIST guideline version 1.1 [1]. Complete response (CR) was defined as disappearance of all target lesions and partial response (PR) as at least a 30 % decrease in the sum of diameters of target lesions, taking as reference the baseline sum diameters. Progressive disease (PD) was defined as at least a 20 % increase in the sum of diameters of target lesion or the appearance of new lesion, and stable disease (SD) as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD. Those cases that had confirmed new lesions before completion of three cycles of chemotherapy were also defined as PD. Patients who demonstrated CR or PR were defined as “responder group,” and those who demonstrated SD or PD were defined as “non-responder group.”
Patient characteristics
The characteristics of the thirty-one patients are summarized in Table 1. The median age was 63 years (range 47–86). Staging was undertaken according to the 2009 International Federation of Gynecology and Obstetrics (FIGO) staging system. The origins of the primary lesion included the uterine cervix in one patient, the vagina in one, the uterine corpus in fifteen and the ovary/fallopian tube/peritoneum in fourteen.
Chemotherapy
As shown in Table 1, the patients received chemotherapy as first-line therapy in four patients, second-line therapy in thirteen, third line in ten, fourth line in one, fifth line in two and seventh line in one. The chemotherapy regimens used were carboplatin/paclitaxel (n = 4), paclitaxel/cisplatin (n = 1), weekly carboplatin/paclitaxel (n = 1), docetaxel/carboplatin (n = 2), pegylated liposomal doxorubicin (PLD) (n = 1), PLD/carboplatin (n = 3), gemcitabine (n = 2), irinotecan (n = 1), gemcitabine/irinotecan (n = 3) and gemcitabine/levofolinate/irinotecan/5-fluorouracil (GLIF) (n = 13). GLIF is a clinical trial regimen and will be described in near future elsewhere.
PET imaging
PET/CT imaging was performed with an integrated scanner (GEMINI GXL, Philips, Eindhoven, the Netherlands). Whole-body images, generally from the top of skull to mid-thighs, were acquired about 60 min after the intravenous injection of 18F-FDG at the dose of 0.10 mCi/kg body weight. The PET portion of the image was acquired using the following parameters: 3D emission scan, 2-min scan/bed position × 11 positions, OSEM reconstruction, 4.0 mm slice thickness/interval. Acquisition parameters for the CT portion of the image (16-slice CT) were as follows: breath-hold during normal expiration from the level of apex of lungs to the lower pole of the kidneys, without intravenous nor oral contrast media, 120 kVp and 50 effective mA, at 5.0 mm slice thickness/4.0-mm interval.
Abnormal FDG uptake was defined as the visually increased FDG uptake as compared to surrounding normal tissue. The standardized uptake value (SUV), which is decay-corrected tissue activity divided by the injected dose per patient body weight, was calculated at the tumor site. The maximum pixel value of SUV within each tumor (SUVmax) was used to express tumor activity semi-quantitatively.
Assessment of the PET response
The PET response was analyzed in terms of the SUVmax of the patient’s single largest lesion, between the baseline reading and after the first cycle of chemotherapy. In the current study, the cutoff value was defined as a 30 % reduction in the SUVmax. A drop of >30 % was defined as “positive response,” and a reduction of <30 % was defined as “negative response.” This definition is consistent with the recent literature [2, 3]. The metabolic changes in SUVmax between the baseline and one cycle after initiation of chemotherapy are presented as percentages. Progression-free survival (PFS) was defined as duration from the initiation of chemotherapy to tumor progression confirmed by diagnostic imaging. The Mann–Whitney U test was used to compare PFS between patients with a positive response in SUVmax (defined as a 30 % metabolism reduction) and patients with a negative response. The Fisher’s exact test was used to compare percent decrease in SUVmax measured in the single largest lesion one cycle after initiation of chemotherapy between responder group (defined as those who demonstrated CR or PR) and non-responder group (defined as those who demonstrated SD or PD). PFS curves with respect to the metabolic changes in SUVmax were constructed using the Kaplan–Meier method and were evaluated for statistical significance by the log-rank test. Statistical analysis was performed using JMP Pro 12 statistical software (SAS Institute, Inc., Cary, NC, USA). Results were considered to be significant when the p value was <0.05.
Results
Percent decrease in SUVmax by measuring the single largest lesion one cycle after initiation of chemotherapy and chemotherapeutic response
The metabolic changes in the SUVmax of the largest tumor of each of the thirteen-one patients are shown as percentages in Fig. 1. As summarized in Table 2, in the responder group (defined as those who demonstrated CR or PR), ten patients showed a positive metabolic response in SUVmax and one showed a negative response (p = 0.006, Fisher’s exact test). In contrast, of the non-responder group (defined as those who demonstrated SD or PD), only one patient showed a positive response and nineteen patients showed a negative response (p = 0.046, Fisher’s exact test). The metabolic change in SUVmax after the initial cycle of chemotherapy, by measuring the change in the single largest lesion, was predictive of eventual tumor response after three cycles in 93.5 % of the patients. In patients treated with platinum-containing regimens, the accuracy of metabolic change for assessing chemotherapeutic response was 100 %. Focusing on patients who received non-platinum chemotherapy, the same tendency was observed. The concordance rate was still 90 % in patients who received non-platinum regimens.
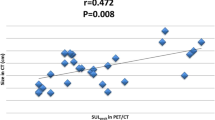
Percent decrease in SUVmax by measuring the single largest lesion one cycle after initiation of chemotherapy and patients’ survival
Of the thirty-one patients we followed, one patient was lost to follow-up and four patients have demonstrated no recurrences after chemotherapy to this date. The PFS was measured in the remaining 26 patients. Figure 2 and Table 3 show the correlation between the changes in SUVmax of the patient’s largest lesion and their PFS. The median PFS of the patients with lower decreases in SUVmax (<30 %) was 4.3 months (range 1–18). In contrast, the median PFS of the patients with higher decreases (≧30 %) was 13 months (range 5–29). There was a statistically significant correlation between metabolic change in SUVmax and PFS (p = 0.002, Mann–Whitney U test).
Figure 3 shows Kaplan–Meier analysis of relationship between PFS probability and the metabolic changes in SUVmax. PFS probability of the patients who demonstrated a positive metabolic response in SUVmax was significantly higher than those who demonstrated a negative metabolic response (p = 0.0043, log-rank test).
Discussion
RECIST version 1.0 (published in 2000) recommended measuring 10 target lesions in total with a maximum of five lesions per organ, for assessing tumor response to treatment [4]. The RECIST guidelines were revised in 2009, and version 1.1 is now the most commonly accepted criteria for assessing tumor response [1]. Although the maximum number of target lesions to be measured has been reduced in total from ten to five, and per organ from five to two [1], measuring five target lesions is still time-consuming and the selection of the target lesions depends on the clinician’s preferences [5].
Several authors have investigated the effectiveness of taking a CT scan measurement of a single lesion per organ for assessing tumor response [5–7]. Kim et al. [6] described the impact of measuring the single largest lesion in each organ in patients with metastatic colorectal cancer, compared with measuring two target lesions per organ, as recommended by the RECIST 1.1. They concluded that in 94.7 % of patients the single-lesion measurement was highly concordant with two-lesion measurements. They also reported that the single-lesion measurements in patients with non-small cell lung cancer and advanced gastric cancer had a concordance rate of 95.3 and 94.1 %, respectively [5, 7]. However, the data shown in these previous studies were retrospectively reviewed and the ideal number of target lesions to be measured has yet to be identified.
It has been shown that metabolic glucose uptake declines more rapidly than tumor size in patients who are responding well to chemotherapy. Egawa-Takata et al. [8] evaluated ovarian cancer cells in vitro for glucose uptake after cisplatin treatment and found an early decrease in glucose uptake that significantly preceded later cell death. Wieder et al. [9] demonstrated that a change in SUV, representing glucose uptake, preceded a reduction in tumor size in esophageal cancer. These initial results suggested that an early assessment by FDG-PET might be a better predictor of tumor response and patients’ survival, than RECIST criteria.
Early assessment by FDG-PET of tumor response to treatment has been described in recent papers [10–13]. Takahashi et al. [11] reported on their early assessment of FDG-PET uptake after 2 days of gefitinib treatment for lung cancer. The authors showed that the patients who demonstrated a diminished metabolic response had significantly prolonged PFS, compared with metabolic non-responders, when they defined a drop of >20 % in the sum of SUVmax as the cutoff value. Another paper described similar results in patients with non-small cell lung cancer treated with erlotinib [12]. A decrease in mean SUVmax (if more than one target lesion was present) at 14 days from baseline scan was associated with improved PFS and overall survival (OS). De Gues-Oei et al. [13] investigated changes in average SUV in lung cancer and showed a strong correlation with metabolic changes, OS and PFS. However, to the best of our knowledge, there have been no data yet published that evaluate change in the SUVmax of the single largest lesion after a single cycle of chemotherapy, as an accurate predictor of chemotherapeutic response and patient survival. Our results compellingly show that measuring metabolic changes in the single largest lesion is a simple, reliable and rapid method to predict patient outcomes.
The patients in the current study received various types of chemotherapy for various types of gynecologic malignancies. As shown in Table 2, we found a strong correlation between the amount of decrease in SUVmax of the single largest lesion and RECIST, regardless of the chemotherapy regimens. We believe that the data from our study might be widely utilized for better evaluating various types of chemotherapy regimens, to reduce lost treatment time from use of ineffective chemotherapies and unnecessary toxicity to patients.
Platinum-containing drugs remain the key option in gynecologic oncology. However, they have limited response rates (RR) as chemotherapy for advanced, recurrent or refractory gynecologic malignancies. In particular, the RR for recurrent, taxane-resistant or platinum-resistant malignancies ends in disappointing results [14–18]. Avoiding providing ineffective chemotherapy is beneficial to patients. Our results show that FDG-PET can predict chemotherapeutic effects far earlier than RECIST, thus enabling avoidance of inappropriate chemotherapy, with less toxicity to the patient, providing potential medical economic benefits, and leading to improved patient QOL.
The limitation of the current study is small sample size. We have evaluated only 31 patients, and a study conducted with larger sample size is necessary to support our results.
We believe this to be the first report of an evaluation of the correlation between FDG-PET scanning and clinical outcomes in patients with various types of gynecologic malignancies. We found that an early FDG-PET assessment of the chemotherapeutic effects of various types of chemotherapeutic regimens, by measuring the response to treatment in the single largest lesion, was a simple yet promising tool for predicting tumor response and patients’ survival. In conclusion, FDG-PET may be utilized for evaluating various types of chemotherapy regimens in a wide range of solid tumors and can be individualized for predicting chemosensitivity, providing benefits to patients.
References
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Wahl RL, Jacene H, Kasamon Y, Lodge MA (2009) From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 50:122S–150S
Farnebo J, Grybäck P, Harmenberg U et al (2014) Volumetric FDG-PET predicts overall and progression: free survival after 14 days of targeted therapy in metastatic renal cell carcinoma. BMC Cancer 14:408
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Kim HS, Kim JH, Yang I (2014) Tumor response assessment by measuring the single largest lesion per organ in patients with advanced non-small cell lung cancer. Lung Cancer 85:385–389
Kim HS, Kim JH (2014) Ideal number of target lesions per organ to measure in metastatic colorectal cancer. Oncol Lett 8:1896–1900
Kim HS, Kim JW, Kim JH et al (2015) Single-lesion measurement per organ for assessing tumor response in advanced gastric cancer. Oncology 88:69–75
Egawa-Takata T, Endo H, Fujita M et al (2010) Early reduction of glucose uptake after cisplatin treatment is a marker of cisplatin sensitivity in ovarian cancer. Cancer Sci 101:2171–2178
Wieder HA, Beer AJ, Lordick F et al (2005) Comparison of changes in tumor metabolic activity and tumor size during chemotherapy of adenocarcinomas of the esophagogastric junction. J Nucl Med 46:2029–2034
Zander T, Scheffler M, Nogova L et al (2011) Early prediction of nonprogression in advanced non-small-cell lung cancer treated with erlotinib by using [18F]fluorodeoxyglucose and [18F] fluorothymidine positron emission tomography. J Clin Oncol 29:1701–1708
Takahashi R, Hirata H, Tachibana I et al (2012) Early [18F]fluorodeoxyglucose positron emission tomography at two days of gefitinib treatment predicts clinical outcome in patients with adenocarcinoma of the lung. Clin Cancer Res 18:220–228
Mileshkin L, Hicks RJ, Hughes BG et al (2011) Changes in 18F-fluorodeoxyglucose and 18F-fluorodeoxythymidine positron emission tomography imaging in patients with non-small cell lung cancer treated with erlotinib. Clin Cancer Res 17:3304–3315
de Geus-Oei LF, van der Heijden HF, Visser EP et al (2007) Chemotherapy response evaluation with 18F-FDG PET in patients with non-small cell lung cancer. J Nucl Med 48:1592–1598
De Geest K, Blessing JA, Morris RT et al (2010) Phase II clinical trial of ixabepilone in patients with recurrent or persistent platinum- and taxane-resistant ovarian or primary peritoneal cancer: a gynecologic oncology group study. J Clin Oncol 28:149–153
Dizon DS, Blessing JA, McMeekin DS et al (2009) Phase II trial of ixabepilone as second-line treatment in advanced endometrial cancer: gynecologic oncology group trial 129-P. J Clin Oncol 27:3104–3108
Garcia AA, Blessing JA, Nolte S, Mannel RS, Gynecologic Oncology Group (2008) A phase II evaluation of weekly docetaxel in the treatment of recurrent or persistent endometrial carcinoma: a study by the Gynecologic Oncology Group. Gynecol Oncol 111:22–26
Di Legge A, Trivellizzi IN, Moruzzi MC et al (2011) Phase 2 trial of nonpegylated doxorubicin (Myocet) as second-line treatment in advanced or recurrent endometrial cancer. Int J Gynecol Cancer 21:1446–1451
Grisham RN, Adaniel C, Hyman DM et al (2012) Gemcitabine for advanced endometrial cancer: a retrospective study of the Memorial Sloan-Kettering Cancer Center experience. Int J Gynecol Cancer 22:807–811
Acknowledgments
We would like to thank Dr. G. S. Buzard for his constructive critique and editing of our manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Tanaka, Y., Ueda, Y., Egawa-Takata, T. et al. Early metabolic change in 18F-FDG-PET by measuring the single largest lesion predicts chemotherapeutic effects and patients’ survival: PEACH study. Cancer Chemother Pharmacol 77, 121–126 (2016). https://doi.org/10.1007/s00280-015-2935-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2935-0